Abstract
Mice are used as models for western equine encephalitis virus (WEEV) infection, but high mortality is generally only seen with intracranial or intranasal challenge, while peripheral inoculation results in approximately 50% mortality, and is not dose dependant. Hamsters were therefore studied as a model for WEEV infection. Hamsters were highly sensitive to intraperitoneal (i.p.) infection with WEEV. Disease progression was rapid, and virus titers in serum, brain, liver, and kidney of infected hamsters peaked between 2 and 4 days post-virus inoculation (dpi). Foci of virus infection were detected in neurons of the cerebral cortex and midbrain. Pre-treatment i.p. with either interferon alfacon-1 (5 μg/kg/d), or with Ampligen® (3.2 mg/kg/d), resulted in complete survival, reduced brain titers, and improved weight gain. This model of WEEV infection in hamsters appears to serve as a suitable model for the evaluation of potential therapeutic agents for the treatment of WEE disease.
Keywords: Western equine encephalitis virus, Alphavirus, Interferon, Infergen, Ampligen®, Antiviral, Hamster, Treatment, Model
Introduction
Western equine encephalitis virus (WEEV) is a pathogen of human concern that is endemic to the Americas, and periodic outbreaks cause morbidity and mortality in different species including equines and humans (Calisher, 1994). While human outbreaks are infrequent, the case fatality rate may be as high as 15%, and the disease is generally more severe in infants that are less than one year old. It is important that therapies be developed for the treatment of this disease (Sidwell and Smee, 2003).
Mice have been used as models for WEEV infection, including antiviral and virulence studies (Bianchi et al., 1993; Hunt and Roehrig, 1985; Monath et al., 1978; Nagata et al., 2005; Nagata et al., 2006; Takehara, 1977). Inoculation through peripheral routes, including intraperitoneal (i.p.), intrademal, and subcutaneous, generally result in approximately 50% mortality and infection through these routes is not dose dependent as compared with more lethal intracranial and intranasal (i.n.) inoculation (Hardy et al., 1997; Liu, C. et al., 1970). In a mouse pathogenesis study, subcutaneous infection of 1–2 day old mice resulted in high mortality, but pathologic changes were limited to muscle, cartilage, and bone marrow, while infection 3-week old mice resulted in diffuse meningoencephalitis and changes in heart, lung, kidney, liver, and brown fat (Aguilar, 1970). Previous work has shown that hamsters serve as suitable models for disease caused by WEEV, and high mortality and lesions of encephalitis were observed in adult hamsters inoculated with a virulent strain of WEEV (Zlotnick et al., 1972). Hamsters have been used as models for WEEV infection for immune clearance and virulence studies, and are susceptible to WEEV disease (Jahrling, 1976; Jahrling et al., 1983).
Interferon alfacon-1 is a consensus-type interferon that has been efficacious in the clinical treatment of hepatitis C virus (HCV) infections in human patients (Melian and Plosker, 2001; Sjogren et al., 2005; Yasuda and Miyata, 2002), as well as in treatment of various viral diseases and cancer in experimental animal models or cell culture models (Hisaka et al., 2004; Morrey et al., 2004a; Takemoto et al., 2004; Yasuda et al., 2000). This cytokine works well in humans as well as in hamsters against different viral diseases (Balan et al., 2006; Gowen et al., 2005; Morrey et al., , 2004a). Ampligen® [polyI:polyC12U] is a double-stranded RNA, which elicits an antiviral response at least through the activation of cellular RNase-L and the production of interferon (Anon., 2004). Ampligen® has been shown to have activity against many different viruses and other diseases in vivo (Essey et al., 2001; Leyssen et al., 2003; Morrey et al., 2004a; Wild et al., 1996). This compound has also been shown to be efficacious in hamster models of viral disease (Gowen et al., 2005; Morrey et al., 2004a; Smee et al., 1993).
The purposes of these studies were to characterize the WEEV infection of hamsters for use as a model for encephalitic alphavirus infection and to then use the model to evaluate the efficacy of pre-exposure treatment of animals with interferon alfacon-1 and Ampligen®. Such studies with known antiviral agents should establish the utility of the hamster WEEV infection model.
Results
Titration of WEEV in hamsters
Titration experiments revealed that hamsters were highly susceptible to infection with WEEV. Disease progression was rapid and mortality was high in this hamster model. A 100% mortality rate was observed in all i.p. challenge groups with 10-fold dilutions of stock from 106.5 down to 103.5 CCID50, while one animal survived in the lowest (103.5 CCID50) dose administered via i.n. inoculation (Table 1, experiment 1). A 60–80% mortality rate occurred in subsequent dilutions down to 101.5 CCID50 with animals administered by i.p. injection (Table 1, experiment 2). Mean day to death (MDD) appeared to be dose responsive, with shorter times tending to be in animals challenged with higher doses of virus. In the second titration study, weight gain was recorded, and weight change between –24 h and 4 dpi was lower in animals infected with higher doses of WEEV, but this difference in weight was not statistically significant from weight gain in sham-infected controls (Table 1). A 101.5 CCID50 dose of virus was selected for use in studies characterizing the model and in experiments evaluating antiviral compounds because infection with this dose resulted in 80% mortality.
