Abstract
Background
Concern about the potential risks associated with hormone replacement therapy (HRT) has left postmenopausal women and healthcare providers searching for safe and effective means for cardiovascular disease (CVD) risk factor reduction.
Methods
The Woman On the Move through Activity and Nutrition (WOMAN) study is a 5-year clinical trial (2002-2006) designed to test whether a lifestyle intervention will reduce measures of subclinical CVD. Participants were randomized at baseline to a health education or lifestyle change group. The impact of lifestyle intervention on CVD risk factors was examined in 240 women who were initially on HRT at baseline and either continued (n=110) or discontinued (n=130) by 18 months.
Results
The lifestyle change group significantly decreased weight, BMI, waist circumference (all p<0.0001), total cholesterol (p=0.02) and LDL-C (p=0.01), improved fat intake (p<0.0001), and increased leisure physical activity (p=0.005) when compared to the health education group. HRT discontinuation resulted in increased total cholesterol (p=0.04) and LDL-C (p=0.009). CVD risk factor changes were further explored by HRT group, stratified by randomized group assignment. Within the health education arm, HRT discontinuers averaged over 22 mg/dL increase in total cholesterol and LDL-C while HRT continuers averaged less than 4 mg/dL (p = 0.004 and 0.002, respectively). No such differences were noted in the lifestyle change group (p = 0.78 and 0.90, respectively).
Conclusions
Lifestyle modification was effective for CVD risk factor reduction in PM women. HRT discontinuation resulted in increased total cholesterol and LDL-C, which were successfully attenuated by a lifestyle intervention incorporating weight loss, physical activity and dietary modification.
INTRODUCTION
After menopause, women experience an increased incidence of cardiovascular disease (CVD).1 This increase is likely explained by the observation that related risk factors, such as dyslipidemia, abdominal adiposity, hypertension, and insulin resistance often worsen during the menopausal transition.2, 3 Hormone replacement therapy (HRT) has been shown to improve certain CVD risk factors4 and, until recently, was widely prescribed for general CVD prevention.5, 6 Results from the Postmenopausal Estrogen/Progestin Interventions (PEPI) trial suggest that, regardless of HRT type, HRT users had increased HDL cholesterol (HDL-C) and decreased LDL cholesterol (LDL-C) and fasting glucose when compared to the placebo group.7
Past observational studies have suggested an inverse association between HRT use and CVD,8, 9 due in part to the beneficial impact on CVD risk factors. However, evidence from recent clinical trials10-12 have failed to confirm the cardio-protective benefits of HRT.8, 9 Following the publication of the Women's Health Initiative (WHI) results, many HRT users were advised by their physicians to taper doses and/or discontinue use.5 To date, there is little information regarding the health impact of HRT discontinuation.
The Women On the Move through Activity and Nutrition (WOMAN) study is a five-year randomized clinical trial of primary CVD prevention designed to test whether an aggressive nonpharmacological lifestyle intervention will improve subclinical CVD measures. Approximately midway through the WOMAN study recruitment process, results were publicized from the estrogen/progestin (E+P) arm of the WHI trial indicating adverse effects among HRT users.11 Women who had already been randomized into the WOMAN study were advised by their health care provider to taper and/or discontinue HRT use according to the new guidelines. The WOMAN study provides a unique opportunity to explore the impact of HRT discontinuation on CVD risk factors and the possible attenuation of this impact by healthy lifestyle behaviors. Based on results from previous studies,7 it was hypothesized that HRT discontinuation will have a negative impact on certain CVD risk factors. It was further hypothesized that a lifestyle intervention could lessen such unfavorable CVD risk factor changes over 18 months of study participation.
