Abstract
Angiotensin II (ANG II)-infused rats exhibit increases in distal nephron renin expressed in principal cells of connecting tubules and collecting ducts. This study was performed to determine whether the augmentation of distal nephron renin involves ANG II type 1 (AT1) receptor activation. Male Sprague-Dawley rats (200 -220 g) were divided into three groups: 1) sham operated (n = 8); 2) ANG II infused (80 ng/min, 13 days, n = 8); and 3) ANG II infused plus AT1 receptor blocker (ARB), olmesartan (5 mg/days, n = 8). ANG II infusion increased systolic blood pressure (BP; 178 ± 4 vs. 122 ± 1 mmHg; P < 0.001) and suppressed plasma renin activity (PRA; 0.08 ± 0.1 vs. 5.3 ± 0.8 ng ANG I · ml-1·h-1). ARB treatment prevented the increase in BP (113 ± 6 mmHg) and led to increases in PRA (15.8 ± 1.5 ng ANG I·ml-1h-1). Renin protein levels measured in the kidney medulla, to avoid contribution from juxtaglomerular appartus cells, were higher in ANG II-infused rats [1.64 ± 0.3 vs. 1.00 ± 0.1 densitometric units (DU) compared with sham-operated rats; P < 0.05], and ARB treatment prevented this increase (1.01 ± 0.1). Similarly, renin immunoreactivity increased in medullary collecting ducts of ANG II-infused compared with sham-operated rats (2.5 ± 0.3 vs. 1.0 ± 0.2 DU; P < 0.001), which was also prevented by ARB (1.01 ± 0.06). Renin qRTPCR in ANG II-infused rats showed higher mRNA levels in the kidney medulla compared with sham-operated rats (5.5 ± 2.3 vs. 0.04 ± 0.02 ratio to GAPDH mRNA levels; P < 0.001); however, renin transcript levels were normalized in the ARB-treated rats. These data demonstrate that the augmentation of distal nephron renin in ANG II-infused hypertensive rats is AT1 receptor mediated. The augmented distal tubular renin may contribute to increased intratubular ANG II levels and distal nephron sodium reabsorption in ANG II-dependent hypertension.
Keywords: distal nephron, immunohistochemistry, Western blotting, qRT-PCR
ANGIOTENSIN II (ANG II) plays an important role in fluid, electrolyte, and blood pressure regulation. In ANG II-dependent hypertension, intrarenal ANG II levels increase to a greater extent than can be explained from the circulating levels (4, 6, 10, 20, 25, 33, 36, 37). The augmented intrarenal ANG II levels are partially due to enhanced uptake of ANG II via an AT1 receptor-mediated mechanism and augmentation of proximal tubule cell angiotensinogen (AGT) formation and secretion (11, 13, 14, 35, 37). Chronically elevated intrarenal ANG II levels exert a stimulatory action on proximal tubule AGT transcript and protein which is mediated by the AT1 receptor (17). The finding that urinary excretion of AGT also increases in a time- and dose-dependent manner indicates that proximally formed AGT is delivered to distal nephron segments, which could thus contribute to intratubular ANG I and ANG II formation in distal nephrons as long as adequate sources of renin and angiotensin-converting enzyme (ACE) are available. Substantial ACE activity in collecting duct fluid and urine have been demonstrated (1), in addition to an increment of intrarenal ACE binding in ANG II-dependent hypertension. In contrast, juxtaglomerular (JG) renin as well as plasma renin activity (PRA) are suppressed by chronic ANG II infusions (20, 32).
Although renin is mainly produced by JG cells, renin transcript and protein are also present in renal tubular segments (2, 5, 9, 19, 27, 29, 30). Renin has been identified in the proximal tubules, and it has been suggested that it may be regulated differently than JG renin, because it can be activated by ACE inhibitors (21, 30, 31). Rohrwasser et al. (29) reported in human and mouse tissues the presence of apical renin immunostaining in connecting tubule (CNT) cells and renin secretion in isolated CNT cells in vitro, suggesting that these cells may secrete renin. It was also demonstrated that renin is present in principal cells of CNTs and of cortical and medullary collecting ducts of normal rat kidneys; furthermore, chronic infusion of ANG II led to an enhancement of renin protein in these distal nephron cells (27). Distal nephron renin could be an additional contributing factor to the development and maintenance of hypertension due to continued intratubular formation of ANG II, despite suppression of JGA renin. Because elevated ANG II levels were associated with increased distal tubular renin levels, further experiments were performed to determine whether the augmentation of distal nephron renin in chronic ANG II-infused hypertensive rats is dependent on an AT1 receptor-mediated mechanism.
