Abstract
Synaptic vesicle endocytosis is stimulated by calcium influx in mature central nerve terminals via activation of the calcium-dependent protein phosphatase calcineurin. However in different neuronal preparations calcineurin activity is either inhibitory, stimulatory or irrelevant to the process. We addressed this inconsistency by investigating the requirement for calcineurin activity in synaptic vesicle endocytosis during development, using vesicle recycling assays in isolated nerve terminals. We show that endocytosis occurs independently of calcineurin activity in immature nerve terminals, and that a calcineurin requirement develops two to four weeks after birth. Calcineurin-independent endocytosis is not due to the absence of calcineurin activity, since calcineurin is present in immature nerve terminals and its substrate dynamin I is dephosphorylated on depolarisation. Calcineurin-independent endocytosis is calcium-dependent, since substitution of the divalent cation barium inhibits the process. Finally we demonstrated that in primary neuronal cultures derived from neonatal rats, endocytosis that was initially calcineurin-independent developed a calcineurin requirement on maturation in culture. Our data account for the apparent inconsistencies regarding the role of calcineurin in synaptic vesicle endocytosis, and we propose that an unidentified calcium sensor exists to couple Ca2+ influx to endocytosis in immature nerve terminals.
Keywords: endocytosis, calcineurin, calcium, nerve terminal, synaptic vesicle, FM2-10
Endocytosis of synaptic vesicles (SVs) is essential for the maintenance of neurotransmission in central nerve terminals. This is because endocytosis retrieves SVs from the neuronal plasma membrane, allowing them to become available for exocytosis after their subsequent recycling and refilling with neurotransmitter. To ensure the efficiency of this retrieval process, SV exocytosis and endocytosis are closely coupled both temporally and spatially in nerve terminals. This high degree of coupling originates from the influx of extracellular calcium (Ca2+) into nerve terminals on their depolarization (Cousin 2000). The vast majority of this Ca2+ influx occurs at active zones. Active zones have a high density of voltage-dependent Ca2+ channels and on nerve terminal depolarisation, intracellular free Ca2+ levels can reach as high as 1mM (Lin and Scheller 2000). Such high levels are required since the proposed Ca2+ sensor for exocytosis, synaptotagmin, has a very low affinity for Ca2+ (Yoshihara et al. 2003). After SV fusion with the plasma membrane, the same Ca2+ influx stimulates SV endocytosis. SV endocytosis occurs in regions surrounding the active zone, called, “endocytosis zones”, however the levels of Ca2+ required to stimulate the process are approximately 100 fold lower (Cousin 2000). The Ca2+ sensor for SV endocytosis in mature central nerve terminals is the Ca2+-dependent protein phosphatase calcineurin (CaN, (Marks and McMahon 1998; Cousin et al. 2001; Cousin and Robinson 2001).
CaN was identified as the Ca2+ sensor in isolated central nerve terminals (synaptosomes) using fluorescent assays of SV recycling (Marks and McMahon 1998; Cousin et al. 2001). However in other neuronal preparations, the CaN-dependence of SV endocytosis differs dramatically. For example, in primary neuronal cultures no requirement for CaN in SV endocytosis has ever been demonstrated, even though the process is Ca2+-dependent (Sankaranarayanan and Ryan 2001). Furthermore, at neuromuscular junctions in Drosophila larvae, CaN inhibits, rather than stimulates SV endocytosis (Kuromi et al. 1997; Kuromi and Kidokoro 1999). Conflicting results on the CaN-dependency of endocytosis in chromaffin cells have been reported, with some forms demonstrating an essential requirement for CaN, whereas in others CaN is inhibitory or has little effect (Artalejo et al. 1996; Engisch and Nowycky 1996; Chan and Smith 2001; Chan et al. 2003). Taken together, these results suggest that there are either multiple forms of SV endocytosis that have differential CaN requirements, or that other as yet unidentified Ca2+ sensors are responsible for this disparity.
We assessed whether the differing requirement for CaN in SV endocytosis between neuronal preparations could result from a change in the coupling of Ca2+ influx to the endocytosis sensor during development. We used a system that has an essential requirement for CaN activity (synaptosomes) and tested whether this was present throughout development, using fluorescent assays of SV recycling. We show that CaN activity only becomes essential for SV endocytosis between the ages of 2 - 4 weeks after birth. The same developmental pattern was also observed in primary neuronal culture. Our study highlights that Ca2+ influx couples to a different Ca2+ sensor to stimulate SV endocytosis in immature and adult animals.
Methods and Materials
Materials
Cyclosporin A and cypermethrin were from CN Biosciences (Nottingham, U.K). FM2-10 was from Molecular Probes (Oregon, USA). Dynamin I antibody (C-16) was from Santa Cruz Biotechnology (California, USA). Calcineurin antibody was from BD Biosciences (California, USA). Coverslips were from Raymond Lamb (Eastbourne, U.K.). All tissue culture plastics were obtained from either Falcon (Plymouth, U.K.) or Griener (Dursley, U.K.). Penicillin / streptomycin, phosphate buffered salts, foetal calf serum and Minimal Essential Medium were obtained from Invitrogen (Paisley, Strathclyde, U.K.). All other reagents were from Sigma (Poole, UK).