METHODS
Study Population
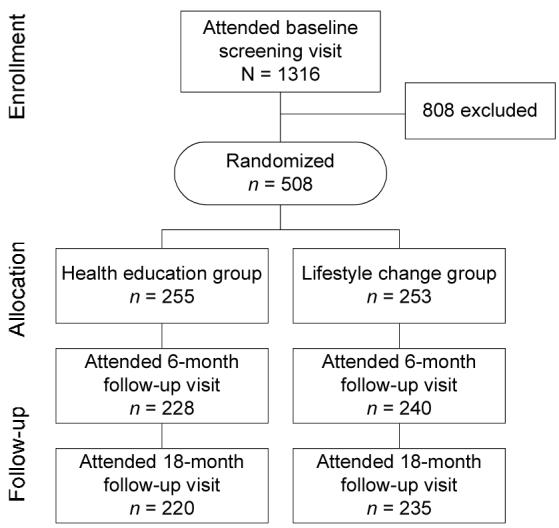
Five hundred and eight postmenopausal women were recruited for the WOMAN study, primarily through direct mailing from selected ZIP codes in Allegheny County, Pennsylvania from April 2002 to October 2003. Only the women who were on HRT at baseline are included in the current report. Briefly, eligibility criteria for study enrollment included waist circumference ≥ 80 cm, body mass index (BMI) between 25 – 39.9 kg/m2, not currently taking lipid lowering drugs and having a low density lipoprotein (LDL-c) level between 100 - 160 mg/dL, no physical limitations that would preclude walking, no known diabetes, and no diagnosed psychotic disorder or depression. All participants provided written informed consent and all protocols were approved by the institutional review board at the University of Pittsburgh. Follow-up visits were held at 6 months post-randomization and yearly thereafter. Results from the current investigation were generated from data collected at baseline and at the 18 month clinic visit.
Group Randomization: Intervention Design
Eligible women were randomized to a health education or a lifestyle change group using a block randomized design (Figure 1). The intervention design of the WOMAN study has been previously reported.13 Briefly, women in the health education group received a core educational series of six lectures offered during the first year and quarterly thereafter. The lifestyle intervention was group based and facilitated by a multidisciplinary team of nutritionists, exercise physiologists, and psychologists. Participant contact within the lifestyle change group was extensive, including 40 visits during the first year and a minimum of monthly visits in year 2 and beyond. The lifestyle intervention was individualized and focused on weight and waist circumference reduction through healthy lifestyle changes. The healthy lifestyle behaviors promoted in the lifestyle change included 150 minutes per week of moderate intensity physical activity similar to brisk walking and a caloric intake of 1300-1500 calories per day, emphasizing an eating pattern low in total (<17%) and saturated fat (<7%).
Figure 1.
Randomization scheme in the WOMAN study
Physical Activity
Physical activity was measured using the past year version of the Modifiable Activity Questionnaire (MAQ), a reliable14, 15 and valid14-16 interviewer-administered questionnaire.15 Although this questionnaire assesses leisure and occupational activities, we focused on leisure activity as there was little reported occupational activity in our study population. Study participants were asked to identify if they participated in a variety of specific activities, such as walking for exercise, at least 10 times over the past year (12 months). For each activity identified, they were asked which months they had participated in that specific activity over the past year and then estimated the number of times each month and length of time that they spent doing the activity. Physical activity was calculated as the product of the duration and frequency of each activity (in hours per week), weighted by an estimate of the metabolic equivalent (MET) of that activity and summed for all activities performed. Physical activity data were expressed as MET hours per week (MET hr-wk).
Objective assessments of physical activity were obtained with the Accusplit Eagle AE120 (Accusplit Inc., Pleasanton, CA) in a random subgroup of WOMAN study participants (n=170; 33.5%) to ensure that the estimates obtained from the MAQ were valid in this specific population. Participants were instructed to wear the pedometer clipped to their waistbands over the dominant hip for one week, and at the end of each day to record the number of steps taken in a diary, which was returned to the investigator at the end of the week. The daily steps were averaged over the week to obtain a seven-day average of steps taken per day.
Hormone Therapy Groups
Information on HRT use was obtained through self-report and from a medication inventory that was completed at both the baseline and 18 month clinic visit. The 240 women identified as current HRT users at baseline were classified into one of two HRT groups: (1) HRT Continuers (HRT user at baseline and 18 months) and (2) HRT Discontinuers (HRT user at baseline and non-user at 18 months).
Clinical Measures
All clinical measures were obtained at the baseline and 18 month clinic visit. Clinical measures included height, body weight, waist circumference, and a fasting (12 hour) blood draw. Height and weight were measured with a stadiometer and calibrated balance beam scale. Waist circumference was measured in the standing position at the navel (horizontal plane at the center of the navel) using a fiberglass retractable tape measure. Total cholesterol, HDL-C, triglycerides, and glucose were determined by conventional methods. LDL-C was estimated by the Friedewald equation and insulin was measured via radioimmunoassay.