METHODS
Animals and tissue preparation
All experimental protocols were approved by the Tulane Institutional Animal Care and Use Committee. Male Sprague-Dawley rats (200 to 220 g, n = 24; Charles River Laboratories, Wilmington, MA) were cage housed and maintained in a temperature-controlled room with a 12:12-h light-dark cycle, with free access to tap water and standard rat chow (Ralston Purina, St. Louis, MO) for 2 wk. For minipump implantation (Alzet model 2002, Alza, Palo Alto, CA), rats were selected at random to be subjected to either sham operation (n = 8) or ANG II infusion (n = 16). For the ANG II infusions, an osmotic minipump containing ANG II (Human ANG II, Sigma, St. Louis, MO; 80 ng/min for 13 days) was implanted subcutaneously at the dorsum of the neck. One group of ANG II-infused rats (n = 8) also received an AT1 receptor blocker (ARB), olmesartan (5 mg/day in the food, Sankyo, Japan) during the period of ANG II infusion. Systolic blood pressure (SBP) was monitored by tail-cuff plethysmography (Visitech, BP2000, Apex, NC) 1 day before and 3, 7, and 11 days following sham operation or minipump implantation as previously described (13-15).
Sample collection, preparation, and evaluation
Blood and kidney samples were harvested on day 13. Trunk blood was collected into two chilled tubes containing 5.0 mmol/l EDTA, which were centrifuged at 4,000 rpm for 30 min at 4°C. Plasma fractions were removed and assayed for PRA as previously described (33). PRA was expressed as nanograms per milliliter per hour of generated ANG I.
After decapsulation, the left kidney was cross sectioned and fixed in zinc-saturated formalin for immunohistochemical studies. Kidneys were embedded in paraffin blocks, sectioned (23 μm), and mounted onto slides with Vectabond (Vector Laboratories, Burlingame, CA). Serial kidney sections were immunostained by the immunoperoxidase technique (with modifications as described previously) (26). Briefly, after being dewaxed in xylene, the kidney sections were rehydrated in 100, 95, to 70% alcohols and treated with methanol and 3% H2O2 for 30 min to block peroxidase activity. Then, kidney sections were washed and sequentially incubated with 1) normal blocking rabbit serum for 20 min; 2) primary antibody, antipeptide renin polyclonal antibody diluted in PBS containing 1% bovine serum albumin at 1:4,000 or 1:8,000 concentrations (100 μl) for 90 min; 3) secondary antibody, biotinconjugated rabbit antimouse IgG for 30 min; and 4) avidin DH, biotinylated horseradish peroxidase H complex using an ABC Elite Vectastain kit (Vector Laboratories) for 45 min. Peroxidase activity was visualized with 0.1% 3,3′diaminobenzidine tetrahydrochloride (DAB; Sigma). The slides were counterstained with hematoxylin, mounted using aqueous mounting media (Biomeda, Fisher Scientific), and coverslipped. Renin immunolocalization was assessed using a polyclonal anti-rat specific renin antibody raised in rabbit (generously provided by Dr. T. Inagami) and an automatic robot system (DAKO, Carpinteria, CA), which allowed identical incubation time of all slides to reagents and antibody as previously described (27). Renin immunoreactivity was determined in 10 different microscopic fields per tissue section per animal, using an Olympus BX51TRF microscope, ×40 magnification, and an integrated Magnafire SP Digital “Firewire” Camera System for image processing. The intensity of distal nephron renin immunoreactivity in ANG II-infused and sham rat kidney sections was analyzed using ImagePro Plus Software (version 4.5.1 for Windows 2000 XP; Media Cybernetics), which allowed a computerized determination of the area of positive staining (μm) and the intensity of immunoreactivity (sum of density of positive tubules in an analyzed area). For this analysis, JGA renin immunostaining was analyzed separately. The results were expressed in arbitrary units of the relative intensity normalized to the renin immunostaining average of the sham group.
To avoid the contribution of JGA renin to distal nephron renin expression, protein and total RNA were extracted from renal medulla dissected from cortex of the right kidney. Renin Western blot analysis using 10 μg of protein against rat renin (1:4,000) was performed with a standard protocol as described previously (14, 27).
For quantitative real-time PCR (qRT-PCR), 50 ng/well of total RNA was used as previously described (14, 27). Although we previously were not able to show upregulation due to inadequate sensitivity, we optimized the conditions and procedures since the previous report (14, 27). Amplifications of the rat renin 1c gene were performed using 1) a specific probe (5′TTCAAAGTCATCTTTGACACGGGTTCAG-3′) labeled with 5′6-FAM and 3′-black hole quencher 1 and 2) a set of primers (sense 5′-AGTACTATGGTGAGATCGGCATT-3′; antisense 5′-AGATTCACAACCTCTATGACTCCTC-3′). In addition, coamplifications of the GAPDH gene were performed using the following primers (sense, 5′-CAGAACATC ATCCCTGCATC-3′; antisense, 5′-CTGCTTCACCACCTTCTTGA-3′; and the probe, 5′-CCT GGA GAA ACC TGC CAA GTA TGA TGA-3′) labeled with 5′-HEX and 3′-black hole quencher-2.
Statistical analysis
Results are expressed as means ± SE. The data were analyzed using a one-way factorial or a two-way repeated ANOVA with post hoc Newman-Keuls and Bonferroni tests, respectively. Statistical significance is defined at a value of P < 0.05.