Table 1.
Titration of WEEV infection in hamsters challenged by intraperitoneal or intranasal inoculation
| Experiment | Virus dose (CCID50)a | Inoculation Route | Alive/totalb | MDD ± SDc | Mean wt. change (g) ± SDd |
|---|---|---|---|---|---|
| 1 | 106.5 | i.n. | 0/5 | 3.0 ± 0.0 | N/D |
| 105.5 | i.n. | 0/5 | 3.4 ± 0.9 | N/D | |
| 104.5 | i.n. | 1/5 | 3.0 ± 0.0 | N/D | |
|
| |||||
| 106.5 | i.p. | 0/5 | 3.0 ± 0.0 | N/D | |
| 105.5 | i.p. | 0/5 | 3.3 ± 0.6 | N/D | |
| 104.5 | i.p. | 0/5 | 4.0 ± 0.0 | N/D | |
|
| |||||
| 2 | 103.5 | i.p. | 0/5 | 4.8 ± 1.1 | −1.2 ± 7.0 |
| 102.5 | i.p. | 2/5 | 4.7 ± 0.6 | 3.6 ± 5.9 | |
| 101.5 | i.p. | 1/5 | 5.0 ± 0.0 | 5.8 ± 2.6 | |
| Sham-infected | i.p. | 3/3 | >21 ± 0.0 | 8.0 ± 4.4 | |
50% cell culture infectious doses in 0.1 ml inoculation volume
Number of animals alive on day 21 per total challenged with virus
Mean day to death ± standard deviation
Mean weight change between –24 h and 4 days post-virus inoculation
Characterization of WEEV infection in Syrian golden hamsters
Tissue samples were taken from selected animals challenged i.p. with WEEV each day after challenge to determine the time-course of disease in hamsters. WEEV titers were significantly elevated in tissues from infected hamsters, with peak titers in brain, liver, and spleen on 4 dpi, while serum titers peaked 2 dpi (Fig 1). Brain virus titers were the highest, with a mean peak titer of 8.6 ± 1.5 log10 CCID50/g tissue. Weight gain was significantly lower on 3, 4, and 5 dpi in hamsters challenged with WEEV as compared with sham infection (Figure 2).
Figure 1.
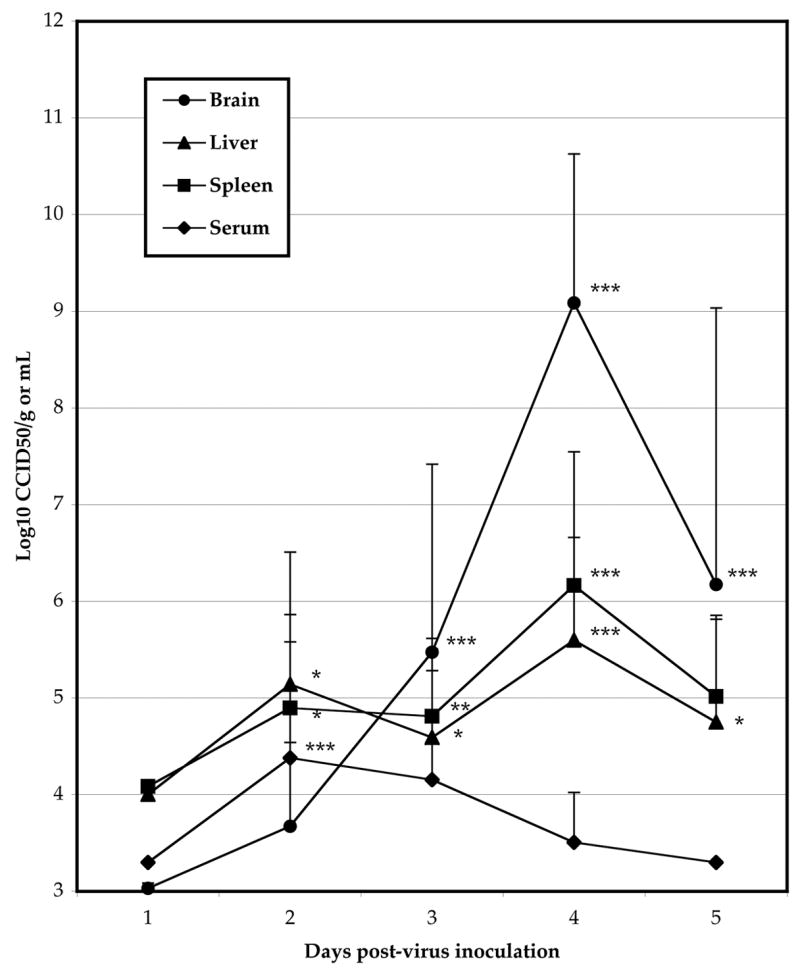
Time-course of WEEV titer in various tissues from infected hamsters (***P<0.001, **P<0.01, *P<0.05 as compared with sham-infected)
Figure 2.
