Abstract
Changes in the permeability of the blood-brain barrier (BBB) were evaluated in two mouse models of viral encephalitis. The ability of sodium fluorescein (NaFl) to cross the BBB from the serum into the central nervous system was assayed in animals inoculated with virulent strains of either Banzi or Semliki Forest viruses. To test the hypothesis that increases in BBB permeability were associated with poor disease outcome subsequent experiments measured BBB permeability in conjunction with treatment with the interferon inducer Ampligen (poly I: poly C12U). A single intraperitoneal injection of Ampligen (1 mg/kg) administered either 24 h or 4-6 h before, but not 24 h after, virus inoculation with Banzi virus provided significant improvements in survival, viral brain titers, weight change, and BBB permeability. In comparison, a similar treatment with Ampligen administered either 24 h or 4-6 h before inoculation with Semliki Forest virus was able to significantly improve weight change, and BBB permeability, but only animals receiving Ampligen 4-6 h pre-virus showed a significantly improved mortality. In general, it was found that evaluation of BBB permeability was a more sensitive indicator of disease outcome and the antiviral efficacy Ampligen than either weight change or brain viral titers.
Keywords: Blood-Brain Barrier, Banzi virus, Semliki Forest virus, Viral Encephalitis, Ampligen
1. Introduction
The blood-brain barrier (BBB) is a functional and structural component of the central nervous system (CNS) vasculature that serves important protective functions for the CNS. It is composed of highly specialized endothelial cells in the CNS vasculature. The physical apposition of astrocyte foot-processes on the abluminal side of the endothelial cells, and the production of various trophic factors by astrocytes directly impacts the function of the BBB(Haseloff et al. 2005). The specialized endothelium of the BBB is able to selectively transport molecules into the CNS (e.g. glucose, insulin) while limiting entry of hydrophilic compounds and toxins as well as circulating leukocytes.
It has long been recognized that the ability of the BBB to exclude substances from the CNS is often compromised during infection and inflammation of the CNS. The exact mechanism of BBB breakdown and its role in the disease progression of viral encephalitis is currently unknown. However, a number of inflammatory cytokines, whose expression levels can be increased in association with viral infection, have been demonstrated to increase permeability of the BBB. Levels of matrix metalloproteinases (MMPs) are increased in the cerebrospinal fluid of patients with HIV encephalitis, as is the permeability of the BBB (Conant et al. 1999). Infection of mice with an avirulent strain of SFV significantly increased the expression of MMP-2 & 9, and increased the permeability of the BBB, while treatment with an inhibitor MMPs significantly improved the condition of the BBB (Keogh et al. 2003).
Arborviruses are the most common known cause of encephalitis world wide (WHO 1985), and the exact role of the BBB in the pathophysiology of viral encephalitis is poorly understood. However, increased permeability of the BBB appears to be a vital component of viral encephalitic pathology. Mortality associated with infection of non-neuroinvasive strains of West Nile virus or Sindbis virus can be greatly enhanced by prior administration of lipopolysaccharide (Lustig et al. 1992) which is known to increase BBB permeability (Lustig et al. 1992; Xiao et al. 2001). Semliki Forest virus (SFV) can infect and damage endothelial cells of the BBB endothelium (Eralinna et al. 1996). Permeability of the BBB is also seen in encephalitis associated with human immunodeficiency virus (HIV) (Dallasta et al. 1999) and in murine models of lymphochoriomeningitis virus (Andersen et al. 1991).
A variety of methods have been used to evaluate the function of the BBB. Leakage of systemic proteins, such as fibrinogen or albumin, into the CNS have been used to assess BBB permeability associated with HIV associated encephalitis (Dallasta et al. 1999), simian immunodeficiency virus encephalitis (Luabeya et al. 2000) and a mouse model of experimental allergic encephalomyelitis (EAE) induced by infection with an avirulent strain of Semliki Forest virus (Eralinna et al. 1996). While these techniques have the benefit of using endogenous proteins they are highly dependent upon the skill of the evaluator, and are semiquantitative at best. Additional methods involve the injection of a radioactively labeled marker, as was used in a mouse model of lymphochoriomeningitis virus (Andersen et al. 1991). Use of radioactive markers can provide highly accurate and detailed information regarding the degree of BBB permeability, as well as giving the ability to spatially identify areas of variable BBB permeability within the CNS. However, use of radioactive materials poses a potential risk to laboratory personnel and requires additional oversight and precautions for disposal of isotope as well as animal remains. Therefore, we chose to use sodium fluorescein (NaFl) as a measurable marker of BBB permeability. NaFl is a small molecular weight marker (MW 367) that has been widely used in a variety of model systems for evaluation of BBB permeability (Gupta et al. 1999; Deli et al. 2000; Hooper et al. 2001; Erdlenbruch et al. 2003; Kozler and Pokorny 2003).
The interferon inducing agent Ampligen™ (poly I: poly C12U) is a known antiviral agent. Ampligen has proven to be effective against the viruses being tested here. The antiviral mechanisms of interferons and interferon inducers has previously been reviewed (De Clercq 2006). It is recognized that the primary mechanism of action of Ampligen is via activation of Toll-like Receptor-3 (TLR-3) on cells. This results in production of alpha/beta interferons. Interferons in turn induce an antiviral state within multiple cell types.
To our knowledge, no studies have been performed to evaluate the degree of BBB permeability, the time course of permeability change, or the correlation of BBB permeability changes with disease outcome in any animal model involving either Banzi virus (BaV) or a virulent strain of SFV. We hypothesized that inoculation of animals with an encephalitic virus would increase BBB permeability, and that the degree of permeability would positively correlate with the progression and severity of the disease. The present experiments were designed to evaluate the effect of viral encephalitis on the permeability of the BBB to the small molecular weight marker sodium fluorescein. Additionally, to our knowledge no studies have been reported in which measurement of BBB permeability was done in conjunction with antiviral therapies. Additional studies compared the degree of BBB permeability in virus inoculated animals following treatment with various regimens of the known immunomodulatory antiviral agent, Ampligen.
2. Materials and Methods
2.1 Viruses and Animals
Banzi virus (BaV) (H336 strain) was obtained from the American Type Culture Collection (ATCC, Manassas, VA). The virus was passaged 3 times in Vero 76 cells. Semliki Forest virus (SFV) (Original strain) was obtained from ATCC. The virus was passaged in 2 times in Vero 76 cells.
Female BALB/c mice 7-8 weeks old (18-20 g) were obtained from Charles River Laboratories (Wilmington, MA). Mice were quarantined at the animal housing facilities at Utah State University for 1 week prior to the beginning of the experiment. All animals were fed standard mouse chow and tap water ad libitum. All experiments were conducted in an AAALAC accredited facility.