RESULTS
SBP and PRA
SBP values were similar among the groups before implantation of the osmotic minipumps. By day 7 of ANG II infusion, SBP was significantly elevated in ANG II-infused rats (157 ± 8 vs. 120 ± 3 mmHg) and was increased further by day 11 (178 ± 4 vs. 122 ± 1 mmHg; P < 0.001). ARB treatment with olmesartan prevented the development of hypertension (110 ± 6 and 113 ± 6 mmHg at days 7 and 11, respectively). PRA was suppressed in ANG II-infused rats (0.1 ± 0.1 vs. 5.3 ± 0.8 ng ANG I·ml-1·h-1) compared with sham-operated rats. ARB treatment with olmesartan led to large increases in PRA (15.8 ± 1.5 ng ANG I·ml-1·h-1).
Renin immunohistochemistry in JGA and in cortical and medullary distal nephron segments
Figures 1 and 2 show renin immunostaining of rat kidney sections stained in the same run using an automatic immunostainer system and a polyclonal rat anti-renin antibody raised in rabbit at 1:8,000 concentration. Figure 1 shows augmentation of renin immunoreactivity in principal cells of cortical CNTs and cortical (cortex, top) and medullary (medulla, bottom) collecting ducts of ANG II-infused rats (Fig. 1, B and E) compared with sham-operated rats [Fig. 1, A and D; 5.0 ± 0.6 vs. 1.0 ± 0.1 densitometric units (DU) cortex; 2.5 ± 0.3 vs. 1.0 ± 0.2 DU medulla; P < 0.001]. Treatment with olmesartan, a specific AT1 receptor blocker, prevented the increases in renin immunoreactivity induced by chronic ANG II infusion (0.1 ± 0.0 DU cortex; 0.1 ± 0.0 DU medulla; Fig. 1, C and F). In contrast, positive renin immunoreactivity observed in JGA cells (Fig. 2) of sham-operated (A), ANG II-infused (B), and ANG II-infused plus ARB treatment (C) rats was significantly suppressed in ANG II-infused rats (B) compared with sham-operated (A) rats (0.1 ± 0.0 vs. 1.0 ± 0.1 DU relative to sham; P < 0.001), but it markedly increased in ARB-treated rats (C; 2.1 ± 0.3 DU relative to sham). In addition, renin-specific immunostaining was observed in medullary interstitial cells of rats infused with ANG II and treated with olmesartan. Densitometric analysis of the intensity of renin immunoreactivity in JGA (Fig. 3A), cortical (Fig. 3B), and medullary (Fig. 3C) collecting duct cells expressed in arbitrary units is presented in the Fig. 3.
Fig. 1.

Renin immunoreactivity in paraffin-embedded rat kidney sections. Positive renin immunostaining is observed in cortical collecting tubule cells (cortex, A-C) and medullary collecting duct cells (medulla, D-F) of sham (n = 8; A and D), ANG II-infused (n = 8; B and E), and ANG II-infused rats treated with AT1 receptor blocker (n = 8; ANG II + ARB, C and F). *Renin immunostaining present in collecting duct cells. Renin antibody concentration 1:4,000. ARB, AT1 receptor blocker; olmesartan, 5 mg/day orally.
Fig. 2.
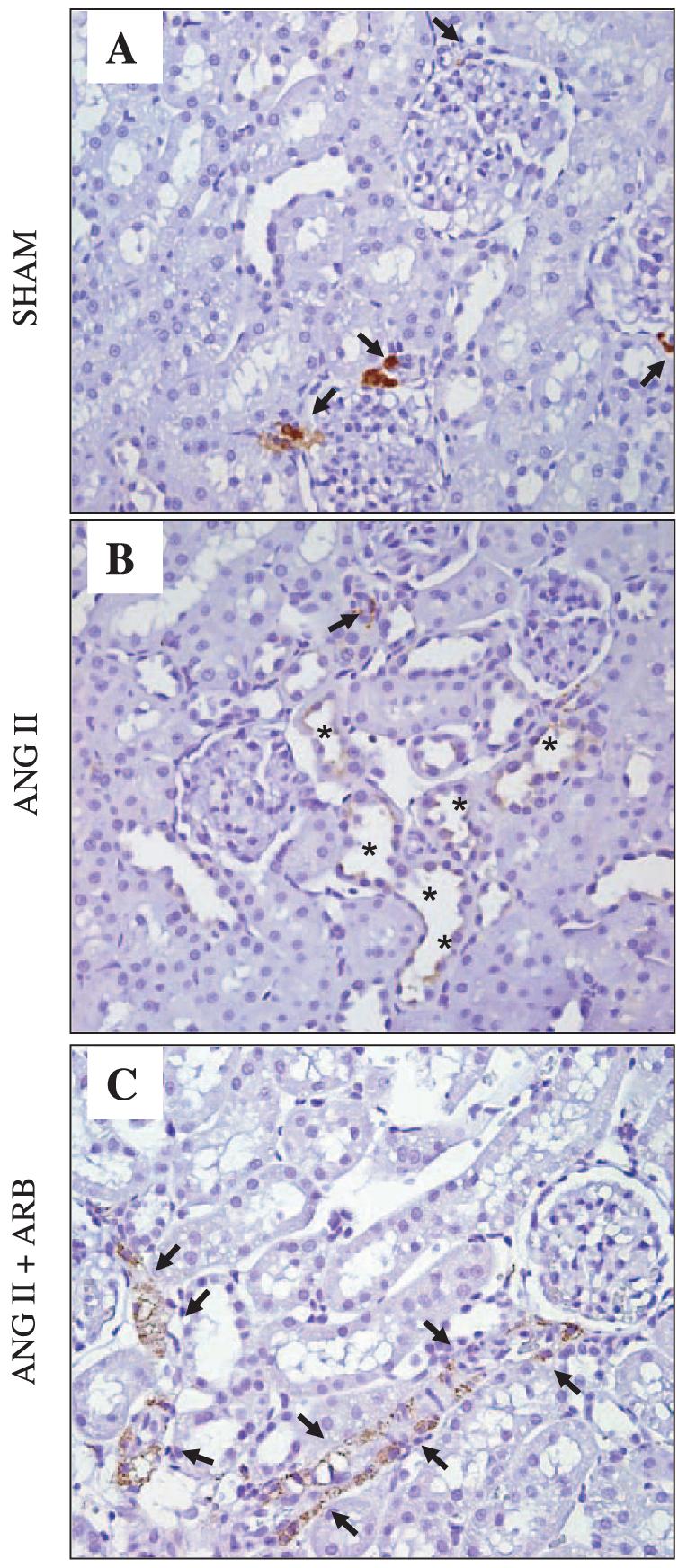