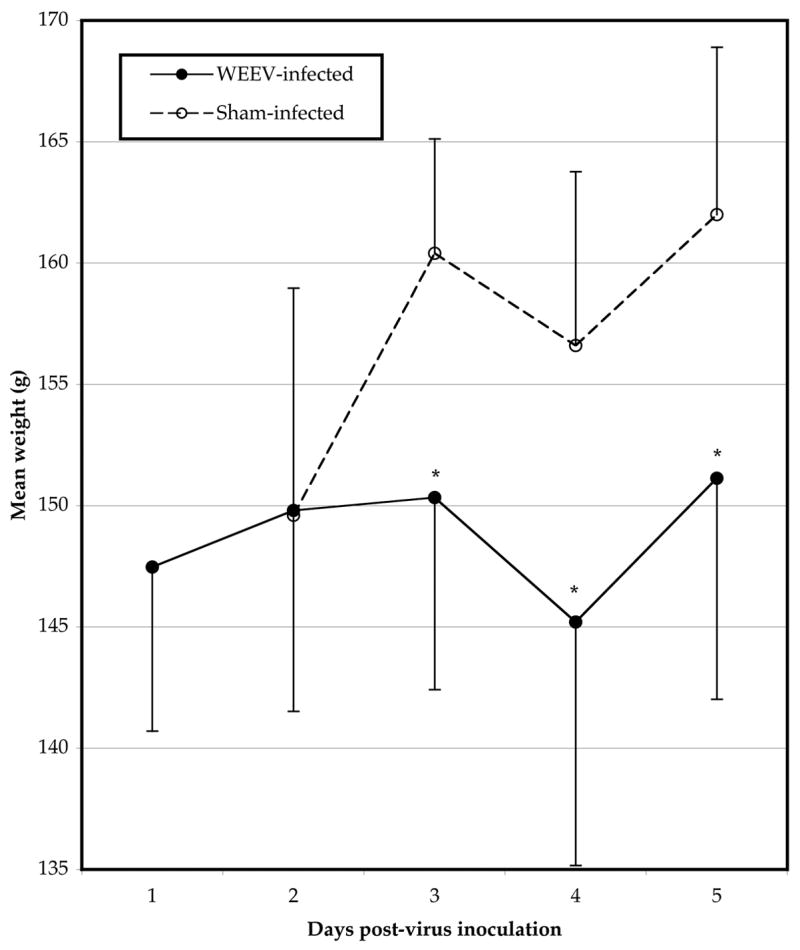
Weight change of golden Syrian hamsters challenged with WEEV (*P<0.05 as compared with sham-infected controls)
In order to determine possible cell types in the brain that are infected with WEEV, brain sections from 5 dpi necropsies were double-stained for WEEV and one of two cell specific markers, neuron specific enolase (NSE) or glial fibrillay acidic protein (GFAP). WEEV-specific staining was observed in the cerebral cortex and mid-brain of infected animals. No specific staining was observed in uninfected control brains. Neurons were the cell type found to be most commonly infected with the virus, and staining with NSE clearly co-localized with WEEV staining (Fig 3, arrow). Brain sections from animals infected with WEEV had altered staining as compared with sections from uninfected animals. These alterations were repeatedly observed to include a lack of NSE staining in the dendrites and axons of infected neurons as compared with normal staining in neurons from uninfected animals. NSE staining of infected neurons was restricted to the cell body. WEEV staining was not found to co-localize with GFAP staining and neuronal morphological aberrations or pathology of brain sections was not apparent in hemotoxylin and eosin-stained sections from infected animals (data not shown)
Figure 3.
Immunofluorescent localization of anti-WEEV and NSE in the brain tissue of uninfected (A–C) and infected (D–I) hamsters. A, D, and G show NSE staining and B, E, and H show the WEEV expression on the cerebral cortex and midbrain of uninfected and infected hamster. NSE expression on the uninfected hamster stains the entire neuron with axons and dendrites (A, arrowhead) whereas infected hamsters did not express NSE in those areas. Arrows (D – F) show the clear co-localization of WEEV with NSE. Scale bar = 20 micron
Treatment of WEEV infection
Pre-treatment with interferon alfacon-1 or Ampligen® 4 h prior to virus challenge resulted in 100% survival of infected animals. Weight gain was observed in infected animals treated with either interferon alfacon-1 or Ampligen®; this was similar to weight gain in sham-infected controls (Table 2). There was no sign of toxicity in drug-treatment groups as measured by weight loss and mortality, and all sham-infected toxicity controls appeared healthy and gained weight.
Table 2.
Effect of interferon alfacon-1 or ampligen on disease parameters of hamsters infected with western equine encephalitis virus
| Toxicity controls
|
Infected, treated
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Treatment | Dosage | Treatment schedule | Alive/total | Mean wt. change (g)a | Alive/total b | MDD ± SD c | Mean wt. change (g) | Mean brain virus titerd |
| Interferon alfacon-1 | 5 μg/kg/d | -4 h to 6 dpi, qd, i.p. | 3/3 | 7.3 | 25/25*** | >21 ± 0.0*** | 7.5 | <3.6 ± 0.0*** |
| Ampligen | 3.2 mg/kg/injection | -4 h and 2 dpi, i.p. | 3/3 | 7.0 | 15/15*** | >21 ± 0.0*** | 10.0 | 4.3 ± 1.3** |
| Saline | -- | -4 h to 6 dpi, qd, i.p. | 3/3 | 8.0 | 9/40 | 5.5 ± 1.1 | 2.0 | 8.1 ± 1.8 |
Mean weight change between –24 h and 4 days after start of treatment
Data taken from 3 different experiments; data for interferon alfacon-1 from 2 experiments and ampligen treatment from 1 experiment, and saline placebo data is combined placebo treatment from all 3 studies.