Synaptosome FM2-10 exocytosis and endocytosis assays
Synaptosomes were prepared from rat cerebral cortex by centrifugation on discontinuous percoll gradients (Dunkley et al. 1986). SV exocytosis was measured using the loading and unloading of FM2-10, as previously described (Cousin and Robinson 2000). Briefly, synaptosomes (0.6 mg of protein in 2 ml) were incubated for 5 min at 37°C in plus Ca2+ Krebs-like solution (118.5 mM NaCl, 4.7 mM KCl, 1.18 mM MgCl2, 0.1 mM K2HPO4, 20 mM Hepes, 1.3 mM CaCl2, 10 mM glucose, pH 7.4). FM2-10 (100 μM) was added 1 min before a standard stimulation with 30 mM KCl (S1). After 2 min of KCl stimulation, synaptosomes were washed twice in plus Ca2+ solution containing 1 mg/ml bovine serum albumin to remove non-internalised FM2-10. Washed synaptosomes were resuspended in either plus or minus (minus 1.3 mM CaCl, supplemented with 1 mM EGTA) Ca2+ Krebs-like solution at 37°C in the presence or absence of cyclosporin A (CsA), placed inside a fluorimeter (Spex FluoroMax, USA) and stimulated with 30 mM KCl. Unloading of accumulated FM2-10 was measured as the decrease in fluorescence upon release of the dye into solution by exocytosis (excitation 488 nm, emission 540 nm).
SV endocytosis was monitored in an identical manner to that described above apart from being loaded with FM2-10 in the presence or absence of either CsA or Ca2+ (Cousin et al. 2001). Accumulated FM2-10 was quantified by subsequent unloading with a standard addition of 30 mM KCl. Results are presented as the difference between unloading in plus and minus Ca2+ solutions for identical stimulation conditions for both exocytosis and endocytosis.
Glutamate release assay
The glutamate release assay was performed using enzyme-linked fluorescent detection of glutamate (Nicholls and Sihra 1986; Cousin and Robinson 2000). Synaptosomes were stimulated with either 1.3 mM CaCl2 or 1.3 mM BaCl2. Increases in fluorescence due to production of NADPH were monitored in a fluorimeter at 340 nm excitation and 460 nm emission. Experiments were standardised by the addition of 4 nmol of glutamate. Data are presented as Ba2+-dependent glutamate release, calculated as the difference between release in BaCl2 and CaCl2 stimulated samples.
SV recycling assays in CGNs
Primary cultures of cerebellar granule neurones (CGNs) were prepared from the cerebella of 7-day old Sprague-Drawley rat pups as previously described (Cousin and Nicholls 1997). SV exocytosis and endocytosis were monitored with FM2-10 using an S1 / S2 protocol which internally controls for responses from individual nerve terminals. This is necessary due to the high degree of variability in dye loading and unloading between individual nerve terminals in culture (Murthy et al. 1997). Cells were removed from culture medium and left to repolarise for 10 min in incubation medium (170 mM NaCl, 3.5 mM KCl, 0.4 mM KH2PO4, 20 mM TES (N-tris[hydroxy-methyl]-methyl-2-aminoethane-sulphonic acid), 5 mM NaHCO3, 5 mM glucose, 1.2 mM Na2SO4, 1.2 mM MgCl2, 1.3 mM CaCl2, pH 7.4). The total recycling pool of SVs was loaded with FM2-10 (100 μM) by evoking SV recycling with 50 mM KCl (50 mM NaCl removed to maintain osmolarity) for 2 min. This corresponds to S1 loading (Fig 5A). CGNs were washed and after 15 min FM2-10 was unloaded by two sequential 30 sec KCl challenges at 20 sec and 120 sec (S1 unloading, Fig 5A). This S1 protocol provides an estimate of the total number of SVs turned over during KCl stimulation. This was repeated for CGNs preincubated with 40 μM CsA for 15 min before and during S2 loading (for SV endocytosis) or S2 unloading (for SV exocytosis). FM2-10 unloading was quantified by monitoring the total decrease in fluorescence after normalising fluorescence in individual nerve terminals to an arbitrary value. FM2-10 unloading was visualised using a Nikon (Japan) epifluorescence microscope (Diaphot-TMD) and x20 objective at 480 nm excitation and > 510 nm emission. Fluorescent images were visualised using a Hammamatsu Orca-ER CCD digital camera (Japan) and offline imaging software (Compix Imaging Systems, USA). Data from at least 3 independent experiments each containing at least 70 nerve terminals were collated and presented as the ratio of the total unloading of FM2-10 at S2 and S1 respectively (S2 / S1).
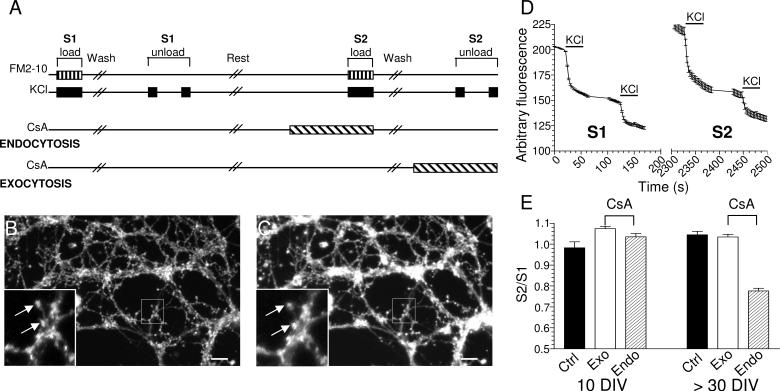
Fig 5. SV endocytosis develops a CaN requirement with age in culture.