Renin immunoreactivity in paraffin-embedded rat kidney sections. Positive renin immunostaining is observed in juxtaglomerular cells (arrows) of sham (n = 8; A), ANG II-infused (n = 8; B), and ANG II-infused rats treated with AT1 receptor blocker (n = 8; ANG II + ARB; C). Recruitment of arterial smooth muscle cells beyond the afferent arterioles positive for renin immunostaining can be seen in the ANG II+ARB rat kidney. Renin immunostaining is also present in cortical collecting duct cells (*) of ANG II-infused rat kidney. Ten microscopic fields per kidney section were analyzed from 5 different kidneys sections/animal in each group. Renin antibody concentration 1:4,000. Olmesartan, 5 mg/day orally.
Fig. 3.

Quantification of intensity of renin immunostaining in juxtaglomerular (JGA; A), cortical collecting duct (B), and medullary collecting duct (C) cells of rat kidneys. A: densitometric analysis of the renin immunostaining in JGA cells. JGA renin immunoreactivity was suppressed in ANG II-infused compared with sham-operated rat kidneys; however, it substantially increased after ARB. B and C: densitometric analysis of renin immunoreactivity in cortex and medulla, respectively. Notice that in contrast to the JGA renin immunoreactivity suppression observed in ANG II-infused rats, an enhancement of renin immunoreactivity is observed in collecting duct cells of the renal cortex (B) as well as of the renal medulla (C). Relative to sham-operated rats (n = 8), densitometric analysis of the renin immunoreactivity in JGA cells, cortical and medullary CCDs of ANG II-infused rats treated (n = 8) or not with ARB (n = 8) was determined in 5 kidney sections/animal; 10 analyzed microscopic fields/kidney section. Values are means ± SE. *P < 0.0001 vs. sham rats.
Renin protein expression of renin in rat kidney cortex and medulla
Analysis of integrated densitometric values (IDV) showed that the ratios for renin protein were significantly suppressed in cortical renal tissues from ANG II-infused hypertensive rats (Fig. 4A; 0.5 ± 0.2 vs. 1.0 ± 0.1 densitometric ratio compared with the average of sham-operated rats; P < 0.05); whereas the renin levels in ANG II-infused hypertensive rats treated with olmesartan increased (1.2 ± 0.3 densitometric ratio compared with the average of sham-operated rats). In contrast, renin protein levels were higher in the kidney medulla samples of ANG II-infused than sham-operated rats (Fig. 4B, 1.6 ± 0.3 vs. 1.0 ± 0.1 densitometric ratio compared with the average of sham-operated rats; P < 0.05). Treatment of ANG II-infused rats with olmesartan prevented the increases (1.0 ± 0.1 densitometric ratio compared with average of sham-operated rats). As a control study to assess for equal loading, membranes were reprobed with an antibody against β-actin. The results showed that IDV levels were unaltered among the groups.
Fig. 4.