Mean day to death of animals dying up to 21 days after virus challenge
Log10 CCID50/g tissue ± SD virus from samples taken 4 days after virus inoculation
P<0.001 as compared with placebo-treated, infected controls
There was a highly significant (P<0.001) reduction in day 4 brain virus titer in animals treated with interferon alfacon-1, where virus was reduced to below the limits of detection (Table 2). Ampligen® was also effective in significantly (P<0.01) reducing virus titer in the brain, although virus was detected in 3 out of 5 animals with a mean titer of 4.3 ± 1.3 log10 CCID50/g tissue (Table 2). The mean brain virus titer in placebo-treated, infected controls was 8.1 ± 1.8 log10 CCID50/g tissue.
Discussion
Hamsters were highly susceptible to WEEV infection when challenged via i.p. or i.n. routes. A high mortality rate was associated with a relatively small virus inoculum, which is similar to infection in seen previously in hamsters as well as in gerbils (Zlotnick et al., 1972; Hayles, 1972). A dose of 101.5 CCID50 was chosen as a dose for antiviral studies due to the longer mean day to death, which allows more time for data collection but is lethal enough to determine significance in treatment studies. Previous work with hamsters has focused on immunological clearance of virus and virulence studies (Jahrling, 1976; Jahrling et al., 1983). The present study represents the first use of hamsters as a model for the treatment of WEEV disease. In general, >3-week old mice are more susceptible to intracranial or i.n. WEEV challenge and are less susceptible to subcutaneous or i.p. challenge, which generally yields approximately 50% mortality regardless of virus concentration (Hardy et al., 1997; Liu, C. et al., 1970). The California strain of WEEV used in this study was also used in mouse titration experiments conducted in our laboratory, and variable mortality rates from experiment to experiment between 40 and 60% were observed with 103.5–105.5 CCID50 challenge doses (data not shown). Hamsters have also been shown to be suitable animal models for other viral encephalitis infections, and often show better disease parameters and susceptibility as compared with mouse models (Georges-Courbot et al., 2006; Morrey et al., 2004a; Morrey et al., 2004b). Yellow fever virus (YFV) causes encephalitis in a mouse model, but in hamsters causes a viscerotropic disease similar to the natural disease in primates and man (Liu, T. and Chambers, 2001; Tesh et al., 2001). Due to the increased susceptibility of hamsters to peripheral infection with the California strain of WEEV as compared with mice, it appears that hamsters will serve as a more robust model for antiviral studies.
Weight loss was observed in animals infected with higher concentrations of WEEV. At lower viral challenges, the animals did not gain or lose weight; however, there was a statistically significant difference in weight change between WEEV-infected and sham-infected hamsters as seen in antiviral experiments. Weight change between –24 h and 4 dpi appears to serve as a useful parameter in evaluating the efficacy of investigational antiviral compounds against WEE disease.
This encephalitic virus infected groups of neurons in the cerebral cortex and in areas of mid-brain. Similar results were seen in cynomolgus macaques infected intranasally with WEEV, where viral antigen was detected by immunohistochemistry (IHC) in the brain, specifically in neurons, microglia, and Purkinje cells (Reed et al., 2005). This also correlates with previous studies involving an attenuated variant of eastern equine encephalitis virus (EEEV), a closely related alphavirus, where neuronal infection was observed in infected hamsters (Dremov et al., 1978). The lack of staining in dendrites and axons of the cerebral cortex and midbrain of infected hamsters suggested viral induced changes. WEEV staining did not co-localize with GFAP staining, indicating that astrocytes did not produce adequate levels of antigen to be detected by IHC in hamsters. It is interesting that viral antigen was not detected by IHC in astrocytes since the closely related Venezuelan equine encephalitis virus (VEEV) has been shown to infect primary astrocytes (Schoneboom et al., 1999). In human cases of EEEV, parykarion and dendrites of neurons were positive for EEE antigen, and mature extracellular virus as well as some morphological evidence for infection was observed (Garen et al., 1999; Kim et al., 1985). West Nile virus, an encephalitic flavivirus, was also found to infect neurons, but not astrocytes in hamster and mouse models of infection (Shrestha et al., 2003; Morrey et al., 2006). From these results, it would appear that WEEV primarily infects neurons, and does not produce detectable antigen levels in astrocytes in this hamster model. Pathology of other organs and further characterization of disease pathology is currently under investigation.