2.2 Compounds
The interferon-inducing compound Ampligen (poly I: poly C12U) is a mismatched double-stranded (ds) RNA molecule with proven efficacy against both alphavirus- and flavivirus-associated encephalitis in experimental animal models (Pinto et al. 1990; Morrey et al. 2004). The compound, (courtesy of David Strayer, Hemispherx Biopharma, Philadelphia, PA) was provided as a viscous 2.4 mg/mL solution (stored at -20°) and was diluted in diethylprocarbonate (DEPC) treated sterile water to the appropriate concentrations.
2.3 Cell culture assays
Virus titers in tissues were assayed using the virus yield assay (Morrey et al. 2002) where a specific volume of tissue homogenate or heparinized serum was added to the first tube of a series of dilution tubes. Serial log10 dilutions were made and added to Vero 76 cells in 96-well microplates. Three days later for SFV, and seven days later in the case of BaV, visual identification of cytopathic effect (CPE) was used to identify the end-point of infection. Four replicates were used to calculate the infectious doses per gram of tissue or milliliter of serum. Results were reported as log10 infectious units/gram of tissue or milliliter of serum.
2.4 Blood-brain barrier permeability assay
The method for evaluation of BBB permeability to the small molecular weight molecule sodium fluorescein has been previously described(Gupta et al. 1999). Briefly, mice were injected intraperitoneally with 10 mg of NaFl (Sigma Aldrich, St. Louis MO) in a volume of 0.1 ml of sterile saline. To obtain serum, animals were anesthetized with ketamine HCl (100-200 mg/kg) i.p. 45 minutes after the NaFl injection. Blood was collected into serum separator tubes (Sarstedt, Newton, NC) by retro-orbital bleeding. Blood samples were stored at 4 °C for approximately 30 minutes to allow for clotting, after which they were centrifuged at 4,500 × g for 7 min. The serum was removed and stored at -70 °C until processing. Animals were sacrificed immediately following blood collection. Transcardial perfusion to remove blood from the vasculature was performed by making an incision in the right atrium of the heart, and gently injecting sterile phosphate-buffered saline (PBS) through the left ventricle until the expelled blood ran clear. The brain was removed, weighed, homogenized in 1 ml of sterile PBS and then stored at -70 °C until processing.
2.5 Measurement of fluorescence
Protein was precipitated out of the samples with trichloroacetic acid (TCA) to remove potential background fluorescence. Serum samples were diluted 1:10 in 20% TCA, while brain samples were first centrifuged at 1, 700 × g for 5 min, after which the resulting supernatant was also diluted 1:10 in 20% TCA. All samples were incubated in TCA at 4 °C for 24 hours. Samples were centrifuged at 10,000 × g for 15 min to remove precipitated protein. The supernatant was removed and diluted with equal volumes of sodium borate buffer (0.05 M, pH 10), resulting in a final concentration of 10% TCA and 0.025 M sodium borate buffer. Samples were analyzed on an fmax 96-well plate fluorometer (Molecular Devices, Sunnyvale, CA) using an excitation wavelength of 480 nm, and fluorescence measured at 538 nm. A standard curve for quantitation of NaFl in the samples was generated by simultaneously analyzing samples of known NaFl concentration in 10% TCA and 0.025 M borate buffer. The degree of BBB permeability was measured as the percentage (w/v) of NaFl in a gram of brain tissue per the amount of NaFl in a milliliter of serum. The comparison of brain and serum fluorescence was intended to normalize the degree of BBB permeability detected across multiple animals.
2.6 Experimental Design: Time course of Infection
For viral infection studies intended to monitor systemic virus dissemination or to evaluate the time course of alterations to BBB permeability animals were inoculated with an LD50 dose of virus diluted in minimum essential medium (MEM). These viral doses correlated to 10 50%-cell culture infectious doses (CCID50) per animal for BaV and 104 CCID50 per animal for SFV. To evaluate virus dissemination to various organs groups of 4 virus-inoculated animals and 1 sham inoculated animal were sacrificed at each time point. Tissues were collected starting 1 day post – virus inoculation (dpi) and continuing daily until 10 dpi, with one additional set of tissues harvested on 14 dpi. Tissue samples collected from each mouse included serum, liver, kidney, spleen, and brain. Samples were homogenized in MEM and then stored at -70°C until being assayed for virus titer.
For experiments to determine the time course of BBB permeability alterations associated with virus infection groups of 3 virus-inoculated animals and 2 sham-inoculated animals were assayed for BBB permeability to NaFl. Animals were sacrificed beginning on 1 dpi, and continuing daily unti 10 dpi. All virus inoculations were performed via the intraperitoneal (i.p.) route.
2.7 Experimental Design: Ampligen efficacy studies
Mice were inoculated with LD90 doses of virus for all experiments wherein mice were treated with Ampligen. This dose of virus correlates to 100 CCID50 per animal for BaV and 105 CCID50 per animal for SFV. Groups of 25 virus-inoculated mice (35 for placebo-treated animals) and 6 sham-inoculated mice were treated i.p. with 1 mg/kg of Ampligen or vehicle. Ten virus-inoculated and 3 sham- inoculated mice were assayed for BBB permeability to NaFl on either 6 dpi (SFV) or 8 dpi (BaV). The necessity of sampling and processing the entire brain for detection of fluorescence prohibited us from measuring fluorescence and viral titers in the same animals. Therefore, on the same day, 5 animals from each group were assayed for brain virus titers. The remaining animals were monitored for weight change and survival until 21 dpi. Ampligen was administered as a single treatment at 24 h before, 4-6 h before or 24 h after virus inoculation.
2.8 Statistical evaluation
Differences in total survivors between the different populations of mice were evaluated by chi square analysis with Yates correction. Differences in tissue virus titer, mean day to death (MDD), BBB permeability and changes in whole body weight were analyzed by unpaired, two-tailed t test.
3. Results
The infectious viral titers in the plasma, spleen, kidney, liver and brain were determined over a course of time in mice inoculated i.p with either BaV or SFV (Table 1). Peak viral brain titers in BaV-infected mice was 8.3 ± 0.8 log10 CCID50/gram of brain tissue on 8 dpi, while peak viral brain titers for SFV infected mice was 7.5 ± 0.5 log10 CCID50/g of tissue on 6 dpi. Similarly, the time course of BBB permeability to NaFl was determined in mice inoculated with either BaV or SFV. The permeability of the BBB in BaV-infected mice began increasing at 4 dpi, with peak penetration of fluorescent dye occurring on 9 dpi (Figure 1). Brain fluorescence reached 12.4% of that in serum (w/v) on 9 dpi, which is greater than 4-fold higher than the 2-3% noted in sham infected animals. In SFV-infected mice BBB permeability began to increase on 5 dpi, with peak permeability occurring on 7 dpi (Figure 2). Peak brain fluorescence reached 8.1% of serum fluorescence (w/v), which was approximately 3-fold higher than the 2-3% fluorescence seen in sham infected animals. Interestingly, in both models peak fluorescence was reached 1 day after expected peak virus titers in the brain. The data were used to identify time of virus entry into the brain as well as peak brain titers and peak fluorescence for subsequent experiments.
TABLE 1.