Nutritional Measures
A saturated fat/cholesterol intake index score (fat intake) was calculated using the Connor Diet Habit Survey, which has been shown to be both a reliable and valid measure for the rapid assessment of eating habits and diet composition. Fat intake was generated by summing the scores for 20 questions related to cholesterol and saturated fat intake.17
Statistical Methods
All variables were assessed for normality. Normally distributed variables were reported as mean ± standard deviations (SD) and non-normally distributed variables as medians with 25th and 75th percentiles. Depending upon the characteristics of the variable, t-tests or Wilcoxon Rank Sum tests were used to compare baseline risk factor levels between HRT groups. The 18 month change in anthropometric, physical activity, fat intake, and CVD risk factors were calculated as the difference between the 18 month follow-up and baseline values. T-tests or Wilcoxon Rank Sum tests were again used to compare cohort characteristics and 18 month change in CVD risk factor values by HRT group, stratified by randomized group.
For each CVD risk factor change variable, an HRT group was evaluated by randomized group (HRGRP*GRP) interaction term using multivariate linear regression (MLR) models. The dependent variable was change in one risk factor (e.g. total cholesterol) and the predictors included HRT group, randomized group, and the HRGRP*GRP interaction term.
Using MLR models, further post-hoc analyses were also conducted to determine the independent effects of specific lifestyle changes on CVD risk factor change. For each of the six models, the dependent variable was the change in one risk factor, with predictors including change in leisure physical activity and fat intake, and after adjusting for HRT group and baseline levels of leisure physical activity, fat intake, and the relevant CVD risk factor. Using Bonferroni adjustment due to multiple testing, a p value of <0.01 should be considered statistically significant. All data was analyzed in 2006 using SAS 9.1 (Cary, NC, 2003).
RESULTS
Of the 508 women randomized into the WOMAN study, 455 (90%) completed the 18 month clinic visit. Of these 455 women, 182 reported not using HRT at baseline and were excluded from these analyses. Also excluded were 14 women initiating a lipid lowering medication during the 18 month interval and 19 with incomplete physical activity, clinical, or diet quality data. Non-HRT users at baseline were more likely to be African American, had lower reported leisure physical activity levels, and higher baseline total cholesterol, LDL-C, insulin, and glucose levels when compared to women who were HRT users at baseline (all p<0.05).
The 240 women included in the present analyses had a mean (SD) age of 58.3 (2.9 years). The mean BMI was 28.5 (4.4) kg/m2 and mean waist circumference was 98.5 (12.1) cm. Approximately 99% had at least a high school degree, 7.5% were African American, and 4.6% were current smokers. At baseline, 100 (41.7%) of the 240 women took estrogen therapy and the remaining 140 (58.3%) took estrogen plus progestogen (E+P) therapy. The median duration of HRT use prior to randomization was 59.5 (28.0, 110.0) months among HRT continuers and 71.0 (39.0, 115.0) months among HRT discontinuers and was not statistically different between HRT groups (p=0.39). At 18 months, 110 (46%) of the 240 women continued HRT use and the remaining 130 (54%) discontinued HRT. Of the 110 participants that continued HRT use, 61 (55.5%) took estrogen therapy and the remaining 49 women took E+P therapy. Among HRT discontinuers, 39 (30%) of the 130 women were on estrogen therapy and the remaining 91 (70%) took E+P therapy. Furthermore, 60% of HRT discontinuers had done so prior to the 6 month follow-up visit.
When comparing baseline CVD risk factor levels between HRT groups, HRT continuers were somewhat (p=0.04) younger than HRT discontinuers and had higher total cholesterol (p=0.05) and lower insulin levels (p=0.02). The HRT groups did not significantly differ at baseline with regard to anthropometric measures, leisure physical activity, fat intake, LDL-C, HDL-C, triglyceride, or glucose levels. They also did not differ with regards to education, race, or smoking status (data not shown).
When compared to HRT continuers, HRT discontinuers had a significantly higher increase in LDL-C (p=0.009) and a moderate increase in total cholesterol (p=0.04). HRT discontinuation did not result in significant changes to weight, BMI, waist circumference, leisure physical activity, fat intake, systolic or diastolic BP, HDL-C, triglyceride, insulin, or glucose (data not shown). Conversely, overall the lifestyle intervention was effective for CVD risk factor reduction. The lifestyle intervention group significantly decreased weight, BMI, waist circumference (all p<0.0001), total cholesterol (p=0.02) and LDL-C (p=0.01), improved fat intake (p<0.0001), and increased leisure physical activity (p=0.005) when compared to the health education group.