It is not surprising that interferon alfacon-1 was effective in the treatment of WEEV if delivered before viral challenge, as this compound is effective in hamster models of Pichindae and West Nile virus infections (Gowen et al., 2005; Morrey et al., 2004a), and is highly inhibitory to WEEV in vitro (unpublished data). Poly ICLC, another double-stranded RNA, has been shown to be effective in the treatment of respiratory infection with WEEV in a mouse model (Wong et al., 2005). The related material, Ampligen®, used in the present study was very effective in preventing disease in this hamster model when injected 4 h before and 2 days after virus inoculation. While these compounds work well when administered prophylactically, it is important that treatments for viral encephalitides are effective therapeutically and show efficacy when given after the development of clinical disease signs, which is the subject of future studies. This model should be useful in determining antiviral compounds that are efficacious in treating WEEV after clinical signs are apparent.
One negative aspect of this model is the short mean day to death observed in infected hamsters, which allows only a small therapeutic window. Another factor to be considered is the greater amount of antiviral compounds necessary for treating hamsters as compared with amounts needed to treat mice. From these studies, it appears as if the hamster may serve effectively as an animal model for study of antiviral therapies for WEEV disease.
Materials and Methods
Animals
Female Syrian golden hamsters weighing between 100 and 110 g were obtained from Charles River Laboratories (Wilmington, MA). They were randomly assigned to cages and individually marked with eartags. Hamsters were fed standard rodent chow and tap water ad libitum and quarantined 48 h prior to use.
Western equine encephalitis virus (WEEV)
WEEV (California strain, VR-70) was obtained from the American Type Culture Collection (Manassas, VA) and used after 3 passages in Vero cells. Stock virus was diluted 10−5 (101.5 50% cell culture infectious doses [CCID50]/0.1 ml) and hamsters were inoculated intraperitoneally (i.p.) with this dilution. All work with this virus was performed in the Biosafety Level 3 (BL-3) area of the AAALAC-accredited Laboratory Animal Research Center (LARC) at Utah State University (USU).
Test Materials
Interferon alfacon-1 was obtained as a 30 mg/ml solution from Intermune (Brisbane, CA). It was diluted in physiological saline and stored at 4°C until use. Ampligen® was provided by Hemispherx Biopharma (Philadelphia, PA) as a 2.4 mg/ml solution. The compound was stored at −20°C until use.
Tissue virus titer determination
The virus titers in organs and serum were assayed using an infectious cell culture assay where a specific volume of organ tissue homogenate or serum was serially diluted and added to a monolayer of Vero cells in a 96-well flat-bottomed microplate. Three days later virus induced cytopathic effect (CPE) was used to identify the infectious virus end-point. Four replicates were used for each sample.
Immunohistochemistry
At the time of necropsy, infected and uninfected animals were perfused directly with PBS and 4% paraformaldehyde during cardiac puncture. Slides made from paraffin embedded samples were subjected to immunohistochemical protocols for staining. Sections were immersed in diluted (1:10) DakoCytomation Target Retrieval Solution (DakoCytomation Inc, Carpinteria, CA) in distilled water and boiled in microwave for 4 cycles of 1 minute or boiled at 125°C for 4 minutes in decloaking chamber (Bio-care Medical, Walnut Creek CA). Sections were permeabilized with 0.5% Triton X 100 in PBS for 5 minutes, and blocked using 10% normal goat serum in 0.2% Triton X 100 in PBS blocking solution. Slides were simultaneously incubated with mouse anti-WEEV (Chemicon, Temecula, CA), polyclonal anti-neuron specific enolase (NSE), and polyclonal Glial Fibrillay Acidic Protein (GFAP) (Chemicon, Temecula, CA) primary monoclonal antibodies (mAb) diluted in 5% normal goat serum and 0.2% Triton X 100 in PBS for 2 hr. After washing with PBS, slides were incubated with diluted Alexa-fluor® 568 goat anti-mouse IgG and Alexa-fluor® 488 goat anti-rabbit IgG secondary antibodies (Molecular Probes, Eugene, OR) (1:200). The slides were washed and mounted with VECTASHIELD® mounting medium (Vector Laboratories, Burlingame, CA). Stained slides were visualized using a Nikon Eclipse TE300 microscope (Nikon) attached with Lambda DG4 (Sutter Instrument Company, Novato, CA) and a Bio-Rad MRC 1024 confocal microscope (Bio-Rad, Hercules, CA). Control and experimental images were collected and processed using the same instrument settings. Sections, stained with hemotoxylin and eosin, were observed to determine if morphology of infected animals was altered.
Experimental design
A titration experiment was initially conducted to determine an effective challenge dose for WEEV infection. Groups of hamsters were challenged by i.p. or intranasal (i.n.) inoculation with 10-fold dilutions of virus stock from 106.5 to 104.5 CCID50. Mortality was observed for 21 days after virus challenge and weight change was recorded –24 hours and 4 days post-virus exposure. An additional titration experiment, from 103.5 to 101.5 CCID50 using i.p. inoculation, was conducted to achieve a higher dynamic range. Weights of each animal were taken as above, and weight change was calculated. Mortality was observed for 21 days.