Time course of tissue viral titers in i.p.-inoculated 7-8 week old BALB/c mice.
| Days post-viral injection – viral titers (log10 infectious doses/g tissue or mL plasma ± S.D.) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Virus | Tissue | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 14 | |||
| BaV | Serum | (1/4)a | (1/4) | (1/4) | (3/4) | (1/4) | (0/4) | (2/4) | (2/4) | (1/4) | (1/4) | (1/4) | |||
| NCb | NC | NC | 3.3 ± 0.8 | NC | NC | 3.7 ± 1.6 | 6.1 ± 1.6 | NC | NC | NC | |||||
| BaV | Spleen | (1/4) | (1/4) | (1/4) | (3/3) | (3/4) | (2/4) | (1/4) | (2/4) | (3/4) | (4/4) | (0/4) | |||
| NC | NC | NC | 3.6 ± 0.5 | 4.9 ± 0.6 | 4.3 ± 0.6 | NC | 3.4 ± 0.1 | 3.5 ± 1.2 | 4.0 ± 0.7 | NC | |||||
| BaV | Kidney | (0/4) | (0/4) | (0/4) | (3/4) | (1/4) | (0/4) | (2/4) | (2/4) | (2/4) | (3/4) | (0/4) | |||
| NC | NC | NC | 3.4 ± 0.5 | NC | NC | 2.8 ± 0.7 | 6.6 ± 4.1 | 4.0 ± 1.0 | 3.9 ± 0.8 | NC | |||||
| BaV | Liver | (0/4) | (1/4) | (1/4) | (2/4) | (2/4) | (0/4) | (0/4) | (2/4) | (2/4) | (3/4) | (0/4) | |||
| NC | NC | NC | 2.6 ± 0.0 | 3.2 ± 0.2 | NC | NC | 4.4 ± 2.0 | 3.8 ± 1.4 | 4.6 ± 2.2 | NC | |||||
| BaV | Brain | (0/4) | (0/4) | (0/4) | (1/4) | (2/4) | (2/4) | (1/4) | (4/4) | 4/4 | (4/4) | (1/4) | |||
| NC | NC | NC | NC | 3.6 ± 0.1 | 3.6 ± 1.4 | NC | 8.3 ± 0.8 | 6.7 ± 2.9 | 7.3 ± 2.0 | NC | |||||
|
| |||||||||||||||
| SFV | Serum | (4/4) | (4/4) | (3/4) | (0/4) | (0/4) | (0/4) | (0/4) | (0/4) | (0/4) | - | (0/4) NC | |||
| 4.2 ± 0.2 | 4.7 ± 0.5 | 2.4 ± 0.8 | NC | NC | NC | NC | NC | NC | NC | ||||||
| SFV | Spleen | (4/4) | (4/4) | (4/4) | (4/4) | (2/4) | (1/4) | (0/4) | (0/4) | (0/4) | - | (0/4) | |||
| 3.6 ± 1.8 | 4.8 ± 0.9 | 4.6 ± 0.5 | 3.9 ± 0.7 | 2.5 ± 0.2 | NC | NC | NC | NC | NC | ||||||
| SFV | Kidney | (4/4) | (4/4) | (4/4) | (2/4) | (1/4) | (1/4) | (0/4) | (0/4) | (0/4) | - | (0/4) | |||
| 3.7 ± 2.1 | 4.1 ± 1.0 | 2.7 ± 0.3 | 2.4 ± 0.2 | NC | NC | NC | NC | NC | NC | ||||||
| SFV | Liver | (4/4) | (4/4) | (4/4) | (1/4) | (0/4) | (0/4) | (0/4) | (0/4) | (0/4) | - | (0/4) | |||
| 3.3 ± 2.0 | 4.6 ± 0.8 | 3.4 ± 0.3 | NC | NC | NC | NC | NC | NC | NC | ||||||
| SFV | Brain | (0/4) | (3/4) | (4/4) | (4/4) | (4/4) | (4/4) | (4/4) | (4/4) | (4/4) | - | (0/4) | |||
| NC | 1.7 ± 1.2 | 5.2 ± 0.3 | 6.4 ± 0.9 | 6.9 ± 1.1 | 7.5 ± 0.5 | 6.9 ± 1.4 | 7.3 ± 0.6 | 5.8 ± 1.0 | NC | ||||||
Number of samples with detectable virus/total animals assayed.
NC- Not Calculated. Means were not calculated when most samples were below the limits of detection.
Figure 1.
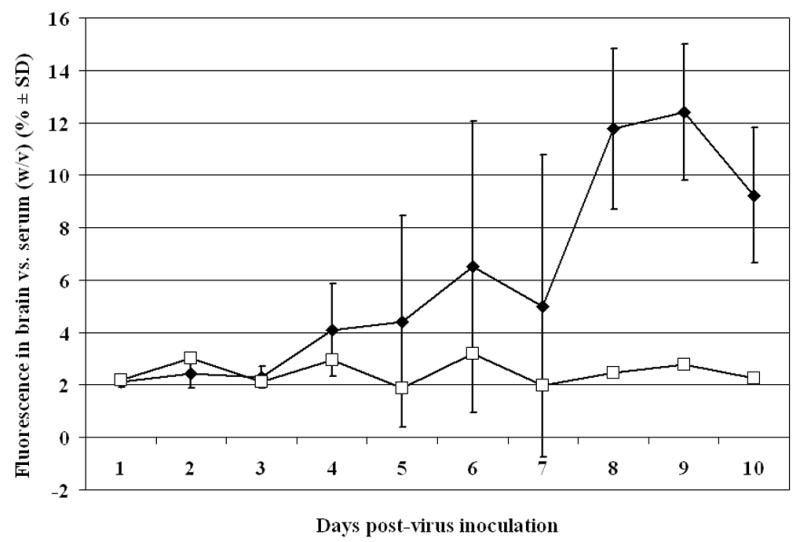
Time course of blood-brain barrier permeability in 7-8 week old BALB/c mice inoculated i.p. with Banzi virus. Percentage of fluorescence measured in 1 g of brain tissue vs. the amount of fluorescence measured in 1 ml of serum 45 min after i.p. injection of sodium fluorescein dye. (♦Banzi-virus inoculated mice, □Sham-inoculated mice)
Figure 2.