Renin Western blot analyses in kidney cortex and medulla from sham-operated, ANG II-infused, and ANG II-infused rats treated with AT1 receptor blocker (ANG II + ARB, olmesartan). Representative autoradiographies of renin kidney cortex (Fig. 3A) and kidney medulla (Fig. 3B) from sham-operated (n = 8), ANG II-infused (n = 8), and ANG II + ARB (n = 8) rats are shown. Densitometric analysis showed that chronic ANG II infusion significantly decreased renin levels in the cortex (Fig. 3A), but in contrast increased renin levels in the medulla (Fig. 3B). ARB treatment prevented these effects. Relative expression of renin protein was corrected by β-actin in all groups in 3 independent experiments. Renin antibody concentration 1:4,000. *P < 0.05 vs. sham rats.
qRT-PCR for renin in rat kidney medulla
Expression of renin mRNA in renal medullary tissues is shown in Fig. 4. ANG II-treated rats showed significantly higher renin mRNA levels compared with sham-operated rats (5.5 ± 2.3 vs. 0.04 ± 0.02 DU; P < 0.001). Treatment with olmesartan prevented ANG II-induced increases in renin mRNA expression (0.1 ± 0.0 DU; Fig. 5). These results indicate that chronic ANG II infusion exerts a stimulatory effect on medullary renin mRNA which reflects the renin mRNA level in the tubular cells as the medulla does not contain JGA cells.
Fig. 5.

Quantitative renin mRNA levels in rat kidney medulla measured by qRT-PCR. Renin qRT-PCR in ANG II-infused rats showed higher mRNA levels in the kidney medulla compared with sham-operated rats; however, renin transcript levels were normalized in the ARB (olmesartan)-treated rat group. Renin mRNA concentration was determined in 3 independent experiments and expressed relative to GAPDH. ARB, olmesartan (5 mg/day × 5 days). * P < 0.001 vs. Sham and ANGII+ARB.
DISCUSSION
This study extends previous findings that chronic ANG II infusions lead to progressive increases in SBP along with suppression of PRA and JGA renin but augmentation of medullary collecting duct renin protein and mRNA levels. These findings are consistent with previous results demonstrating that ANG II-infused hypertensive rats have increases of renin immunoreactivity in principal cells of cortical CNT cells and cortical and medullary collecting duct cells (27). The novel issue addressed in the present study is whether the augmentation of distal tubular renin in ANG II-dependent hypertension is mediated by an AT1 receptor mechanism. To test this hypothesis, ANG II-infused rats were treated with olmesartan, a specific AT1 receptor blocker. The data demonstrate that the increases in distal nephron renin mRNA and protein expression were prevented by treatment with ARB. Therefore, AT1 receptor activation is involved in the mechanism by which increases in circulating and intrarenal ANG II levels stimulate renin in distal nephron segments. In contrast to the increases in PRA and JGA renin caused by blockade of ANG II-induced inhibition of JGA renin release, ARB treatment markedly reduced the expression of renin in distal nephron segments. Despite the fact that ARB-treated rats exhibited substantial increases in PRA and renin immunoexpression by recruited cells from the afferent arteriole, there was suppression of renin in medullary collecting tubules. These findings provide further evidence that renin in principal cells of distal nephron segments is differentially regulated and is not the consequence of uptake of renin produced by JGA cells. Renin mRNA levels measured in the kidney medulla samples may reflect renin mRNA not only in tubular epithelial cells but also in endothelial and interstitial cells. Renin immunoreactivity was detected in medullary interstitial cells particularly under conditions of olmesartan treatment; however, no consistent trends were observed in the present study. Nevertheless, it is possible that interstitial cell renin expression could be upregulated in certain conditions such as ANG II inhibition with ACE inhibitors or under ANG II receptor blockade as has been reported in renin expressing cells in the preglomerular vasculature (7, 8, 23). In addition, these data firmly establish that medullary renin mRNA is also upregulated by the chronic ANG II infusions and that this increase is prevented by ARB treatment. Although it has also been shown that aldosterone exerts positive effects on renin gene expression in JGA cells probably by stabilizing renin mRNA via a mineralocorticoid receptor-mediated mechanism (12), the present study did not rule out the aldosterone effects on distal nephron renin. Whether aldosterone exerts a positive action on distal tubular renin is an issue that remains to be investigated.
Previous studies demonstrated that the augmented intrarenal ANG II levels after chronic ANG II infusions are due to an enhanced internalization of ANG II (11, 35, 37) coupled with stimulation of AGT expression (13, 14, 17), both involving activation of AT1 receptors. Additionally, it has also been reported that chronic ARB treatment limits the increases in AGT protein levels in renal cortex as well as its urinary excretion rate (15, 16). Our data extend these findings, demonstrating that chronic treatment with olmesartan blocks the increases in renin protein expression in principal cells of CNTs and collecting ducts and renin mRNA in the renal medulla as well. Therefore, the chronic effect of ARB treatment to block the internalization of ANG II into kidney cells (11, 35, 37), the stimulation of intrarenal AGT expression (13, 14), as well as the increases of renin in distal nephron segments may all be acting in a synchronized manner to ameliorate the enhanced intrarenal ANG II content seen during ANG II-dependent hypertension.