Table 1 further explores the 18 month change in CVD risk factors by HRT group, stratified by randomized group. Regardless of HRT status at 18 months, the lifestyle intervention was successful in implementing behavior change, reducing weight, and improving CVD risk factors. CVD risk factor changes in the lifestyle change versus health education groups were primarily noted among HRT discontinuers. In HRT discontinuers, women in the health education group had worsened total cholesterol, LDL-C, glucose, and triglycerides while those in the lifestyle change group maintained their baseline values. HDL-C, insulin, and blood pressure changes did not significantly differ between randomized groups in either HRT group.
Table 1.
18-month change in cardiovascular risk factors by hormone therapy group (HTGRP) and randomized group assignment (GRP) (n=240).
| HRT continuers (n=110) |
HRT discontinuers (n=130) |
p value for HTGRPa GRP interaction |
|||||
|---|---|---|---|---|---|---|---|
| HE (n=46) |
LC (n=64) |
p value | HE (n=60) |
LC (n=70) |
p value | ||
| Age at 18-month visit, years | 58.2 (2.9) | 57.7 (2.7) | 0.15 | 59.0 (2.8) | 58.4 (3.1) | 0.13 | |
| High school graduate (%) | 100 | 96.9 | 0.38 | 98.3 | 100 | 0.14 | |
| African American (%) | 8.7 | 9.4 | 0.62 | 11.7 | 1.4 | 0.04 | |
| Married (%) | 65.2 | 73.4 | 0.88 | 66.7 | 78.6 | 0.49 | |
| Current smoker at 18-month visit (%) | 4.4 | 3.1 | 0.88 | 5.0 | 5.7 | 0.47 | |
| Duration of hormone therapy use at baseline, months |
47.5 (27.0, 105.0) |
77.0 (31.0, 134.0) |
0.07 | 71.5 (39.5, 104.0) |
70.5 (39.0, 119.0) |
0.77 | |
| Weight, lbs. | −1.9 (−11.6, 1.0) |
−17.3 (− 26.8, −6.9) |
<0.0001 | −2.5 (−10.0, 2.5) |
−16.0 (− 27.0, −7.0) |
<0.0001 | 0.73 |
| BMI, kg/m2 | −0.4 (−1.7, 0.2) |
−3.0 (−4.9, − 1.3) |
<0.0001 | −0.8 (−1.8, 0.2) |
−2.8 (−5.0, − 1.1) |
<0.0001 | 0.98 |
| Waist circumference, cm | −5.3 (6.3) | −10.9 (7.6) | <0.0001 | −3.9 (6.3) | −11.1 (8.5) | <0.0001 | 0.40 |
| Leisure physical activity, MET hr-wk |
0.4 (17.0) | 4.1 (11.0) | 0.18 | 1.1 (12.3) | 6.7 (10.1) | 0.006 | 0.55 |
| Saturated fat/cholesterol Intake index |
8.2 (11.0) | 19.1 (14.1) | <0.0001 | 5.1 (8.5) | 20.3 (16.5) | <0.0001 | 0.20 |
| Insulin, mg/dL | 1.2 (4.1) | −0.7 (4.2) | 0.03 | 1.1 (8.3) | −0.3 (5.2) | 0.32 | 0.73 |
| Triglyceride, mg/dL | −10.0 (43.3) | −12.0 (52.8) | 0.83 | 3.1 (50.7) | −16.9 (47.7) | 0.0233 | 0.16 |
| Glucose, mg/dL | 5.5 (7.7) | 1.9 (9.8) | 0.03 | 8.2 (13.1) | 3.6 (9.1) | 0.004 | 0.72 |
| HDL cholesterol, mg/dL | 0.6 (12.9) | 2.0 (10.0) | 0.55 | −0.6 (11.0) | 0.02 (11.0) | 0.7671 | 0.79 |
| Systolic blood pressure, mmHg | 2.0 (−12.0, 10.0) |
−3.0 (−12.6) | 0.57 | −3.0 (−12.0, 6.0) |
−4.0 (−12.0, 6.0) |
0.5171 | 0.76 |
| Diastolic blood pressure, mmHg | 0.0 (9.7) | −0.03 (9.1) | 0.99 | −0.7 (7.1) | −1.8 (7.9) | 0.4322 | 0.65 |
| Total cholesterol, mg/dL | 3.3 (31.2) | 4.9 (25.7) | 0.78 | 21.9 (32.9) | 4.3 (30.2) | 0.004 | 0.015 |
| LDL cholesterol, mg/dL | 4.7 (26.2) | 5.3 (22.5) | 0.90 | 22.3 (29.3) | 6.9 (24.9) | 0.002 | 0.018 |
18-month change in leisure physical activity levels presented as median (25th, 75th percentile); the remaining 18 month change variables as mean (SD).
p values comparing HRT groups stratified by randomized group assignment (HE and LC).