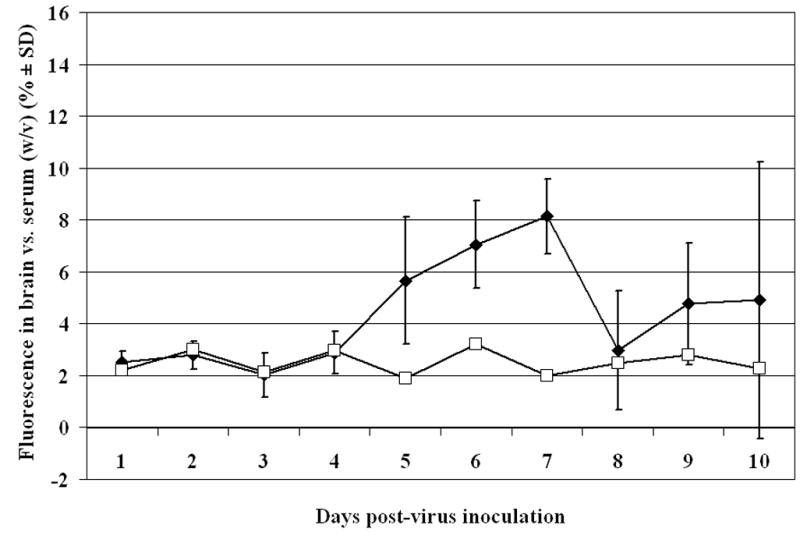
Time course of blood-brain barrier permeability in 7-8 week old BALB/c mice inoculated i.p with Semliki Forest virus. Percentage of fluorescence measured in 1 g of brain tissue vs. the amount of fluorescence measured in 1 ml of serum 45 min after i.p. injection of sodium fluorescein dye. (♦Semliki Forest virus-inoculated mice, □Sham-inoculated mice)
Treatment with Ampligen significantly improved the outcome for mice inoculated with BaV, but only if it was administered prior to virus inoculation (Table 2). Placebo-treated animals had 0% survival following BaV inoculation, with a MDD of 10.4 ± 1.8, whereas, mice receiving a single 1 mg/kg dose of Ampligen 24 h prior to virus inoculation had 90% survival (p<0.001) with a single mouse dying on 14 dpi, while those treated 4-6 h before virus exposure had 70% survival (p<0.001) with a MDD of 12.3 ± 1.5. Delaying treatment to 24 h post-virus inoculation resulted in 0% survival, although the MDD of 12.8 ± 3.1 was significantly (p<0.05) later than that seen in placebo-treated animals.
Table 2.
Effects of a single dose of Ampligen administered at various times in relation to virus exposure on survival and brain virus titers in BALB/c mice inoculated with Banzi virus or Semliki Forest virus. All mice received an approximately LD90 dose of virus inoculum.
| Uninfected toxicity control | Infected, treated | |||||
|---|---|---|---|---|---|---|
| Compound
Virus |
Virus | Dose | Surv/Total | Surv/Total | MDDa | Brain Virus
Titersbc |
| Ampligen (24 h pre) | Banzi | 1 mg/kg | 3/3 | 9/10*** | 14.0 | 2.3 ± 0.1*** |
| Ampligen (4-6 h pre) | Banzi | 1 mg/kg | 3/3 | 7/10*** | 12.3 ± 1.5 | 3.8 ± 1.0*** |
| Ampligen (24 h post) | Banzi | 1 mg/kg | 3/3 | 0/10 | 12.8 ± 3.1* | 7.0 ± 2.1 |
| Placebo (24 h pre) | Banzi | - | 3/3 | 0/20 | 10.4 ± 1.8 | 9.1 ± 0.1 |
|
| ||||||
| Normal Control | - | - | 3/3 | - | - | - |
|
| ||||||
| Ampligen (24 h pre) | Semliki Forest | 1 mg/kg | 3/3 | 4/10 | 9.0 ± 1.1* | 6.2 ± 1.9 |
| Ampligen (4-6 h pre) | Semliki Forest | 1 mg/kg | 3/3 | 7/10*** | 7.7 ± 1.5 | 5.5 ± 1.5+ |
| Ampligen (24 h post) | Semliki Forest | 1 mg/kg | 3/3 | 3/10 | 7.4 ± 1.8 | 7.9 ± 1.1 |
| Placebo (24 h pre) | Semliki Forest | - | 3/3 | 2/20 | 7.6 ± 1.3 | 7.4 ± 1.2 |
|
| ||||||
| Normal Control | - | - | 3/3 | - | - | - |
Mean day to death of mice dying before day 21
Log10 infectious doses/g of tissue.
Banzi virus inoculated animals assayed on 8 dpi. Semliki Forest virus inoculated animals assayed on 6 dpi.
p<0.05,
p<0.001 compared to placebo treated controls,
p<0.05 compared to Ampligen treatment 24 h after virus inoculation
Ampligen treatment prior to virus exposure resulted in significantly reduced viral titers in the brains of BaV-inoculated mice on 8 dpi. Placebo-treated mice had viral brain titers of 9.1 ± 0.1 log10 CCID50/g of brain tissue. Mice treated with Ampligen 24 h prior to virus inoculation had titers of 2.3 ± 0.1 log10 CCID50/g (p<0.001), and those treated 4-6 h before virus injection had titers of 3.8 ± 1.0 log10 CCID50/g (p<0.001). When treatment was delayed until 24 h after virus inoculation titers rose to 7.0 ± 2.1 log10 CCID50/g, which was not significantly different than placebo-treated animals. However, delaying treatment to 24 h after virus inoculation did cause titers to be significantly higher than animals receiving Ampligen 24 h (p<0.01), and 4-6 h before virus exposure (p<0.05).
The beneficial effects of Ampligen treatment on survival and brain virus titer of mice challenged with BaV were mirrored in its ability to significantly improve weight change in virus-inoculated mice (Figure 3). Placebo-treated animals had an average weight change of −13.1% ± 8.8% on 8 dpi. In contrast, animals receiving 1 mg/kg of Ampligen 24 h prior to virus inoculation had an average weight change of +1.3% ± 4.4% (p<0.001), while animals treated with Ampligen 4-6 h prior to virus exposure had a weight change of +1.1% ± 7.2% (p<0.001). Mice treated with Ampligen 24 h after virus exposure had an average weight change of −1.4% ± 5.2% on 8 dpi, which was also significantly different than placebo-treated animals (p<0.001).
Figure 3.

Average percentage weight change from initial body weight of Banzi virus-inoculated mice after a single i.p. dose of 1 mg/kg of Ampligen administered at various times in relation to virus exposure. ***p<0.001 compared to placebo treated animals (♦Ampligen administered 24 h before virus exposure, □Ampligen administered 4-6 h before virus exposure, ▲Ampligen administered 24 h after virus exposure, ○Placebo-treated mice (Placebo was administered 24 h before virus exposure).
Treatment with a single 1 mg/kg dose of Ampligen was able to significantly improve the outcome for mice inoculated with SFV (Table 2). Placebo-treated animals displayed 10% survival, with a MDD of 7.6 ± 1.3. Animals that received 1 mg/kg of Ampligen 24 h prior to virus exposure had 40% survival, which was not significantly higher than placebo treated animals. However, the MDD of mice receiving Ampligen 24 h before virus exposure was 9.0 ± 1.1, which was significantly longer (p<0.05) than placebo-treated animals. Mice treated with Ampligen 4-6 h prior to virus inoculation had a significantly higher survival rate, with 70% of the animals surviving (p<0.001), while animals treated with Ampligen 24 h after virus inoculation had 30% survival which was not significantly higher than placebo-treated animals.