Renin in distal nephron segments may provide a possible pathway for intratubular ANG I generation in distal nephron segments. Intrarenal AGT mRNA and protein have been localized to proximal tubule cells, indicating that the intratubular ANG II could be derived from locally formed and secreted AGT (3, 13, 14, 22). Furthermore, intrarenal AGT is regulated by an amplification mechanism such that proximal tubule AGT mRNA and protein are Enhanced during chronic ANG II infusions associated with increased passage of AGT in the urine (16). This effect helps to maintain or even increase further the production of intrarenal ANG II in ANG II-dependent hypertension (13, 14). The present findings suggest that ANG II mediates enhancement of tubular renin through an AT1 receptor-dependent mechanism. Once ANG I is formed, intratubular ANG II formation can occur because ACE is present in distal nephron segments as well as on the brush border of proximal tubule cells (1). Indeed, ACE production has been demonstrated in isolated inner medullary collecting duct cells, suggesting a new site of ACE secretion in collecting duct in the nephron (28). Therefore, intratubular ANG II formation may occur not only in the proximal tubule but also beyond the CNT. The increased intratubular ANG II formation may partially lead to augmented ANG II renal content and to enhancement of fractional sodium reabsorption rate characteristic of the ANG II-infused rat model.
Recent studies showed that ANG II directly stimulates epithelial sodium channel activity in cortical collecting duct cells (24) and that there is intraluminal conversion of ANG I to ANG II in cortical collecting ducts (18). Thus renin in distal nephron segments may synergistically contribute to the ANG II stimulatory effect on distal tubular renin and could help explain the marked stimulation of sodium reabsorption and suppression of the pressure-natriuresis relationship observed in ANG II-infused hypertensive rats (34). Therefore, distal nephron renin may play a crucial role in the sustained high intrarenal ANG II levels and hence contribute to the progressive high blood pressure observed in ANG II-dependent hypertension.
In summary, these data demonstrate that chronic ANG II infusions to normal rats significantly increase renin protein and mRNA levels in principal cells of connecting ducts and collecting tubules. This augmentation is dependent on activation of AT1 receptors. Although PRA and JGA renin are markedly suppressed in ANG II-induced hypertension, increased distal nephron renin associated with an increased proximal tubular AGT production and spillover into the distal nephron segments may collectively contribute to elevated and sustained intratubular ANG I and ANG II formation in this hypertensive model. ANG II actions on the distal nephron could be acting synergistically with aldosterone to increase distal nephron sodium reabsorption and contribute to overall enhanced sodium reabsorption. These results provide an additional mechanism by which ARB treatment may exert a protective effect on the kidney.
ACKNOWLEDGMENTS
We thank Dr. T. Inagami (Vanderbilt University) for providing the rat anti-renin antibody and Sankyo for supplying Olmesartan. The authors express gratitude to M. Rauv and H. Pappas LeBau for excellent technical assistance. Digital images of histological specimens were obtained at the Imaging Core Facility of Hypertension and Renal Center of Excellence at Tulane University Health Sciences Center.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL-26371 and National Institutes of Health Grant P-20RR-017659 from the IdeA of NCRR, American Heart Association Grant 0325269, and the Louisiana Board of Regents Millennium Health Excellence Fund 20010607.
REFERENCES
- 1.Casarini DE, Boim MA, Stella RCR, Krieger-Azzolini MH, Krieger JE, Schor N. Angiotensin I converting enzyme activity in tubular fluid along the rat nephron. Am J Physiol Renal Physiol. 1997;272:F405–F409. doi: 10.1152/ajprenal.1997.272.3.F405. [DOI] [PubMed] [Google Scholar]
- 2.Chen M, Harris MP, Rose D, Smart A, He XR, Kretzler M, Briggs JP, Schnermann J. Renin and renin mRNA in proximal tubules of the rat kidney. J Clin Invest. 1994;94:237–243. doi: 10.1172/JCI117312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Darby IA, Sernia C. In situ hybridization and immunohistochemistry of renal angiotensinogen in neonatal and adult rat kidneys. Cell Tissue Res. 1995;281:197206. doi: 10.1007/BF00583388. [DOI] [PubMed] [Google Scholar]