(A) Protocol for quantifying the effect of CsA on SV exocytosis and endocytosis in CGNs. Image of CGN neurite field from a 10 days in vitro (DIV) culture loaded with FM2-10 at S1 (B) and S2 (C). Inset displays the reproducible loading of nerve terminals during repetitive stimuli (arrows). Scale bar denotes 1 μm. (D) Example of FM2-10 unloading from 10 DIV nerve terminals loaded at S1 and S2. The fluorescence decrease from 2 repetitive 50 mM KCl stimuli (bars) are summed and taken as the total amount of accumulated FM2-10 at S1 or S2 respectively. (E) S2 / S1 ratio of the effect of CsA (40 μM for 15 min) on either SV exocytosis (open bars) or endocytosis (hatched bars) in young (10 DIV) or mature (> 30 DIV) cultures. Experiments were ≥3 (10 DIV - control n = 221, CsA exocytosis n = 345, CsA endocytosis n = 246; > 30 DIV - control n = 252, CsA exocytosis n = 351, CsA endocytosis n = 215; all ± SEM).
Western blotting
The amount of dynamin I, calcineurin or phosphorylated dynamin I in synaptosomes was monitored by Western blotting using antibodies directed against either dynamin I, calcineurin or phospho-serine-774 and phospho-serine-778 (both on dynamin I (Tan et al. 2003)). Blots were scanned and analysed using GeneSnap and GeneTools (SynGene, USA) software.
Results
SV endocytosis is CaN-dependent in adult but not young animals
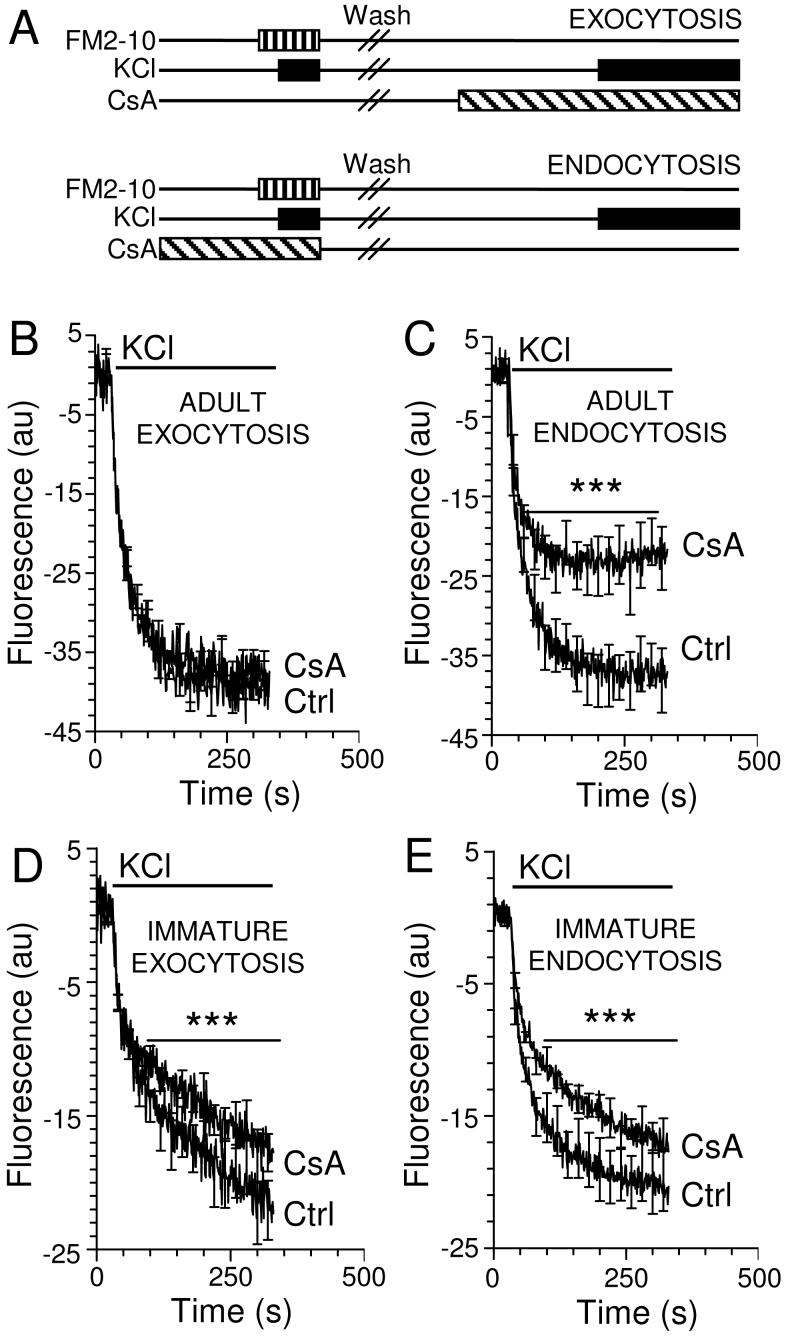
SV endocytosis in mature central nerve terminals is dependent upon CaN activity (Marks and McMahon 1998; Cousin et al. 2001). To determine whether CaN activity is also essential for SV endocytosis in immature nerve terminals we performed exocytosis and endocytosis assays using the styryl dye FM2-10 (Cousin and Robinson 2000). Fig 1A displays the protocol for monitoring the effect of the CaN antagonist CsA on both SV exocytosis and endocytosis. When CsA was applied to adult synaptosomes that received a standard load of FM2-10, it had no significant effect on KCl-evoked unloading and thus exocytosis (Fig 1B). This confirms previous studies where CsA had no effect on KCl-evoked Ca2+-dependent glutamate release in adult nerve terminals (Nichols et al. 1994; Marks and McMahon 1998; Cousin et al. 2001; Baldwin et al. 2003). When CsA or a different CaN antagonist, cypermethrin, were applied prior to FM2-10 loading, a significant inhibition of KCl-evoked unloading was observed (Fig 1C and data not shown). Previous studies have confirmed that this inhibition is a direct result of a block in SV endocytosis and not subsequent recycling (Marks and McMahon 1998). Thus CsA inhibits the accumulation of FM2-10 by endocytosis, since exocytosis is unaffected by the drug.