Treatment with Ampligen did not significantly reduce brain viral titers on 6 dpi as compared to placebo treatment in SFV inoculated mice (Table 2). However, it is interesting to note that mice treated with Ampligen 4-6 h before virus exposure had a brain viral titer of 5.4 ±1.5 log10 CCID50/g, which was significantly (p<0.05) lower than the brain viral titer observed in mice that received virus 24 h after virus exposure, which was of 7.8 ± 1.1 log10 CCID50/g.
Ampligen treatment also significantly improved weight change in SFV inoculated mice (Figure 4). Placebo treated animals had an average weight change of −22.3% ± 9.7% on 7 dpi. In contrast, animals receiving 1 mg/kg of Ampligen 24 h prior to virus inoculation had an average weight change of −9.6% ± 8.8% (p<0.01), while animals treated with Ampligen 4-6 h prior to virus exposure had a weight change of −4.7% ± 9.7% (p<0.001). Mice treated with Ampligen 24 h after virus exposure had an average weight change of −20.1% ± 9.6% on 7 dpi, which was not significantly different than placebo treated animals.
Figure 4.

Average percentage weight change from initial body weight of Semliki Forest virus-inoculated mice after a single i.p. dose of 1 mg/kg of Ampligen administered at various times in relation to virus exposure. *p<0.05. **p<0.01, ***p<0.001 compared to placebo treated animals. +p<0.05, ++p<0.01, +++p<0.001 compared to mice receiving Ampligen 24 h post-virus exposure. (♦Ampligen administered 24 h before virus exposure, □Ampligen administered 4-6 h before virus exposure, ▲Ampligen administered 24 h after virus exposure, ○Placebo-treated mice (Placebo was administered 24 h before virus exposure).
Blood-brain barrier permeability was assayed in SFV inoculated mice on 6 dpi, and in BaV inoculated mice on 8 dpi using i.p. injections of NaFl. In both SFV and BaV inoculated mice BBB permeability was assayed 1 day prior to expected peak permeability to avoid the death loss of a substantial number of placebo treated mice. A time of 45 minutes between administration of NaFl and collection of serum and brain samples for assay was based on previously published reports using a similar technique(Gupta et al. 1999). Treatment with Ampligen prior to virus exposure was able to significantly decrease BBB permeability associated with BaV induced encephalitis (Figure 5). The brains of placebo treated animals had 9.4% ± 3.7% (w/v) of the fluorescence found in their serum. Mice receiving Ampligen 24 h before virus exposure had a measurement of brain fluorescence of 2.8% ± 1.0% (p<0.001), and mice receiving Ampligen 4-6 h before virus exposure had a measurement of brain fluorescence of 4.0% ± 2.7% (p<0.01). In those animals treated with Ampligen 24 h after virus inoculation the brain fluorescence was 5.8% ± 2.3% of serum fluorescence, which was not significantly lower than placebo treated animals, but was significantly higher than animals treated with Ampligen 24 h prior to virus inoculation (p<0.05).
Figure 5.
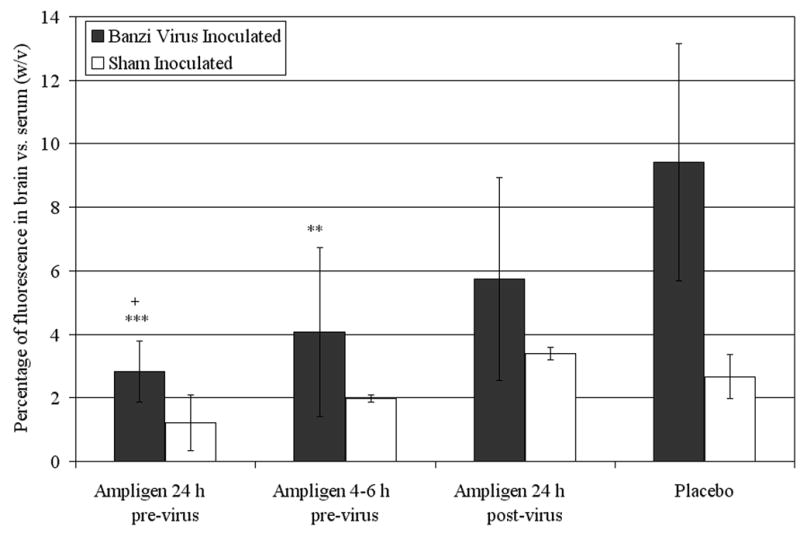
Percentage of fluorescence detected in the brain vs. the serum (w/v) on 8 dpi of mice inoculated with Banzi virus and treated with a single i.p. dose of 1 mg/kg of Ampligen at various times in relation to virus exposure. **p<0.01, ***p<0.001 compared to placebo. +p<0.05 compared to Ampligen administration at 24 h post-virus inoculation
Treatment with Ampligen prior to virus exposure was also able to significantly decrease BBB permeability associated with SFV induced encephalitis (Figure 6). The brains of placebo treated animals had 11.3% ± 2.3% (w/v) of the fluorescence found in their serum. Administration of Ampligen to mice 24 h before virus exposure resulted in brain fluorescence of 6.2% ± 3.4% (p<0.01), and animals treated with Ampligen 4-6 h before virus exposure displayed a measurement of brain fluorescence of 4.7% ± 2.9% (p<0.001). In those animals treated with Ampligen 24 h after virus inoculation the brain fluorescence was 8.8% ± 3.3% of serum fluorescence, which was not significantly lower than placebo treated animals, but was significantly higher than animals treated with Ampligen 4-6 h prior to virus inoculation (p<0.01).
Figure 6.
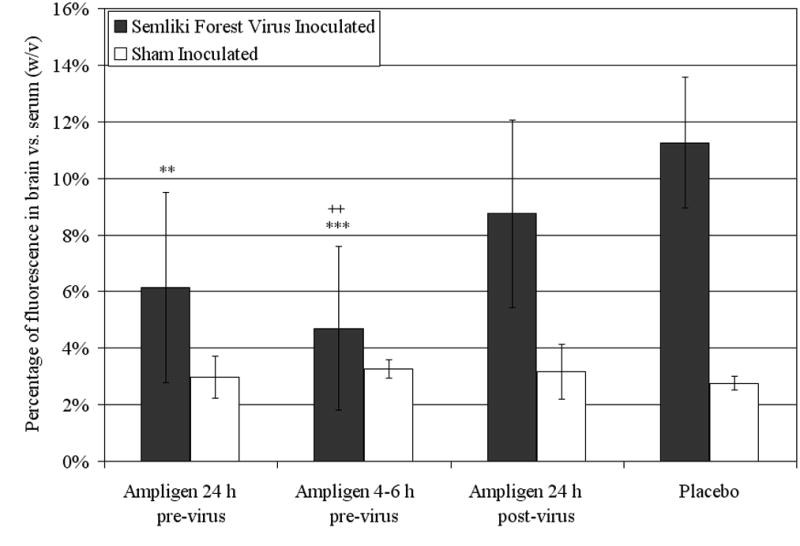
Percentage of fluorescence detected in the brain vs. the serum (w/v) on 6 dpi of mice inoculated with Semliki Forest virus and treated with a single i.p. dose of 1 mg/kg of Ampligen at various times in relation to virus exposure. **p<0.01, ***p<0.001 compared to placebo. ++p<0.01 compared to Ampligen administration at 24 h post-virus inoculation
No significant differences in weight change or BBB permeability were detected between groups of sham inoculated mice, regardless of treatment regimen.