- 4.DeNicola L, Keiser JA, Blantz RC, Gabbai FB. Angiotensin II and renal functional reserve in rats with Goldblatt hypertension. Hypertension. 1992;19:790–794. doi: 10.1161/01.hyp.19.6.790. [DOI] [PubMed] [Google Scholar]
- 5.Gilbert RE, Wu LL, Kelly DJ, Cox A, Wilkinson-Berka JL, Johnston CI, Cooper ME. Pathological expression of renin and angiotensin II in the renal tubule after subtotal nephrectomy. Implications for the pathogenesis of tubulointerstitial fibrosis. Am J Pathol. 1999;155:429–440. doi: 10.1016/S0002-9440(10)65139-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldblatt PJ. The Goldblatt experiment: a conceptual paradign. In: Laragh JH, Brenner BM, editors. Hypertension: Pathophysiology, Diagnosis and Management. Raven; New York: 1995. p. 2335. [Google Scholar]
- 7.Gomez RA, Chevalier RL, Everett AD, Elwood JP, Peach MJ, Lynch KR, Carey RM. Recruitment of renin geneexpressing cells in adult rat kidney. Am J Physiol Renal Fluid Electrolyte Physiol. 1990;259:F660–F665. doi: 10.1152/ajprenal.1990.259.4.F660. [DOI] [PubMed] [Google Scholar]
- 8.Gomez RA, Lynch KR, Chevalier RL, Everett AD, Johns DW, Wilfong N, Peach MJ, Carey RM. Renin and angiotensinogen gene expression and intrarenal renin distribution during ACE inhibition. Am J Physiol Renal Fluid Electrolyte Physiol. 1988;254:F900–F906. doi: 10.1152/ajprenal.1988.254.6.F900. [DOI] [PubMed] [Google Scholar]
- 9.Henrich WL, McAllister EA, Eskue A, Miller T, Moe OW. Renin regulation in cultured proximal tubular cells. Hypertension. 1996;27:1337–1340. doi: 10.1161/01.hyp.27.6.1337. [DOI] [PubMed] [Google Scholar]
- 10.Huang WC, Bell PD, Harvey D, Mitchell KD, Navar LG. Angiotensin influences on tubuloglomerular feedback mechanism in hypertensive rats. Kidney Int. 1988;34:631–637. doi: 10.1038/ki.1988.227. [DOI] [PubMed] [Google Scholar]
- 11.Ingert C, Grima M, Coquard C, Barthelmebs M, Imbs JL. Contribution of angiotensin II internalization to intrarenal angiotensin II levels in rats. Am J Physiol Renal Physiol. 2002;283:F1003–F1010. doi: 10.1152/ajprenal.00322.2001. [DOI] [PubMed] [Google Scholar]
- 12.Klar J, Vitzthum H, Kurtz A. Aldosterone enhances renin gene expression in juxtaglomerular cells. Am J Physiol Renal Physiol. 2004;286:F349–F355. doi: 10.1152/ajprenal.00411.2002. [DOI] [PubMed] [Google Scholar]
- 13.Kobori H, Harrison-Bernard LM, Navar LG. Enhancement of angiotensinogen expression in angiotensin II-dependent hypertension. Hypertension. 2001;37:1329–1335. doi: 10.1161/01.hyp.37.5.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kobori H, Harrison-Bernard LM, Navar LG. Expression of angiotensinogen mRNA and protein in angiotensin IIdependent hypertension. J Am Soc Nephrol. 2001;12:431–439. doi: 10.1681/asn.v123431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kobori H, Harrison-Bernard LM, Navar LG. Urinary excretion of angiotensinogen reflects intrarenal angiotensinogen production. Kidney Int. 2002;61:579–585. doi: 10.1046/j.1523-1755.2002.00155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kobori H, Nishiyama A, Harrison-Bernard LM, Navar LG. Urinary angiotensinogen as an indicator of intrarenal Angiotensin status in hypertension. Hypertension. 2003;41:42–49. doi: 10.1161/01.hyp.0000050102.90932.cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kobori H, Prieto-Carrasquero MC, Ozawa Y, Navar LG. AT1 receptor mediated augmentation of intrarenal angiotensinogen in angiotensin II-dependent hypertension. Hypertension. 2004;43:1126–1132. doi: 10.1161/01.HYP.0000122875.91100.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Komlosi P, Fuson AL, Fintha A, Peti-Peterdi J, Rosivall L, Warnock DG, Bell PD. Angiotensin I conversion to angiotensin II stimulates cortical collecting duct sodium transport. Hypertension. 2003;42:195–199. doi: 10.1161/01.HYP.0000081221.36703.01. [DOI] [PubMed] [Google Scholar]
- 19.Lantelme P, Rohrwasser A, Gociman B, Hillas E, Cheng T, Petty G, Thomas J, Xiao S, Ishigami T, Herrmann T, Terreros DA, Ward K, Lalouel JM. Effects of dietary sodium and genetic background on angiotensinogen and Renin in mouse. Hypertension. 2002;39:1007–1014. doi: 10.1161/01.hyp.0000016177.20565.a0. [DOI] [PubMed] [Google Scholar]