All models to determine significant hormone therapy group by randomized group (HRGRP*GRP) interaction were adjusted for hormone therapy group, randomized group, and HRGRP*GRP interaction term.
BMI, body mass index; HDL, high-density lipoprotein; HE, health education; HRT, hormone replacement therapy; LC, lifestyle change; LDL, low-density lipoprotein; MET, metabolic equivalent
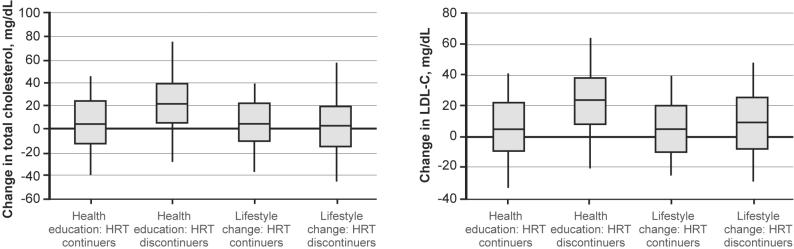
Table 1 also presents the combined effect of HRT discontinuation and randomized group on CVD risk factor change. The HRGRP*GRP interaction term was only significant for total cholesterol and LDL-C change (p = 0.015 and 0.018, respectively). Within the health education arm, HRT discontinuers averaged over 22 mg/dL increase in these two measures while HRT continuers averaged less than 4 mg/dL (p = 0.004 and 0.002, respectively). No such differences were noted in the lifestyle change group (p = 0.78 and 0.90, respectively) (Table 1/Figure 2).
Figure 2.
18-month change in total cholesterol and LDL-C by randomized group assignment and HRT group.
HRT, hormone replacement therapy
Since it appeared that lifestyle intervention had a major impact on CVD risk factors, post-hoc analysis was performed to begin to examine the role of the specific components of the intervention on risk factor reduction (Table 2). After adjustment for HRT use at the 18 month clinic visit, baseline leisure physical activity, fat intake, and relevant CVD risk factor level (i.e. adjustment for baseline HDL-C when exploring HDL-C change), an increase in leisure physical activity was significantly related to increases in HDL-C (p<0.0001). With regards to dietary behavior, a decrease in fat intake was significantly related to decreases in total cholesterol (p<0.0001), LDL-C (p<0.0001), triglycerides (p<0.001), insulin (p<0.0001), and glucose (p<0.01).
Table 2.
18-month change in CVD risk factors after adjustment (MLR models) (n=240).