4. Discussion
Our initial hypothesis was that the permeability of the blood-brain barrier of mice to the small molecular weight marker sodium fluorescein would increase during the course of disease following inoculation with a viral encephalitide. This hypothesis was confirmed by measuring up to four-fold increases in BBB permeability in virus inoculated animals when compared to sham inoculated animals. An additional hypothesis was that BBB permeability would correlate with disease outcome following treatment with the interferon inducer Ampligen, an antiviral agent previously shown to be effective against the viruses tested. This hypothesis was confirmed by measurement of significantly decreased permeability of the BBB and significantly improved weight change and survival of mice which received treatment with the immunomodulatory agent Ampligen prior to virus exposure.
Treatment of viral encephalitis presents a clinical challenge, with few effective therapies. Improved understanding of the role of the BBB in both health and disease is vital to understanding the challenges associated with treating viral encephalitis, and will also aid in developing new strategies for treatment of viral encephalitis. We believe the results presented here highlight several aspects of the role of the BBB in viral encephalitis including that increases in permeability of the BBB is a pathophysiologic event common to many forms of viral encephalitis, including arboviruses, that the degree of BBB permeability correlates directly with disease severity, and that increased permeability of the BBB may play an important role in the pathophysiology of viral encephalitis.
Unique aspects of the data reported here are the quantification of BBB permeability and the monitoring of changes in BBB permeability over the time course of the disease. Banzi and Semliki Forest viruses are often used as surrogate pathogens in virus studies in lieu of using other viruses with a significant risk of causing human disease. Showing the ability of two disparate viral species to produce similar changes in the permeability of the BBB to NaFl reinforces the probability that breakdown of the BBB is a pathophysiological event common to many forms of viral encephalitis. This suggests that similar changes may occur in humans infected with encephalitic viruses from the Flavivirus or Togavirus families of viruses, such as West Nile or Venezuelan Equine Encephalitis viruses.
In addition to quantifying the degree of increased BBB permeability we have shown a positive correlation between increased degrees of BBB permeability and increased disease severity. This was shown in studies in which Ampligen treatment significantly decreased the degree of BBB permeability while simultaneously decreasing mortality and weight loss associated with virus inoculations. Ampligen has been proven to be effective against many viruses in a wide range of animal models(Pinto et al. 1988; Morahan et al. 1991; Sidwell et al. 1994; Leyssen et al. 2003; Morrey et al. 2004). Ampligen is a double-stranded RNA molecule, and its antiviral activity is recognized to be due to its ability to induce the expression of alpha/beta interferons via stimulation of the Toll-like Receptor 3 which responds to the presence of double-stranded RNA. The exact means by which Ampligen treatment of virus inoculated animals resulted in a lessening of the degree of BBB permeability is not known. We postulate the decreased BBB permeability measured in virus inoculated animals associated with Ampligen treatment is most like due to systemic antiviral effects of the drug, and not due to any direct effect of either Ampligen or interferon on the BBB itself. Reducing systemic as well as nervous tissue titers of virus would most likely result in a blunting or decreased inflammatory response, which in turn will minimize associated pathological changes throughout the animal. This view is supported by the result that administration of Ampligen 24 h after virus challenge did not improve outcome, regardless of the absence of detectable virus in central nervous system tissues or detectable changes in BBB permeability at the time of Ampligen administration.
The correlation between high levels of BBB permeability and poor disease outcomes appears to be clear. We suggest that measuring BBB permeability provides a CNS specific and quantifiable marker of disease in animal models of viral encephalitis. Identification of appropriate disease markers is necessary to better characterize viral encephalitis in animal models, and is vital for evaluating the efficacy of antiviral compounds and therapies. A variety of assays for assessing the condition of animals inoculated with encephalitic viruses have been used. But, with the exception of histopathology, sensitive markers of disease which are specific for involvement of the CNS have been difficult to identify in animal models of viral encephalitis Common human symptoms of CNS infection with an encephalitic virus include headache, nuchal rigidity, and altered mental status (Asnis et al. 2001; Pepperell et al. 2003). It is difficult, if not impossible, to evaluate and characterize similar disease syndromes in laboratory animals. Virus titers in the target tissue are a common measurement tool in virological studies. However, as seen here with SFV inoculated animals (Table 2), high viral load in the CNS does not always correlate to a poor disease outcome for experimental animals. Standard serum biochemical analyses are unable to detect abnormalities in the CNS. Changes in body weight can be easily measured and, as seen in the current study, weight loss may correlate with the progression of disease while amelioration of weight loss can indicate efficacy of an antiviral agent. However, weight change is a non-specific marker of general health that can be affected by environmental conditions, reproductive status, and administration of drugs or experimental therapeutic compounds, and does not provide any information specific to the CNS. Measurement of CNS function via neurological exams and evaluation of motor functions has been previously used in animal models of viral encephalitis (Morrey et al. 2004). While these techniques can provide valuable information, they are subjective and their value may depend upon the skill of the observer. Histopathological evaluation of CNS tissues is an ideal technique, both for animal model development, disease characterization and for drug efficacy studies. However, this requires the services or training of an experienced pathologist, and its use may be limited by availability of pathological services or cost concerns. Similarly, specialized imaging techniques such as computerized tomography or magnetic resonance imaging may be useful for examining changes in the CNS of infected animals, but they are not widely available because of cost, and biocontainment concerns.
As with all assays, the measurement of BBB permeability to NaFl or a similar sized marker has certain limitations, and should not be considered a replacement for other established assays. The comparison of NaFl in the brain to that in the serum using a single 45 minute post-injection time point was not intended to fully describe the movement of small molecular weight molecules in animals with viral encephalitis. However, it did provide a quantifiable means for evaluating the function of the BBB. Similarly, the ability of NaFl to cross the BBB should not necessarily be considered indicative of how larger substances, such as proteins or virions, will behave in animals with viral encephalitis. Due to the small molecular weight of NaFl it may cross the BBB much more readily than larger molecules, and we would suggest that changes in the BBB permeability to NaFl may be the earliest and most sensitive indicators of alterations in BBB function. We observed that BBB permeability gradually increased over the course of the disease and appeared to peak shortly before the majority of animals began to die from virus infection. Permeability levels in virus-inoculated animals at the apparent peak of disease severity were up to four-fold higher than the remarkably consistent degree of low BBB permeability measured in sham-inoculated animals.