Fig 1. CsA inhibits SV endocytosis, but not exocytosis, from mature nerve terminals.
(A) Scheme of SV exocytosis and endocytosis assays using FM2-10 in synaptosomes. (B & D) Synaptosomes were loaded with 100 μM FM2-10 during stimulation with 30 mM KCl. After washing, synaptosomes were preincubated with or without extracellular Ca2+ for 5 min and FM2-10 unloading was stimulated with 30 mM KCl. (C & E) Synaptosomes were preincubated with or without extracellular Ca2+ for 5 min and then loaded with 100 μM FM2-10 during stimulation with 30 mM KCl. After washing, synaptosomes were preincubated in extracellular Ca2+ and FM2-10 unloading was stimulated with 30 mM KCl. Ca2+-dependent unloading of accumulated FM2-10 is displayed in (B-E). Where indicated, synaptosomes were preincubated with 40 μM CsA for 5 min before and during FM2-10 unloading (B & D) or before FM2-10 loading (C & E). Synaptosomes were prepared from either 8 month (B & C) or 2 week (D & E) animals. Upper bar indicates period of KCl stimulation, n = 3 ± SEM. Lower bar indicates *** = p < 0.001, 2-way ANOVA.
To determine whether CaN was essential for SV endocytosis through development, we repeated these assays using synaptosomes derived from 2 week old animals. CsA produced a small, equivalent and significant inhibition of both FM2-10 unloading and loading in these immature nerve terminals (Fig 1D, E). Cypermethrin also had a similar effect on FM2-10 loading in immature nerve terminals (data not shown). This small inhibition of FM2-10 loading in the endocytosis assay was a direct result of an upstream inhibition of SV exocytosis and not SV endocytosis, since the CsA inhibited FM2-10 unloading (SV exocytosis) to the same extent. CsA had similar effects on Ca2+-dependent glutamate release from immature synaptosomes (data not shown). Since SV endocytosis is dependent on the prior amount of exocytosis, the decrease in the number of SVs loaded with FM2-10 was due to a decrease in availability of SVs to retrieve. Thus at 2 weeks SV endocytosis is independent of CaN activity.
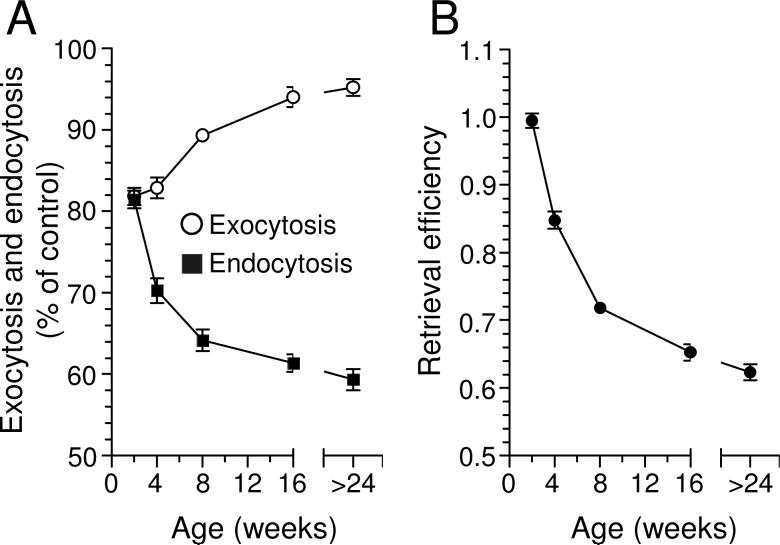
To investigate when a requirement for CaN activity develops, we examined the effect of CsA on SV exocytosis and endocytosis in a range of animal ages (Fig 2A). To obtain an accurate estimate of SV endocytosis, we used the retrieval efficiency calculation (endocytosis / exocytosis (Cousin and Robinson 1998)), since the amount of SV endocytosis is dependent on the prior amount of exocytosis. We determined that SV endocytosis becomes CaN-dependent 4 weeks after birth (Fig 2B). By 16 weeks the requirement for CaN starts to plateau until maturity is reached, where almost half of all SV endocytosis is dependent on CaN activity. Thus immature nerve terminals do not require CaN activity for SV endocytosis, and this requirement develops with age.
Fig 2. SV endocytosis develops a requirement for CaN with age.
(A) Effect of CsA on FM2-10 unloading (SV exocytosis) and FM2-10 loading (SV endocytosis). Results are expressed as a percentage of control (i.e. % remaining after CaN inhibition). The difference in the effect of CsA on exocytosis and endocytosis becomes significantly different at 4 weeks (P < 0.001 student’s t test). (B) Retrieval efficiency (endocytosis / exocytosis) from synaptosomes derived from rats of increasing age. For both A and B, n ≥ 3 ± SEM.