| Δ Total Cholesterol, mg/dL |
Δ LDL Cholesterol, mg/dL |
Δ HDL Cholesterol, mg/dL |
Δ Triglycerides, mg/dL |
Δ Insulin, mg/dL (n=211) |
Δ Glucose, mg/dL (n=236) |
|
|---|---|---|---|---|---|---|
| Hormone therapy use at 18 months |
0.07 (−2.7, 10.9) |
0.11 (−0.03, 11.5) |
−0.09 (−4.5, 0.4) |
0.03 (−8.9, 14.0) |
0.09 (−0.3, 2.3) |
0.15 (0.7, 5.4)* |
| Δ Leisure physical activity (LPA), MET hr- wk |
−0.06 (−5.7, 2.1) |
−0.12 (−6.5, 0.1) |
0.26 (1.6, 4.4) *** |
−0.16 (−14.8, −1.6) (0.0153) |
−0.09 (−1.4, 0.2) |
−0.11 (−2.5, 0.2) |
| Δ Saturated fat/cholesterol intake score |
−0.35 (−1.0, −0.5)*** |
−0.30 (−0.8, −0.3) *** |
−0.03 (−0.1, 0.1) |
−0.23 (−1.2, −0.4)** |
−0.29 (−0.2, −0.1) *** |
−0.20 (−0.2, −0.1)* |
| Baseline LPA, MET hr-wk |
0.00 (−2.1, 2.2) |
−0.08 (−3.1, 0.6) |
0.27 (1.0, 2.5) *** |
−0.03 (−4.5, 2.8) |
−0.08 (−0.7, 0.1) |
−0.13 (−1.5, −0.04) |
| Baseline saturated fat/cholesterol intake score |
−0.06 (−0.4, 0.1) |
−0.06 (−0.4, 0.1) |
−0.06 (−0.2, 0.05) |
0.02 (−0.4, 0.6) |
−0.15 (−0.1, −0.02)* |
−0.08 (−0.2, 0.04) |
| Baseline CVD risk factor level |
−0.43 (−0.7, −0.4)*** |
−0.45 (−0.7, −0.4) *** |
−0.47 (−0.4, −0.3) *** |
−0.34 (−0.31, −0.15) *** |
−0.57 (−0.6, −0.4) *** |
−0.46 (−0.7, −0.4) *** |
| R2 | 0.31 | 0.31 | 0.31 | 0.21 | 0.36 | 0.26 |
Data presented as standardized beta estimates (95% confidence limits). All models were adjusted for HT use at 18 months, change (Δ) in leisure physical activity levels, Δ in saturated fat/cholesterol intake score, baseline leisure physical activity levels, baseline saturated fat/cholesterol intake score, and baseline levels of relevant CVD risk factor.
p<0.01
p<0.001
p<0.0001.
BMI, body mass index; CVD, cardiovascular disease; HDL, high-density lipoprotein; HE, health education; LC, lifestyle change; LDL, low-density lipoprotein; MET, metabolic equivalent
DISCUSSION
The present investigation explored the effect of lifestyle intervention on CVD risk factors in postmenopausal women that were on HRT at baseline and either continued or discontinued use after 18 months of study participation. Prior to the WOMAN study, the beneficial effect of improved lifestyle behaviors for CVD risk factor reduction did not account for concurrent changes in HRT status, an issue which has become relevant in the post-WHI era. Considering the controversies regarding HRT, the findings from the present report are timely. In the current report, HRT discontinuation was associated with an average increase of over 20 mg/dL in total and LDL-C and was successfully counteracted by an intensive lifestyle intervention. These results have important public health implications and suggest that a non-pharmacological lifestyle approach is both safe and effective for CVD risk factor reduction in postmenopausal women, especially those who discontinued HRT use.
Lifestyle intervention was successful in implementing behavior change. Women randomized to the lifestyle intervention significantly increased leisure physical activity, improved glucose, and decreased weight, BMI, waist circumference, saturated fat/cholesterol intake, and LDL-C when compared to the health education group. The overall success of the WOMAN study intervention was consistent with prior findings suggesting the efficacy of lifestyle based approaches for risk factor reduction and chronic disease prevention in populations at risk for diabetes18-21 and heart disease.22 Specifically in postmenopausal women, lifestyle intervention was demonstrated to prevent weight gain23 and to determine a rise in LDL-C24 during the menopausal transition.
CVD continues to be the leading cause of morbidity and mortality among women in westernized countries1. However, CVD in women has been underappreciated by the public25 and undertreated by health care providers.26, 27 Concern about the possible risks associated with HRT has left women and their heath care providers searching for safe and effective means to reduce CVD risk factors. One potential consequence of diminished HRT use is increased use of pharmacological agents, such as statins and aspirin; however, both are associated with side effects.29, 30 Based on the findings of the current investigation, special attention should be paid to encouraging lifestyle strategies that are likely to impart more benefit and less risk than drug therapies.
The present investigation has a number of limitations. The self-reported estimates of physical activity and dietary intake may be subject to recall bias. However, in a subgroup of women, subjective activity estimates were significantly (p<0.0001) correlated with pedometer step counts (rho = 0.30). Also, for the purposes of this report, fat intake was used as general measure of diet composition. Secondly, study participants were not randomized to continue or discontinue HRT use at 18 months, which may influence the findings. Moreover, the duration of prior HRT usage and/or time since discontinuation were not considered in these analyses. Finally, data from the current report were generated from women who volunteered to enroll in a clinical trial, which may limit the overall generalizability of the findings to a more diverse population.