Permeability measurements in virus-inoculated animals appeared to be a sensitive indicator of the antiviral efficacy of Ampligen. Mice treated with Ampligen 24 h after inoculation with Banzi virus had no improvements in survival (Table 2), but had post-virus weight change that was highly significantly different, and presumably better, than placebo treated animals (Figure 3). Meanwhile, no statistically significant difference was detected between the BBB permeability measurements of the same Ampligen treated animals and placebo treated animals. (Figure 5). Similarly, in the case of mice inoculated with Semliki Forest virus animals receiving Ampligen 4-6 h before virus had highly significantly improved survival compared to placebo treated animals, but also had brain virus titers nearly identical to those detected in placebo treated animals (Table 2). However, when BBB permeability was measured in the same group of SFV inoculated mice, Ampligen treated animals had significantly lower permeability levels than that measured in placebo treated animals. In both animal models the BBB permeability assay showed some benefits over currently available assays by identifying a false positive result in the BaV model and a false negative result in the SFV model. The use of a BBB permeability assay in no way replaces the use of established assay techniques. Indeed, the results described here highlight the importance of using multiple assay methods to fully characterize animal models of viral disease and antiviral effects of potential therapeutics. Future studies, such as comparing BBB permeability and histopathological changes in the CNS of virus-inoculated animals and evaluation of BBB permeability to various different sizes of marker molecules, will be a vital component in developing our understanding of the interplay between virus invasion of the CNS, nervous system pathology, and function of the BBB. Therefore, although we did not attempt to evaluate different time points after NaFl injection, different markers of BBB function, or changes in histopathology, we believe the findings reported in this study are an accurate, albeit narrow, representation of the function of the BBB in animals inoculated with encephalitic viruses. Consequently, we believe that measurement of BBB permeability provides a sensitive assay that can be easily and safely performed with minimal equipment, and is specific for the functionality of one portion of the CNS. Furthermore, the use of BBB permeability markers may provide an effective assay for future antiviral studies on animal models of viral encephalitis, supplementing assays currently being used to assess the efficacy of potential therapeutic agents, and enhance our ability to evaluate antiviral efficacy of experimental drugs when used in conjunction with established assays.
One of the functions of the BBB is to limit entry of foreign molecules into the CNS. It does so by severely limiting the penetration of hydrophilic or charged molecules that lack a specific transport system, such as that for glucose. The result of this barrier function is generally poor distribution to CNS tissue of most pharmacological compounds, including the majority of currently available antiviral agents. It has been suggested that investigating means to increase permeability of the BBB to enhance entry of antiviral agents into the CNS may provide a therapeutic strategy for the treatment of viral encephalitis. Such a strategy has proven effective in an animal model of herpes virus infections in the brain in which Acyclovir treatment was administered in conjunction with the use of a synthetic bradykinin analog used to temporarily permeabilize the BBB (Bidanset et al. 2001). However, the data presented here, as well as other reports evaluating the connection between BBB permeability and viral encephalitis (Lustig et al. 1992; Ben-Nathan et al. 2000), would indicate that any therapy aimed at increasing BBB permeability would be contraindicated in BaV and SFV animal models, as well as in individuals infected with related members of the Flavivirus- and Togavirus families of viruses. Subsequent studies designed to study the function of the BBB in the presence of virus will be necessary to fully understand how blood-brain barrier function can alter the outcome in cases of viral encephalitis.
In conclusion, we have shown that inoculation of mice with encephalitic viruses will cause increased permeability of the BBB to the small molecular weight marker NaFl, and that permeability increases in a manner that is progressive with the viral infection in vivo. Similar increases in permeability demonstrated by two disparate virus species indicates that increased permeability of the BBB may be a pathophysiological event common to many forms of viral encephalitis.
In agreement with published data we have also shown that administration of the immunomodulatory agent Ampligen prior to, but not after, virus exposure can significantly improve the survival, weight change, and tissue viral titers in mice inoculated with viral encephalitides. Ampligen treatment prior to virus exposure also results in virus infected animals showing improved BBB function as indicated by a concomitant decrease in BBB permeability. This improved function appears to be positively correlated with improvements in disease outcome such as weight change, tissue viral titers, and survival. The improvement in BBB function was lost if Ampligen treatment was delayed until after virus inoculation. These results support using measurement of BBB permeability to NaFl or similar small molecular weight markers in models of viral encephalitis to evaluate BBB function, and support the use of a BBB permeability assay in conjunction with other established virological and physiological assays as indicators of antiviral drug efficacy.
Acknowledgments
We thank Kristiina Shafer and Luci Wandersee for their cell culture and animal technical support. This work was supported by Contract NO1-AI-15435 from the Virology Branch, NIAID, NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersen IH, Marker O, Thomsen AR. Breakdown of blood-brain barrier function in the murine lymphocytic choriomeningitis virus infection mediated by virus-specific CD8+ T cells. J Neuroimmunol. 1991;31(2):155–63. doi: 10.1016/0165-5728(91)90021-x. [DOI] [PubMed] [Google Scholar]
- Asnis DS, Conetta R, Waldman G, Teixeira AA. The West Nile virus encephalitis outbreak in the United States (1999-2000): from Flushing, New York, to beyond its borders. Ann N Y Acad Sci. 2001;951:161–71. doi: 10.1111/j.1749-6632.2001.tb02694.x. [DOI] [PubMed] [Google Scholar]
- Ben-Nathan D, Kobiler D, Rzotkiewicz S, Lustig S, Katz Y. CNS penetration by noninvasive viruses following inhalational anesthetics. Ann N Y Acad Sci. 2000;917:944–50. doi: 10.1111/j.1749-6632.2000.tb05460.x. [DOI] [PubMed] [Google Scholar]