- 20.Mitchell KD, Jacinto SM, Mullins JJ. Proximal tubular fluid, kidney, and plasma levels of angiotensin II in hypertensive ren2 transgenic rats. Am J Physiol Renal Physiol. 1997;273:F246–F253. doi: 10.1152/ajprenal.1997.273.2.F246. [DOI] [PubMed] [Google Scholar]
- 21.Moe OW, Ujiie K, Star RA, Miller RT, Widell J, Alpern RJ, Henrich WL. Renin expression in renal proximal tubule. J Clin Invest. 1993;91:774–779. doi: 10.1172/JCI116296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Navar LG, Harrison-Bernard LM, Wang CT, Cervenka L, Mitchell KD. Concentrations and actions of intraluminal angiotensin II. J Am Soc Nephrol. 1999;10:S189–S195. [PubMed] [Google Scholar]
- 23.Pentz ES, Lopez ML, Kim HS, Carretero O, Smithies O, Gomez RA. Ren1d and Ren2 cooperate to preserve homeostasis: evidence from mice expressing GFP in place of Ren1d. Physiol Genomics. 2001;6:45–55. doi: 10.1152/physiolgenomics.2001.6.1.45. [DOI] [PubMed] [Google Scholar]
- 24.PetiPeterdi J, Warnock DG, Bell PD. Angiotensin II directly stimulates ENaC activity in the cortical collecting duct via AT1receptors. J Am Soc Nephrol. 2002;13:1131–1135. doi: 10.1097/01.asn.0000013292.78621.fd. [DOI] [PubMed] [Google Scholar]
- 25.Ploth DW. Angiotensin-dependent renal mechanisms in twokidney one-clip renal vascular hypertension. Am J Physiol Renal Fluid Electrolyte Physiol. 1983;245:F131–F141. doi: 10.1152/ajprenal.1983.245.2.F131. [DOI] [PubMed] [Google Scholar]
- 26.Prieto M, Dipp S, Meleg-Smith S, El-Dahr SS. Ureteric bud derivatives express angiotensinogen and AT1 receptors. Physiol Genomics. 2001;6:29–37. doi: 10.1152/physiolgenomics.2001.6.1.29. [DOI] [PubMed] [Google Scholar]
- 27.Prieto-Carrasquero MC, Harrison-Bernard LM, Kobori H, Ozawa Y, Hering-Smith KS, Hamm LL, Navar LG. Enhancement of collecting duct renin in angiotensin II-dependent hypertensive rats. Hypertension. 2004;44:223–229. doi: 10.1161/01.HYP.0000135678.20725.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quinto BM, Pesquero JB, Casarini DE. Identification of a new site of angiotensin Iconverting enzyme (ACE) production in IMCD cells and study of interaction between the bradykinin receptor B2 and ACE using ACE inhibitor. J Hypertens. 2002;20:S195. [Google Scholar]
- 29.Rohrwasser A, Morgan T, Dillon HF, Zhao L, Callaway CW, Hillas E, Zhang S, Cheng T, Inagami T, Ward K, Terreros DA, Lalouel JM. Elements of a paracrine tubular reninangiotensin system along the entire nephron. Hypertension. 1999;34:1265–1274. doi: 10.1161/01.hyp.34.6.1265. [DOI] [PubMed] [Google Scholar]
- 30.Tank JE, Henrich WL, Moe OW. Regulation of glomerular and proximal tubule renin mRNA by chronic changes in dietary NaCl. Am J Physiol Renal Physiol. 1997;273:F892–F898. doi: 10.1152/ajprenal.1997.273.6.F892. [DOI] [PubMed] [Google Scholar]
- 31.Taugner R, Mannek E, Nobiling R, Buhrle CP, Hackenthal E, Ganten D, Inagami T, Schroder Coexistence of renin and angiotensin II in epitheloid cell secretory granules of rat kidney. Histochemistry. 1984;81:39–45. doi: 10.1007/BF00495399. [DOI] [PubMed] [Google Scholar]
- 32.Von Thun AM, El-Dahr SS, Vari RC, Navar LG. Modulation of reninangiotensin and kallikrein gene expression in experimental hypertension. Hypertension. 1994;23(Suppl I):I131–I136. doi: 10.1161/01.hyp.23.1_suppl.i131. [DOI] [PubMed] [Google Scholar]
- 33.Von Thun AM, Vari RC, El-Dahr SS, Navar LG. Augmentation of intrarenal angiotensin II levels by chronic angiotensin II infusion. Am J Physiol Renal Fluid Electrolyte Physiol. 1994;266:F120–F128. doi: 10.1152/ajprenal.1994.266.1.F120. [DOI] [PubMed] [Google Scholar]
- 34.Wang CT, Chin SY, Navar LG. Impairment of pressurenatriuresis and renal autoregulation in ANG II-infused hypertensive rats. Am J Physiol Renal Physiol. 2000;279:F319–F325. doi: 10.1152/ajprenal.2000.279.2.F319. [DOI] [PubMed] [Google Scholar]
- 35.Zhuo JL, Imig JD, Hammond TG, Orengo S, Benes E, Navar LG. ANG II accumulation in rat renal endosomes during ANG II-induced hypertension: role of AT1 receptor. Hypertension. 2002;39:116–121. doi: 10.1161/hy0102.100780. [DOI] [PubMed] [Google Scholar]
- 36.Zimmerman BG, Arendshorst WJ, DiBona GF, Hostetter TH, Ploth DW, Raij L. Renal functional derangements in hypertension. Fed Proc. 1986;45:2661–2664. [PubMed] [Google Scholar]
- 37.Zou L, Imig JD, Von Thun AM, Hymel A, Ono H, Navar LG. Receptor mediated intrarenal ANG II augmentation in ANG II-infused rats. Hypertension. 1996;28:669–677. doi: 10.1161/01.hyp.28.4.669. [DOI] [PubMed] [Google Scholar]