In conclusion, little is known about the widespread impact of the WHI results on CVD risk factor change in postmenopausal women. We have demonstrated that HRT discontinuation resulted in increases in total cholesterol and LDL-C and that these increases were successfully attenuated by a non-pharmacological lifestyle intervention. More research and improved delivery systems are needed to facilitate dissemination and maintenance of lifestyle-based programs in postmenopausal women.
ACKNOWLEDGMENTS
The authors would like to acknowledge the 508 dedicated WOMAN study participants and the contributions of the WOMAN study staff, including Alhaji Buhari, Eileen Cole, Phyllis Jones, Laura Kinzel, Barbara Kolodziej, Wm. Scott Pappert, and Darcy Underwood. This research was funded by National Heart, Lung, and Blood Institute grant R01-HL-6646.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No financial conflict of interest was reported by the authors of this paper.
REFERENCES
- 1.American Heart Association . Heart Disease and Stroke Statistics - 2005 Update. Dallas, TX: 2004. [Google Scholar]
- 2.Matthews KA, Meilahn E, Kuller LH, Kelsey SF, Caggiula AW, Wing RR. Menopause and risk factors for coronary heart disease. N Engl J Med. 1989;321:641–6. doi: 10.1056/NEJM198909073211004. [DOI] [PubMed] [Google Scholar]
- 3.Carr MC. The emergence of the metabolic syndrome with menopause. J Clin Endocrinol Metab. 2003;88:2404–11. doi: 10.1210/jc.2003-030242. [DOI] [PubMed] [Google Scholar]
- 4.Rosano GM, Fini M. Postmenopausal women and cardiovascular risk: impact of hormone replacement therapy. Cardiol Rev. 2002;10:51–60. doi: 10.1097/00045415-200201000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Hersh AL, Stefanick ML, Stafford RS. National use of postmenopausal hormone therapy: annual trends and response to recent evidence. JAMA. 2004;291:47–53. doi: 10.1001/jama.291.1.47. [DOI] [PubMed] [Google Scholar]
- 6.Majumdar SR, Almasi EA. Stafford RS. Promotion and prescribing of hormone therapy after report of harm by the Women's Health Initiative. JAMA. 2004;292:1983–8. doi: 10.1001/jama.292.16.1983. [DOI] [PubMed] [Google Scholar]
- 7.Effects of estrogen or estrogen/progestin regimens on heart disease risk factors in postmenopausal women. The Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial. The Writing Group for the PEPI Trial. JAMA. 1995;273:199–208. [PubMed] [Google Scholar]
- 8.Stampfer MJ, Colditz GA. Estrogen replacement therapy and coronary heart disease: a quantitative assessment of the epidemiologic evidence. Prev Med. 1991;20:47–63. doi: 10.1016/0091-7435(91)90006-p. [DOI] [PubMed] [Google Scholar]
- 9.Hu FB, Grodstein F. Postmenopausal hormone therapy and the risk of cardiovascular disease: the epidemiologic evidence. Am J Cardiol. 2002;90:26F–29F. doi: 10.1016/s0002-9149(01)02219-6. [DOI] [PubMed] [Google Scholar]
- 10.Anderson GL, Limacher M, Assaf AR, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women's Health Initiative randomized controlled trial. JAMA. 2004;291:1701–12. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 11.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–33. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 12.Hulley SGD, Bush T, Furberg C, Herrington D, Riggs B, Vittinghoff E. Randomized Trial of Estrogen Plus Progestin for Secondary Prevention of Coronary Heart Disease in Postmenopausal Women. JAMA. 1998;280:605–613. doi: 10.1001/jama.280.7.605. [DOI] [PubMed] [Google Scholar]
- 13.Kuller LH, Kinzel LS, Pettee KK, et al. Lifestyle Intervention and Coronary Heart Disease Risk Factor Changes over 18 Months in Postmenopausal Women: The Women On the Move through Activity and Nutrition (WOMAN Study) Clinical Trial. Journal of Women's Health. 2006;15:964–976. doi: 10.1089/jwh.2006.15.962. [DOI] [PubMed] [Google Scholar]
- 14.Kriska AM, Knowler WC, LaPorte RE, et al. Development of questionnaire to examine relationship of physical activity and diabetes in Pima Indians. Diabetes Care. 1990;13:401–11. doi: 10.2337/diacare.13.4.401. [DOI] [PubMed] [Google Scholar]