For the time-course experiment, tissue samples and weights were taken from 10 hamsters on each day after virus challenge. Samples were titered for virus by infectious cell culture assay, and blood parameters, including PCV and WBC count, were measured.
For evaluation of interferon alfacon-1 and Ampligen®, animals were randomly assigned to treatment or control groups, individually identified, and weighed. Animals in the Ampligen® treatment group were treated with 3.2 mg/kg/d on –4 h and 2 dpi by i.p. injection. Interferon alfacon-1 was given i.p. once daily, beginning –4 h and continuing through 8 dpi at a dose of 5 μg/kg/d. Each animal was challenged i.p. with 101.5 CCID50 of WEEV or sham-infected with dilution media. Brain samples were taken at necropsy from 5–10 animals from each group on 4 days post-virus inoculation (dpi) and assayed for virus titer. Ten hamsters were left in each group to measure survival daily through 21 days. Hamsters were weighed on 4 dpi.
Statistical analysis
Survival data were analyzed using the Wilcoxon log-rank survival analysis (JMP™ Software, The Statistical Discovery Software, SAS Institute, Inc). All other statistical analysis was done using one-way Students t-test.
Acknowledgments
We thank Luci Wandersee, Maysun Ali, and Isaac Wong for their work. We also thank HEMISPHERx for providing the Ampligen®. This work was supported by NO1-AI-15435 from the Virology Branch, NIAID, NIH, and 1-U54 AI06357-01 from the Rocky Mountain Regional Centers of Excellence, NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Justin G. Julander, Institute for Antiviral Research, Utah State University, Logan, UT. 84322-5600. Ph (435)-797-7215, Fax (435)-797-3959, e-mail jgjulander@cc.usu.edu..
Venkatraman Siddharthan, Institute for Antiviral Research, Utah State University..
Lawrence M. Blatt, InterMune, Inc., Brisbane, CA.
Kristiina Schafer, Institute for Antiviral Research, Utah State University..
Robert W. Sidwell, Institute for Antiviral Research, Utah State University..
John D. Morrey, Institute for Antiviral Research, Utah State University..
References
- Aguilar MJ. Pathological changes in brain and other target organs of infant and weanling mice after infection with non-neuroadapted western equine encephalitis virus. Infect and Immun. 1970;2:533–542. doi: 10.1128/iai.2.5.533-542.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anon Mismatched double-stranded RNA: polyI:polyC12U. Drugs R D. 2004;5:297–304. doi: 10.2165/00126839-200405050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balan V, Nelson DR, Sulkowski MS, Everson GT, Lambiase LR, Wiesner RH, Dickson RC, Post AB, Redfield RR, Davis GL, Neumann AU, Osborn BL, Freimuth WW, Subramanian GM. A Phase I/II study evaluating escalating doses of recombinant human albumin-interferon-alpha fusion protein in chronic hepatitis C patients who have failed previous interferon-alpha-based therapy. Antivir Ther. 2006;11:35–45. [PubMed] [Google Scholar]
- Bianchi TI, Aviles G, Monath TP, Sabattini MS. Western equine encephalomyelitis: virulence markers and their epidemiologic significance. Am J Trop Med Hyg. 1993;49:322–328. doi: 10.4269/ajtmh.1993.49.322. [DOI] [PubMed] [Google Scholar]
- Calisher CH. Medically important arboviruses of the United States and Canada. Clin Microbiol Rev. 1994;7:89–116. doi: 10.1128/cmr.7.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dremov DP, Solyanik RG, Miryutova TL, Laptakova LM. Attenuated variants of eastern equine encephalomyelitis virus: pathomorphological, immunofluorescence and virological studies of infection in Syrian hamsters. Acta Virol. 1978;22:139–145. [PubMed] [Google Scholar]
- Essey RJ, McDougall BR, Robinson WE., Jr Mismatched double-stranded RNA (polyI-polyC(12)U) is synergistic with multiple anti-HIV drugs and is active against drug-sensitive and drug-resistant HIV-1 in vitro. Antiviral Res. 2001;51:189–202. doi: 10.1016/s0166-3542(01)00150-4. [DOI] [PubMed] [Google Scholar]
- Garen PD, Tsai TF, Powers JM. Human eastern equine encephalitis: immunohistochemistry and ultrastructure. Mod Pathol. 1999;12:646–652. [PubMed] [Google Scholar]