SV endocytosis in young animals is Ca2+-dependent
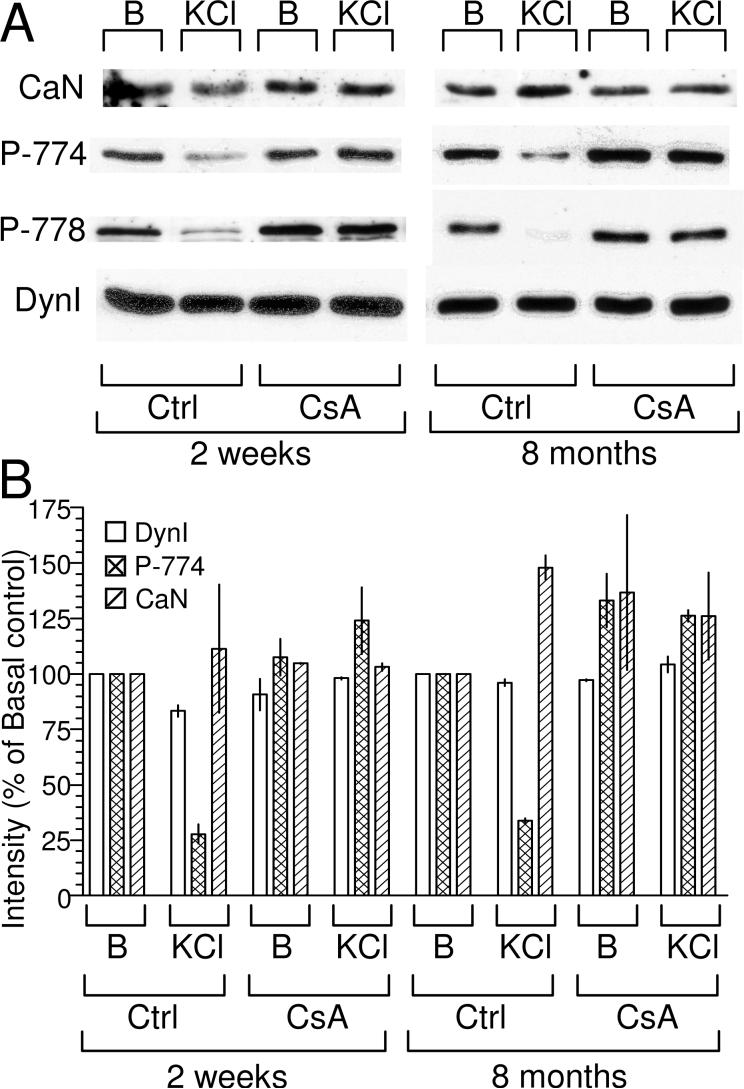
One reason for the lack of CaN dependence of endocytosis in immature nerve terminals could be that the enzyme is either not present or not active. To test this possibility we probed lysates from both immature and mature nerve terminals for the presence of CaN. Fig 3 shows that there is no difference in the amount of CaN present between immature and mature nerve terminals. To test whether the CaN present in immature nerve terminals is active, we examined the phosphorylation status of one of its substrates, dynamin I. Dynamin I is coordinately dephosphorylated by CaN in mature nerve terminals at two sites on nerve terminal depolarization (Tan et al. 2003; Larsen et al. 2004). When probed with phopho-specific antibodies directed against these sites, dynamin I was dephosphorylated on both during depolarization of immature nerve terminals and this dephosphorylation was abolished by CsA and cypermethrin (Fig 3, data not shown). Thus CaN is present and active in immature nerve terminals.
Fig 3. CaN is present and active in immature nerve terminals.
(A) Synaptosome lysates derived from either 2 week or 8 month old rats were blotted for either CaN, phosphorylated (P-774 & P-778) or total (DynI) dynamin I. Lysates were prepared from either basal (B) or depolarised (30 mM KCl) synaptosomes with or without preincubation with 40 μM CsA (5 min) as indicated. (B) Relative intensity of signal from 2 independent experiments displayed as a percentage of the basal / control condition for both 2 week and 8 month samples. Open bars denote total dynamin I (Dyn I), cross-hatched bars denote phosphorylated (P-774) and hatched bars denote CaN. Error bars denote the range of intensities.
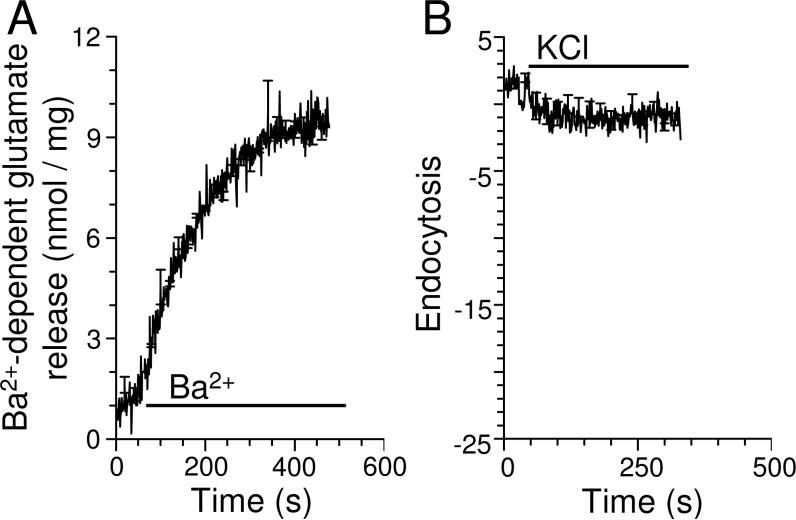
SV endocytosis may not require CaN activity in immature nerve terminals because the process may not be Ca2+-dependent at this stage of development. To test this possibility we stimulated SV recycling with the divalent cation barium (Ba2+). Ba2+ inhibits a presynaptic voltage-sensitive K+ channel, inducing transient plasma membrane depolarizations similar to action potentials (Marks and McMahon 1998; Cousin and Robinson 1998). It also stimulates SV exocytosis by entering nerve terminals through voltage-dependent Ca2+ channels and substituting for Ca2+. However Ba2+ does not support SV endocytosis in mature nerve terminals, due to its inability to activate CaM / CaN (Marks and McMahon 1998; Cousin and Robinson 1998). Ca2+ cannot inhibit K+ channels and therefore is unable to evoke SV exocytosis independently. We assayed Ba2+-evoked release of endogenous glutamate, since Ba2+ has complex effects on FM2-10 unloading (data not shown). Ba2+ efficiently stimulated glutamate release (Fig 4A) but could not evoke SV endocytosis in immature nerve terminals (Fig 4B). Thus SV endocytosis in immature nerve terminals is robustly Ca2+-dependent, suggesting that a different Ca2+ sensor must activate the process.