- 15.Pereira MA, FitzerGerald SJ, Gregg EW, et al. A collection of Physical Activity Questionnaires for health-related research. Med Sci Sports Exerc. 1997;29:S1–205. [PubMed] [Google Scholar]
- 16.Schulz LOHI, Smith CJ, Kriska AM, Ravussin E. Energy intake and physical activity in Pima Indians: comparison with energy expenditure measured by doubly-labeled water. Obes Res. 1994;2:541–548. doi: 10.1002/j.1550-8528.1994.tb00103.x. [DOI] [PubMed] [Google Scholar]
- 17.Connor SL, Gustafson JR, Sexton G, Becker N, Artaud-Wild S, Connor WE. The Diet Habit Survey: a new method of dietary assessment that relates to plasma cholesterol changes. J Am Diet Assoc. 1992;92:41–7. [PubMed] [Google Scholar]
- 18.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pan XR, Li GW, Hu YH, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care. 1997;20:537–44. doi: 10.2337/diacare.20.4.537. [DOI] [PubMed] [Google Scholar]
- 20.Erikkson KFLF. Prevention of type 2 (non-insulin dependent) diabetes mellitus by diet and physical activity. Diabetologia. 1991;34:891–898. doi: 10.1007/BF00400196. [DOI] [PubMed] [Google Scholar]
- 21.Tuomilehto J, Lindstrom J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343–50. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 22.Ornish D, Scherwitz LW, Billings JH, et al. Intensive lifestyle changes for reversal of coronary heart disease. JAMA. 1998;280:2001–7. doi: 10.1001/jama.280.23.2001. [DOI] [PubMed] [Google Scholar]
- 23.Simkin-Silverman LR, Wing RR, Boraz MA, Kuller LH. Lifestyle intervention can prevent weight gain during menopause: results from a 5-year randomized clinical trial. Ann Behav Med. 2003;26:212–20. doi: 10.1207/S15324796ABM2603_06. [DOI] [PubMed] [Google Scholar]
- 24.Kuller LHS-SL, Wing RR, Meilahn EN, Ives DG. Women's Healthy Lifestyle Project: A Randomized Clinical Trial - Results at 54 Months. Circulation. 2001;103:32–37. doi: 10.1161/01.cir.103.1.32. [DOI] [PubMed] [Google Scholar]
- 25.Mosca L, Ferris A, Fabunmi R, Robertson RM. Tracking women's awareness of heart disease: an American Heart Association national study. Circulation. 2004;109:573–9. doi: 10.1161/01.CIR.0000115222.69428.C9. [DOI] [PubMed] [Google Scholar]
- 26.Vittinghoff E, Shlipak MG, Varosy PD, et al. Risk factors and secondary prevention in women with heart disease: the Heart and Estrogen/progestin Replacement Study. Ann Intern Med. 2003;138:81–9. doi: 10.7326/0003-4819-138-2-200301210-00007. [DOI] [PubMed] [Google Scholar]
- 27.King KB, Mosca L. Prevention of heart disease in women: recommendations for management of risk factors. Prog Cardiovasc Nurs. 2000;15:36–42. doi: 10.1111/j.0889-7204.2000.080396.x. [DOI] [PubMed] [Google Scholar]
- 28.Association AH Go Red for Women Campaign. 2006 [Google Scholar]
- 29.McKenney JM, Davidson MH, Jacobson TA, Guyton JR. Final conclusions and recommendations of the National Lipid Association Statin Safety Assessment Task Force. Am J Cardiol. 2006;97:89C–94C. doi: 10.1016/j.amjcard.2006.02.030. [DOI] [PubMed] [Google Scholar]
- 30.Kimmey MB. Cardioprotective effects and gastrointestinal risks of aspirin: maintaining the delicate balance. Am J Med. 2004;117(Suppl 5A):72S–78S. doi: 10.1016/j.amjmed.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 31.Ridker PM, Cook NR, Lee IM, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med. 2005;352:1293–304. doi: 10.1056/NEJMoa050613. [DOI] [PubMed] [Google Scholar]
- 32.Baigent C, Keech A, Kearney PM, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–78. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 33.Pignone M, Earnshaw S, Tice JA, Pletcher MJ. Aspirin, statins, or both drugs for the primary prevention of coronary heart disease events in men: a cost-utility analysis. Ann Intern Med. 2006;144:326–36. doi: 10.7326/0003-4819-144-5-200603070-00007. [DOI] [PubMed] [Google Scholar]