- Georges-Courbot MC, Contamin H, Faure C, Loth P, Baize S, Leyssen P, Neyts J, Deubel V. Poly(I)-poly(C12U) but not ribavirin prevents death in a hamster model of Nipah virus infection. Antimicrob Agents Chemother. 2006;50:1768–1772. doi: 10.1128/AAC.50.5.1768-1772.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowen BB, Barnard DL, Smee DF, Wong MH, Pace AM, Jung KH, Winslow SG, Bailey KW, Blatt LM, Sidwell RW. Interferon alfacon-1 protects hamsters from lethal pichinde virus infection. Antimicrob Agents Chemother. 2005;49:2378–2386. doi: 10.1128/AAC.49.6.2378-2386.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy JL, Presser SB, Chiles RE, Reeves WC. Mouse and baby chicken virulence of enzootic strains of western equine encephalomyelitis virus from California. Am J Trop Med Hyg. 1997;57:240–244. doi: 10.4269/ajtmh.1997.57.240. [DOI] [PubMed] [Google Scholar]
- Hayles LB. Susceptibility of the Mongolian gerbil (Meriones unguiculatus) to Western equine encephalitis. Can J Microbiol. 1972;18:941–944. doi: 10.1139/m72-145. [DOI] [PubMed] [Google Scholar]
- Hisaka T, Yano H, Ogasawara S, Momosaki S, Nishida N, Takemoto Y, Kojiro S, Katafuchi Y, Kojiro M. Interferon-alphaCon1 suppresses proliferation of liver cancer cell lines in vitro and in vivo. J Hepatol. 2004;41:782–789. doi: 10.1016/j.jhep.2004.07.012. [DOI] [PubMed] [Google Scholar]
- Hunt AR, Roehrig JT. Biochemical and biological characteristics of epitopes on the E1 glycoprotein of western equine encephalitis virus. Virology. 1985;142:334–346. doi: 10.1016/0042-6822(85)90342-3. [DOI] [PubMed] [Google Scholar]
- Jahrling PB. Virulence heterogeneity of a predominantly avirulent Western equine encephalitis virus population. J Gen Virol. 1976;32:121–128. doi: 10.1099/0022-1317-32-1-121. [DOI] [PubMed] [Google Scholar]
- Jahrling PB, Hesse RA, Anderson AO, Gangemi JD. Opsonization of alphaviruses in hamsters. J Med Virol. 1983;12:1–16. doi: 10.1002/jmv.1890120102. [DOI] [PubMed] [Google Scholar]
- Kim JH, Booss J, Manuelidis EE, Duncan CC. Human eastern equine encephalitis. Electron microscopic study of a brain biopsy. Am J Clin Pathol. 1985;84:223–227. doi: 10.1093/ajcp/84.2.223. [DOI] [PubMed] [Google Scholar]
- Leyssen P, Drosten C, Paning M, Charlier N, Paeshuyse J, De Clercq E, Neyts J. Interferons, interferon inducers, and interferon-ribavirin in treatment of flavivirus-induced encephalitis in mice. Antimicrob Agents Chemother. 2003;47:777–782. doi: 10.1128/AAC.47.2.777-782.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Voth DW, Rodina P, Shauf LR, Gonzalez G. A comparative study of the pathogenesis of western equine and eastern equine encephalomyelitis viral infections in mice by intracerebral and subcutaneous inoculations. J Infect Dis. 1970;122:53–63. doi: 10.1093/infdis/122.1-2.53. [DOI] [PubMed] [Google Scholar]
- Liu T, Chambers TJ. Yellow fever virus encephalitis: properties of the brain-associated T-cell response during virus clearance in normal and gamma interferon-deficient mice and requirement for CD4+ lymphocytes. J Virol. 2001;75:2107–2118. doi: 10.1128/JVI.75.5.2107-2118.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melian EB, Plosker GL. Interferon alfacon-1: a review of its pharmacology and therapeutic efficacy in the treatment of chronic hepatitis C. Drugs. 2001;61:1661–1691. doi: 10.2165/00003495-200161110-00009. [DOI] [PubMed] [Google Scholar]
- Monath TP, Kemp GE, Cropp CB, Chandler FW. Necrotizing myocarditis in mice infected with Western equine encephalitis virus: Clinical, electrocardiographic, and histopathologic correlations. J Infect Dis. 1978;138:59–66. doi: 10.1093/infdis/138.1.59. [DOI] [PubMed] [Google Scholar]
- Morrey JD, Day CW, Julander JG, Blatt LM, Smee DF, Sidwell RW. Effect of interferon-alpha and interferon-inducers on West Nile virus in mouse and hamster animal models. Antiviral Chem and Chemother. 2004a;15:67–75. doi: 10.1177/095632020401500202. [DOI] [PubMed] [Google Scholar]
- Morrey JD, Day CW, Julander JG, Olsen AL, Sidwell RW, Cheney CD, Blatt LM. Modeling hamsters for evaluating West Nile virus therapies. Antiviral Res. 2004b;63:41–50. doi: 10.1016/j.antiviral.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Morrey JD, Siddharthan V, Olsen AL, Roper GY, Wang HC, Baldwin TJ, Koenig S, Johnson S, Nordstrom JL, Diamond MS. Humanized monoclonal antibody against West Nile virus E protein administered after neuronal infection protects against lethal encephalitis in hamsters. J of Infect Dis. 2006 doi: 10.1086/508293. (accepted) [DOI] [PubMed] [Google Scholar]