Fig 4. SV endocytosis in immature nerve terminals is Ca2+-dependent.
(A) Glutamate release from 2 week old animals was evoked by either 1.3 mM CaCl2 or 1.3 mM BaCl2. Ba2+-dependent glutamate release is displayed (BaCl2 minus CaCl2, n=3 ± SEM). Bar denotes period of BaCl2 addition. (B) FM2-10 was loaded into 2 week old synaptosomes using an S1 stimulation of either 1.3 mM CaCl2 or 1.3 mM BaCl2. The amount of accumulated FM2-10 was quantified with an unloading stimulus of 30 mM KCl (bar). Graph displays Ba2+-dependent FM2-10 loading (BaCl2 minus CaCl2, n=3 ± SEM).
SV endocytosis develops CaN-dependency with age in primary neuronal cultures
To demonstrate the developing requirement for CaN in SV endocytosis, we used primary cultures of cerebellar granule neurones (CGNs). Primary neuronal cultures are usually derived from embryonic or neonatal tissue, possibly explaining why a requirement for CaN in SV endocytosis has never been reported. We would predict that SV endocytosis would be initially CaN-independent and then develop a requirement for CaN on maturation in culture. We devised a protocol to test this hypothesis (Fig 5A). CGNs are loaded with FM2-10 using KCl stimulation and the accumulated dye is then unloaded with 2 KCl challenges (S1). The same nerve terminals are loaded with CsA present either before (endocytosis) or after (exocytosis) the S2 loading stimulus. Inhibition of exocytosis or endocytosis by CsA is estimated as the ratio of total FM2-10 unloaded at S2 to that at S1.
Figs 5 B-D show a CGN field after S1 loading (B) and S2 loading (C) and unloading data from a typical control experiment (D). Note that the total amount of unloading for both S1 and S2 are equal, reflecting the reproducibility of loading and unloading. CsA had no effect on the S2 / S1 ratio for either SV exocytosis or endocytosis in CGNs after 10 days in vitro (Fig 5E). Thus in immature CGN cultures both SV exocytosis and endocytosis is CaN-independent. To determine if a requirement for CaN in SV endocytosis develops with age, we performed the same protocol using CGNs older than 30 days in vitro. A robust and reproducible inhibition of SV endocytosis by CsA was observed, with no effect on SV exocytosis (Fig 5E). Thus in both primary neuronal cultures and acutely isolated nerve terminals a requirement for CaN activity in SV endocytosis develops on nerve terminal maturation.
Discussion
Ca2+ influx stimulates both SV exocytosis and endocytosis in mature nerve terminals (Marks and McMahon 1998; Cousin and Robinson 1998; Cousin 2000). The protein phosphatase CaN is activated by this influx and the subsequent dephosphorylation of a group of proteins called the dephosphins is essential for SV endocytosis (Cousin and Robinson 2001). We demonstrate that the requirement for CaN activity develops 2 - 4 weeks after birth using nerve terminals prepared from both immature and adult animals. The lack of CaN-dependence of SV endocytosis in immature nerve terminals is not explained by an absence or inactivation of CaN, since dynamin I is still dephosphorylated on nerve terminal depolarisation. Likewise it is not because of a lack of Ca2+-dependency, since SV endocytosis is abolished by substituting the divalent cation Ba2+ for Ca2+ (Marks and McMahon 1998; Cousin and Robinson 1998). Finally we have shown that SV endocytosis is CaN-dependent in mature but not immature primary neuronal cultures.
A developmental change in the CaN-dependence of SV endocytosis
A developmental requirement for CaN activity in SV endocytosis explains the apparent contradictions in the published literature. The only other experimental system to demonstrate an obligatory requirement for CaN activity is the bovine chromaffin cell (Chan and Smith 2001; Chan et al. 2003). Two forms of endocytosis were observed in cells derived from adult animals during stimulation with action potential waveforms: a CaN-independent form that predominated at basal frequencies (termed type I endocytosis) and a CaN-dependent form that was activated by higher firing frequencies (termed type II endocytosis) (Chan and Smith 2001). In a different study using adult chromaffin cells, CaN antagonists abolished the Ca2+-dependency of endocytosis evoked by square waveform depolarizations (termed compensatory retrieval) (Engisch and Nowycky 1996). This loss of Ca2+-dependency was explained by a selective inhibition of type II endocytosis, since compensatory retrieval is likely to be a mixture of both type I and type II retrieval (Chan and Smith 2001). In contrast, chromaffin cells derived from immature animals have no requirement for CaN. Various approaches to disrupt CaN function all resulted in an acceleration of a rapid and excess retrieval of membrane that was stimulated by high intensity square waveform pulses (Artalejo et al. 1996). This suggests that CaN activity may inhibit endocytosis in younger animals. A similar inhibitory role for CaN is apparent at the neuromuscular junction of Drosophila larvae, where CaN antagonists greatly increase FM1-43 uptake into nerve terminals (Kuromi et al. 1997; Kuromi and Kidokoro 1999). This form of SV endocytosis was strictly Ca2+-dependent, as was the excess retrieval pathway reported in calf chromaffin cells (Artalejo et al. 1996). When these results are considered with our data it appears that SV endocytosis is Ca2+-dependent at all ages of animal, from pre- to post-natal. However during development the requirement for CaN changes from a putative inhibitory role in immature nerve terminals to a stimulatory role in mature nerve terminals.