- Bidanset DJ, Placidi L, Rybak R, Palmer J, Sommadossi JP, Kern ER. Intravenous infusion of cereport increases uptake and efficacy of acyclovir in herpes simplex virus-infected rat brains. Antimicrob Agents Chemother. 2001;45(8):2316–23. doi: 10.1128/AAC.45.8.2316-2323.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conant K, McArthur JC, Griffin DE, Sjulson L, Wahl LM, Irani DN. Cerebrospinal fluid levels of MMP-2, 7, and 9 are elevated in association with human immunodeficiency virus dementia. Ann Neurol. 1999;46(3):391–8. doi: 10.1002/1531-8249(199909)46:3<391::aid-ana15>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Dallasta LM, Pisarov LA, Esplen JE, Werley JV, Moses AV, Nelson JA, Achim CL. Blood-brain barrier tight junction disruption in human immunodeficiency virus-1 encephalitis. Am J Pathol. 1999;155(6):1915–27. doi: 10.1016/S0002-9440(10)65511-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Clercq E. Interferon and its inducers--a never-ending story: “old” and “new” data in a new perspective. J Infect Dis. 2006;194(Suppl 1):S19–26. doi: 10.1086/505351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deli MA, Nemeth L, Falus A, Abraham CS. Effects of N,N-diethyl-2-[4-(phenylmethyl)phenoxy]ethanamine on the blood-brain barrier permeability in the rat. Eur J Pharmacol. 2000;387(1):63–72. doi: 10.1016/s0014-2999(99)00796-7. [DOI] [PubMed] [Google Scholar]
- Eralinna JP, Soilu-Hanninen M, Roytta M, Hukkanen V, Salmi AA, Salonen R. Blood-brain barrier breakdown and increased intercellular adhesion molecule (ICAM-1/CD54) expression after Semliki Forest (A7) virus infection facilitates the development of experimental allergic encephalomyelitis. J Neuroimmunol. 1996;66(12):103–14. doi: 10.1016/0165-5728(96)00031-8. [DOI] [PubMed] [Google Scholar]
- Erdlenbruch B, Alipour M, Fricker G, Miller DS, Kugler W, Eibl H, Lakomek M. Alkylglycerol opening of the blood-brain barrier to small and large fluorescence markers in normal and C6 glioma-bearing rats and isolated rat brain capillaries. Br J Pharmacol. 2003;140(7):1201–10. doi: 10.1038/sj.bjp.0705554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A, Agarwal R, Shukla GS. Functional impairment of blood-brain barrier following pesticide exposure during early development in rats. Hum Exp Toxicol. 1999;18(3):174–9. doi: 10.1177/096032719901800307. [DOI] [PubMed] [Google Scholar]
- Haseloff RF, Blasig IE, Bauer HC, Bauer H. In search of the astrocytic factor(s) modulating blood-brain barrier functions in brain capillary endothelial cells in vitro. Cell Mol Neurobiol. 2005;25(1):25–39. doi: 10.1007/s10571-004-1375-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper DC, Kean RB, Scott GS, Spitsin SV, Mikheeva T, Morimoto K, Bette M, Rohrenbeck AM, Dietzschold B, Weihe E. The central nervous system inflammatory response to neurotropic virus infection is peroxynitrite dependent. J Immunol. 2001;167(6):3470–7. doi: 10.4049/jimmunol.167.6.3470. [DOI] [PubMed] [Google Scholar]
- Keogh B, Sheahan BJ, Atkins GJ, Mills KH. Inhibition of matrix metalloproteinases ameliorates blood-brain barrier disruption and neuropathological lesions caused by avirulent Semliki Forest virus infection. Vet Immunol Immunopathol. 2003;94(34):185–90. doi: 10.1016/s0165-2427(03)00082-5. [DOI] [PubMed] [Google Scholar]
- Kozler P, Pokorny J. Altered blood-brain barrier permeability and its effect on the distribution of Evans blue and sodium fluorescein in the rat brain applied by intracarotid injection. Physiol Res. 2003;52(5):607–14. [PubMed] [Google Scholar]
- Leyssen P, Drosten C, Paning M, Charlier N, Paeshuyse J, De Clercq E, Neyts J. Interferons, interferon inducers, and interferon-ribavirin in treatment of flavivirus-induced encephalitis in mice. Antimicrob Agents Chemother. 2003;47(2):777–82. doi: 10.1128/AAC.47.2.777-782.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luabeya MK, Dallasta LM, Achim CL, Pauza CD, Hamilton RL. Blood-brain barrier disruption in simian immunodeficiency virus encephalitis. Neuropathol Appl Neurobiol. 2000;26(5):454–62. doi: 10.1046/j.1365-2990.2000.00275.x. [DOI] [PubMed] [Google Scholar]
- Lustig S, Danenberg HD, Kafri Y, Kobiler D, Ben-Nathan D. Viral neuroinvasion and encephalitis induced by lipopolysaccharide and its mediators. J Exp Med. 1992;176(3):707–12. doi: 10.1084/jem.176.3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morahan PS, Pinto A, Stewart D, Murasko DM, Brinton MA. Varying role of alpha/beta interferon in the antiviral efficacy of synthetic immunomodulators against Semliki Forest virus infection. Antiviral Res. 1991;15(3):241–54. doi: 10.1016/0166-3542(91)90070-8. [DOI] [PubMed] [Google Scholar]
- Morrey JD, Day CW, Julander JG, Blatt LM, Smee DF, Sidwell RW. Effect of interferon-alpha and interferon-inducers on West Nile virus in mouse and hamster animal models. Antivir Chem Chemother. 2004;15(2):101–9. doi: 10.1177/095632020401500202. [DOI] [PubMed] [Google Scholar]
- Morrey JD, Day CW, Julander JG, Olsen AL, Sidwell RW, Cheney CD, Blatt LM. Modeling hamsters for evaluating West Nile virus therapies. Antiviral Res. 2004;63(1):41–50. doi: 10.1016/j.antiviral.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Morrey JD, Smee DF, Sidwell RW, Tseng C. Identification of active antiviral compounds against a New York isolate of West Nile virus. Antiviral Res. 2002;55(1):107–16. doi: 10.1016/s0166-3542(02)00013-x. [DOI] [PubMed] [Google Scholar]
- Pepperell C, Rau N, Krajden S, Kern R, Humar A, Mederski B, Simor A, Low DE, McGeer A, Mazzulli T, Burton J, Jaigobin C, Fearon M, Artsob H, Drebot MA, Halliday W, Brunton J. West Nile virus infection in 2002: morbidity and mortality among patients admitted to hospital in southcentral Ontario. Cmaj. 2003;168(11):1399–405. [PMC free article] [PubMed] [Google Scholar]
- Pinto AJ, Morahan PS, Brinton M, Stewart D, Gavin E. Comparative therapeutic efficacy of recombinant interferons-alpha, -beta, and -gamma against alphatogavirus, bunyavirus, flavivirus, and herpesvirus infections. J Interferon Res. 1990;10(3):293–8. doi: 10.1089/jir.1990.10.293. [DOI] [PubMed] [Google Scholar]
- Pinto AJ, Morahan PS, Brinton MA. Comparative study of various immunomodulators for macrophage and natural killer cell activation and antiviral efficacy against exotic RNA viruses. Int J Immunopharmacol. 1988;10(3):197–209. doi: 10.1016/0192-0561(88)90050-1. [DOI] [PubMed] [Google Scholar]
- Sidwell RW, Huffman JH, Barnard DL, Smee DF, Warren RP, Chirigos MA, Kende M, Huggins J. Antiviral and immunomodulating inhibitors of experimentally-induced Punta Toro virus infections. Antiviral Res. 1994;25(2):105–22. doi: 10.1016/0166-3542(94)90100-7. [DOI] [PubMed] [Google Scholar]
- WHO. Arthropod-borne and rodent-borne viral diseases. Geneva: World Health Organization; 1985. pp. 1–115. [PubMed] [Google Scholar]
- Xiao SY, Guzman H, Zhang H, Travassos da Rosa AP, Tesh RB. West Nile virus infection in the golden hamster (Mesocricetus auratus): a model for West Nile encephalitis. Emerg Infect Dis. 2001;7(4):714–21. doi: 10.3201/eid0704.010420. [DOI] [PMC free article] [PubMed] [Google Scholar]