- Nagata LP, Hu WG, Masri SA, Rayner GA, Schmaltz FL, Das D, Wu J, Long MC, Chan C, Proll D, Jager S, Jebailey L, Suresh MR, Wong JP. Efficacy of DNA vaccination against western equine encephalitis virus infection. Vaccine. 2005;23:2280–2283. doi: 10.1016/j.vaccine.2005.01.032. [DOI] [PubMed] [Google Scholar]
- Nagata LP, Hu WG, Parker M, Chau D, Rayner GA, Schmaltz FL, Wong JP. Infectivity variation and genetic diversity among strains of Western equine encephalitis virus. J Gen Virol. 2006;87:2353–2361. doi: 10.1099/vir.0.81815-0. [DOI] [PubMed] [Google Scholar]
- Reed DS, Larsen T, Sullivan LJ, Lind CM, Lackemeyer MG, Pratt WD, Parker MD. Aerosol exposure to western equine encephalitis virus causes fever and encephalitis in cynomolgus macaques. J Infect Dis. 2005;192:1173–1182. doi: 10.1086/444397. [DOI] [PubMed] [Google Scholar]
- Schoneboom BA, Fultz MJ, Miller TH, McKinney LC, Grieder FB. Astrocytes as targets for Venezuelan equine encephalitis virus infection. J Neurovirol. 1999;5:342–354. doi: 10.3109/13550289909029475. [DOI] [PubMed] [Google Scholar]
- Shrestha B, Gottlieb D, Diamond MS. Infection and injury of neurons by West Nile encephalitis virus. J Virol. 2003;77:13203–13213. doi: 10.1128/JVI.77.24.13203-13213.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidwell RW, Smee DF. Viruses of the Bunya- and Togaviridae families: potential as bioterrorism agents and means of control. Antiviral Res. 2003;57:101–111. doi: 10.1016/s0166-3542(02)00203-6. [DOI] [PubMed] [Google Scholar]
- Sjogren MH, Sjogren R, Holtzmuller K, Winston B, Butterfield B, Drake S, Watts A, Howard R, Smith M. Interferon alfacon-1 and ribavirin versus interferon alpha-2b and ribavirin in the treatment of chronic hepatitis C. Dig Dis Sci. 2005;50:727–732. doi: 10.1007/s10620-005-2564-2. [DOI] [PubMed] [Google Scholar]
- Smee DF, Gilbert J, Leonhardt JA, Barnett BB, Huggins JH, Sidwell RW. Treatment of lethal Pichinde virus infections in weanling LVG/Lak hamsters with ribavirin, ribamidine, selenazofurin, and ampligen. Antiviral Res. 1993;20:57–70. doi: 10.1016/0166-3542(93)90059-r. [DOI] [PubMed] [Google Scholar]
- Takehara M. Antiviral effects of double-stranded RNA from rice dwarf virus on infection of mice with western equine encephalitis virus. Microbiol Immunol. 1977;21:309–315. doi: 10.1111/j.1348-0421.1977.tb00292.x. [DOI] [PubMed] [Google Scholar]
- Takemoto Y, Yano H, Momosaki S, Ogasawara S, Nishida N, Kojiro S, Kamura T, Kojiro M. Antiproliferative effects of interferon-alphaCon1 on ovarian clear cell adenocarcinoma in vitro and in vivo. Clin Cancer Res. 2004;10:7418–7426. doi: 10.1158/1078-0432.CCR-04-0279. [DOI] [PubMed] [Google Scholar]
- Tesh RB, Guzman H, da Rosa AP, Vasconcelos PF, Dias LB, Bunnell JE, Zhang H, Xiao SY. Experimental yellow fever virus infection in the Golden Hamster (Mesocricetus auratus). I. Virologic, biochemical, and immunologic studies. J Infect Dis. 2001;183:1431–1436. doi: 10.1086/320199. [DOI] [PubMed] [Google Scholar]
- Wild JS, Hyde DM, Hubbell HR, Giri SN. Dose-related effects of Ampligen (poly(I).poly(C12U)), a mismatched double-stranded RNA, in a bleomycin-mouse model of pulmonary fibrosis. Exp Lung Res. 1996;22:375–391. doi: 10.3109/01902149609031781. [DOI] [PubMed] [Google Scholar]
- Wong JP, Nagata LP, Christopher ME, Salazar AM, Dale RM. Prophylaxis of acute respiratory virus infections using nucleic acid-based drugs. Vaccine. 2005;23:2266–2268. doi: 10.1016/j.vaccine.2005.01.037. [DOI] [PubMed] [Google Scholar]
- Yasuda S, Huffman JH, Smee DF, Sidwell RW, Miyata K. Spectrum of virus inhibition by consensus interferon YM643. Antivir Chem Chemother. 2000;11:337–341. doi: 10.1177/095632020001100504. [DOI] [PubMed] [Google Scholar]
- Yasuda S, Miyata K. Interferon alfacon-1 (Advaferon): a novel synthetic interferon for the treatment of hepatitis C, its pharmacological and clinical profile. Nippon Yakurigaku Zasshi. 2002;120:421–426. doi: 10.1254/fpj.120.421. [DOI] [PubMed] [Google Scholar]
- Zlotnick I, Peacock S, Grant DP, Batter-Hatton D. The pathogenesis of western equine encephalitis virus (W.E.E.) in adult hamsters with special reference to the long and short term effects on the C.N.S. of the attenuated clone 15 variant. Br J Exp Path. 1972;53:59–77. [PMC free article] [PubMed] [Google Scholar]