An unidentified Ca2+ sensor for SV endocytosis in immature nerve terminals
We suggest that the lack of CaN-dependency in immature nerve terminals is due to a switch in the Ca2+ coupling of SV endocytoisis during development. In other words, Ca2+ influx is still required to stimulate SV endocytosis, but the sensor that transduces its effect is different. A requirement for this unidentified sensor may continue into adulthood, since CaN antagonists do not always abolish SV endocytosis ((Cousin et al. 2001), but see (Marks and McMahon 1998)). One possibility is that Ca2+ influx controls essential interactions between endocytosis proteins, such as that observed for dynamin I with both endophilin and synaptophysin (Daly and Ziff 2002; Chen et al. 2003). Another is that the Ca2+ binding protein calmodulin (CaM) is the Ca2+ sensor, since excess membrane retrieval in calf adrenal chromaffin cells has an absolute requirement for its activity (Artalejo et al. 1996). Possible downstream targets are still unclear, however CaM may act via the release of a Ca2+-dependent binding partner such as GAP-43, rather than the activation of a Ca2+-dependent target. In cortical neurones overexpression of GAP-43 mutants that constitutively bind CaM disrupted SV endocytosis, whereas GAP-43 mutants unable to bind CaM enhanced the process (Neve et al. 1998). Thus the release of a Ca2+-independent CaM-interacting protein may be a possible mechanism to control SV endocytosis. This has previously been observed in yeast, where the release of the Ca2+-independent binding partner unconventional myosin I (Geli et al. 1998) and the Ca2+-dependent activation of the actin binding protein Arp2/3 are both essential for endocytosis (Kubler et al. 1994; Schaerer-Brodbeck and Riezman 2000). Thus CaM may play a dual role in the control of SV endocytosis by activating CaN and releasing a Ca2+-independent binding partner.
We have uncovered a developmental switch in the Ca2+ coupling of SV endocytosis in central nerve terminals. In immature animals SV endocytosis has no requirement for CaN activity however this requirement develops from four weeks after birth through to adulthood. Our data explain previous inconsistencies in the published data and indicate that a different Ca2+ sensor must stimulate the process in immature nerve terminals.
Acknowledgements
We thank Prof. Phil Robinson for the gift of the phospho-specific dynamin I antibodies. This work was supported by grants from The Wellcome Trust (Ref: GR070569), the Cunningham Trust and a University of Edinburgh Medical Faculty Scholarship (to KJS).
Abbreviations
- CaN
calcineurin
- CsA
cyclosporin A
- SV
synaptic vesicle
- CGN
cerebellar granule neurone
- CaM
calmodulin
- Ca2+
calcium
- Ba2+
barium
Reference List
- Artalejo CR, Elhamdani A, Palfrey HC. Calmodulin is the divalent cation receptor for rapid endocytosis, but not exocytosis, in adrenal chromaffin cells. Neuron. 1996;16:195–205. doi: 10.1016/s0896-6273(00)80036-7. [DOI] [PubMed] [Google Scholar]
- Baldwin ML, Rostas JA, Sim AT. Two modes of exocytosis from synaptosomes are differentially regulated by protein phosphatase types 2A and 2B. J Neurochem. 2003;85:1190–1199. doi: 10.1046/j.1471-4159.2003.01779.x. [DOI] [PubMed] [Google Scholar]
- Chan SA, Chow R, Smith C. Calcium dependence of action potential-induced endocytosis in chromaffin cells. Pflugers Arch. 2003;445:540–546. doi: 10.1007/s00424-002-0966-y. [DOI] [PubMed] [Google Scholar]
- Chan SA, Smith C. Physiological stimuli evoke two forms of endocytosis in bovine chromaffin cells. J Physiol. 2001;537:871–885. doi: 10.1111/j.1469-7793.2001.00871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Deng L, Maeno-Hikichi Y, Lai M, Chang S, Chen G, Zhang JF. Formation of an endophilin-Ca2+ channel complex is critical for clathrin-mediated synaptic vesicle endocytosis. Cell. 2003;115:37–48. doi: 10.1016/s0092-8674(03)00726-8. [DOI] [PubMed] [Google Scholar]
- Cousin MA. Synaptic vesicle endocytosis: calcium works overtime in the nerve terminal. Mol Neurobiol. 2000;22:115–128. doi: 10.1385/MN:22:1-3:115. [DOI] [PubMed] [Google Scholar]
- Cousin MA, Nicholls DG. Synaptic vesicle recycling in cultured cerebellar granule cells: Role of vesicular acidification and refilling. J Neurochem. 1997;69:1927–1935. doi: 10.1046/j.1471-4159.1997.69051927.x. [DOI] [PubMed] [Google Scholar]
- Cousin MA, Robinson PJ. Ba2+ does not support synaptic vesicle retrieval in rat isolated presynaptic nerve terminals. Neurosci Lett. 1998;253:1–4. doi: 10.1016/s0304-3940(98)00610-7. [DOI] [PubMed] [Google Scholar]
- Cousin MA, Robinson PJ. Two mechanisms of synaptic vesicle recycling in rat brain nerve terminals. J Neurochem. 2000;75:1645–1653. doi: 10.1046/j.1471-4159.2000.0751645.x. [DOI] [PubMed] [Google Scholar]
- Cousin MA, Robinson PJ. The dephosphins: Dephosphorylation by calcineurin triggers synaptic vesicle endocytosis. Trends Neurosci. 2001;24:659–665. doi: 10.1016/s0166-2236(00)01930-5. [DOI] [PubMed] [Google Scholar]
- Cousin MA, Tan TC, Robinson PJ. Protein phosphorylation is required for endocytosis in nerve terminals. Potential role for the dephosphins dynamin I and synaptojanin, but not AP180 or amphiphysin. J Neurochem. 2001;76:105–116. doi: 10.1046/j.1471-4159.2001.00049.x. [DOI] [PubMed] [Google Scholar]
- Daly C, Ziff EB. Ca2+-dependent formation of a dynamin-synaptophysin complex: potential role in synaptic vesicle endocytosis. J Biol Chem. 2002;277:9010–9015. doi: 10.1074/jbc.M110815200. [DOI] [PubMed] [Google Scholar]
- Dunkley PR, Jarvie PE, Heath JW, Kidd GJ, Rostas JA. A rapid method for isolation of synaptosomes on Percoll gradients. Brain Res. 1986;372:115–129. doi: 10.1016/0006-8993(86)91464-2. [DOI] [PubMed] [Google Scholar]
- Engisch KL, Nowycky MC. Calcium dependence of large dense-cored vesicle exocytosis evoked by calcium influx in bovine adrenal chromaffin cells. J Neurosci. 1996;16:1359–1369. doi: 10.1523/JNEUROSCI.16-04-01359.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geli MI, Wesp A, Riezman H. Distinct functions of calmodulin are required for the uptake step of receptor-mediated endocytosis in yeast: the type I myosin myo5p is one of the calmodulin targets. EMBO J. 1998;17:635–647. doi: 10.1093/emboj/17.3.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubler E, Schimmoller F, Riezman H. Calcium-independent calmodulin requirement for endocytosis in yeast. EMBO J. 1994;13:5539–5546. doi: 10.1002/j.1460-2075.1994.tb06891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuromi H, Kidokoro Y. The optically determined size of exo/endo cycling vesicle pool correlates with the quantal content at the neuromuscular junction of Drosophila larvae. J Neurosci. 1999;19:1557–1565. doi: 10.1523/JNEUROSCI.19-05-01557.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuromi H, Yoshihara M, Kidokoro Y. An inhibitory role of calcineurin in endocytosis of synaptic vesicles at nerve terminals of Drosophila larvae. Neurosci Res. 1997;27:101–113. doi: 10.1016/s0168-0102(96)01132-7. [DOI] [PubMed] [Google Scholar]
- Larsen MR, Graham ME, Robinson PJ, Roepstorff P. Improved detection of hydrophilic phosphopeptides using graphite powder micro-columns and mass spectrometry: Evidence for in vivo doubly phosphorylated dynamin I and dynamin III. Mol Cell Proteomics. 2004;3:456–65. doi: 10.1074/mcp.M300105-MCP200. [DOI] [PubMed] [Google Scholar]
- Lin RC, Scheller RH. Mechanisms of synaptic vesicle exocytosis. Annu Rev Cell Dev Biol. 2000;16:19–49. doi: 10.1146/annurev.cellbio.16.1.19. [DOI] [PubMed] [Google Scholar]
- Marks B, McMahon HT. Calcium triggers calcineurin-dependent synaptic vesicle recycling in mammalian nerve terminals. Curr Biol. 1998;8:740–749. doi: 10.1016/s0960-9822(98)70297-0. [DOI] [PubMed] [Google Scholar]
- Murthy VN, Sejnowski TJ, Stevens CF. Heterogeneous release properties of visualized individual hippocampal synapses. Neuron. 1997;18:599–612. doi: 10.1016/s0896-6273(00)80301-3. [DOI] [PubMed] [Google Scholar]
- Neve RL, Coopersmith R, McPhie DL, Santeufemio C, Pratt KG, Murphy CJ, Lynn SD. The neuronal growth-associated protein GAP-43 interacts with rabaptin-5 and participates in endocytosis. J Neurosci. 1998;18:7757–7767. doi: 10.1523/JNEUROSCI.18-19-07757.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls DG, Sihra TS. Synaptosomes possess an exocytotic pool of glutamate. Nature. 1986;321:772–773. doi: 10.1038/321772a0. [DOI] [PubMed] [Google Scholar]
- Nichols RA, Suplick GR, Brown JM. Calcineurin-mediated protein dephosphorylation in brain nerve terminals regulates the release of glutamate. J Biol Chem. 1994;269:23817–23823. [PubMed] [Google Scholar]
- Sankaranarayanan S, Ryan TA. Calcium accelerates endocytosis of vSNAREs at hippocampal synapses. Nat Neurosci. 2001;4:129–136. doi: 10.1038/83949. [DOI] [PubMed] [Google Scholar]
- Schaerer-Brodbeck C, Riezman H. Functional interactions between the p35 subunit of the Arp2/3 complex and calmodulin in yeast. Mol Biol Cell. 2000;11:1113–1127. doi: 10.1091/mbc.11.4.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan TC, Valova VA, Malladi CS, Graham ME, Berven LA, Jupp OJ, Hansra G, McClure SJ, Sarcevic B, Boadle RA, Larsen MR, Cousin MA, Robinson PJ. Cdk5 is essential for synaptic vesicle endocytosis. Nat Cell Biol. 2003;5:701–710. doi: 10.1038/ncb1020. [DOI] [PubMed] [Google Scholar]
- Yoshihara M, Adolfsen B, Littleton JT. Is synaptotagmin the calcium sensor? Curr Opin Neurobiol. 2003;13:315–323. doi: 10.1016/s0959-4388(03)00063-1. [DOI] [PubMed] [Google Scholar]