Abstract
In luteinizing granulosa cells, prostaglandin E2 (PGE2) can exert luteotrophic actions, apparently via the cAMP signalling pathway. In addition to stimulating progesterone synthesis, PGE2 can also stimulate oxidation of the physiological glucocorticoid, cortisol, to its inactive metabolite, cortisone, by the type 1 11β-hydroxysteroid dehydrogenase (11βHSD1) enzyme in human granulosa–lutein cells. Having previously shown these human ovarian cells to express functional G-protein coupled, E-series prostaglandin (PTGER)1, PTGER2 and PTGER4 receptors, the aim of this study was to delineate the roles of PTGER1 and PTGER2 receptors in mediating the effects of PGE2 on steroidogenesis and cortisol metabolism in human granulosa–lutein cells. PGE2-stimulated concentration-dependent increases in both progesterone production and cAMP accumulation (by 1·9±0·1- and 18·7±6·8-fold respectively at 3000 nM PGE2). While a selective PTGER1 antagonist, SC19220, could partially inhibit the steroidogenic response to PGE2 (by 55·9±4·1% at 1000 nM PGE2), co-treatment with AH6809, a mixed PTGER1/PTGER2 receptor antagonist, completely abolished the stimulation of progesterone synthesis at all tested concentrations of PGE2 and suppressed the stimulation of cAMP accumulation. Both PGE2 and butaprost (a preferential PTGER2 receptor agonist) stimulated concentration-dependent increases in cortisol oxidation by 11βHSD1 (by 42·5±3·1 and 40·0±3·0% respectively, at PGE2 and butaprost concentrations of 1000 nM). Co-treatment with SC19220 enhanced the ability of both PGE2 and butaprost to stimulate 11βHSD1 activity (by 30·2±0·2 and 30·5±0·6% respectively), whereas co-treatment with AH6809 completely abolished the 11βHSD1 responses to PGE2 and butaprost. These findings implicate the PTGER2 receptor–cAMP signalling pathway in the stimulation of progesterone production and 11βHSD1 activity by PGE2 in human granulosa–lutein cells.
Introduction
Prostaglandins (PGs) have been implicated in the co-ordination of ovarian function, particularly in ovulation and in regulating the functional lifespan of the corpus luteum. For example, PGF2α has luteolytic effects and can trigger functional luteal regression in a range of species, including humans and non-human primates (reviewed by Michael et al. 1994, Olofsson & Leung 1994), whereas selected PGs, like PGE2, can exert luteotrophic actions in human and non-human primate ovarian cells, stimulating progesterone synthesis apparently via the cAMP signalling pathway (Richardson 1986, Michael et al. 1993, 1994, Olofsson & Leung 1994). Although PGs are established as major paracrine agents in ovarian physiology, their exact modes of action still remain to be elucidated.
Glucocorticoid steroids are also known to be important for gonadal function and have been implicated in the control of oocyte maturation (Greeley et al. 1986, Harlow et al. 1997, Yang et al. 1999, Chen et al. 2000). In potential target tissues, the physiological glucocorticoids, cortisol and corticosterone, are oxidised to inert metabolites (cortisone and 11-dehydrocorticosterone respectively) by 11β-hydroxysteroid dehydrogenase (11βHSD) enzymes (Seckl & Walker 2001, Seckl 2004, Draper & Stewart 2005). These enzymes are expressed and appear to play significant physiological roles in reproductive tissues (reviewed by Michael et al. (2003)). For example, in the uterine–placental complex, both the relatively low-affinity NADP(H)-dependent type 1 11βHSD enzyme (11βHSD1) and the high-affinity NAD+-dependent type 2 11βHSD enzyme (11βHSD2) have recently been implicated in the physiological mechanism of labour (Challis et al. 2000, Whittle et al. 2001), although prior studies of human placenta and decidua had found no significant changes in cortisol metabolism by 11βHSD at parturition (López Bernal et al. 1982a,b).
Previously, we have established that luteinizing human granulosa cells, recovered from the ovarian follicular aspirates of women undergoing oocyte retrieval for in vitro fertilisation, exclusively express 11βHSD1 with no detectable expression of 11βHSD2 at either the mRNA or the protein level (Michael et al. 1997, Thurston et al. 2003a). Although 11βHSD1 generally acts as a 11-ketosteroid reductase to regenerate cortisol from cortisone (Seckl & Walker 2001, Seckl 2004, Draper & Stewart 2005), we have previously reported that in intact human granulosa–lutein cells in primary culture, 11βHSD1 acts predominantly as a 11β-dehydrogenase enzyme to catalyse the oxidative inactivation of cortisol (Michael et al. 1997, Thurston et al. 2003a). Since the utero-placental feedback loop for the induction of parturition (at least in sheep) appears to require stimulation of 11βHSD1 by PGs (Alfaidy et al. 2001, Challis et al. 1997), we have investigated the possible role for local PGs in control of 11βHSD1 in human granulosa–lutein cells in vitro. We recently reported that inhibition of local PG synthesis (using four structurally distinct inhibitors of PGH synthase) could suppress cortisol oxidation by 11βHSD1 in these human cells, but that this enzyme activity could be stimulated by PGE2 (Jonas et al. 2006).
At the cellular level, PGE2 actions are mediated through G protein-coupled, E-series PG (PTGER) receptors. To date, four subtypes of PTGER receptors have been cloned, designated PTGER1, PTEGER2, PTGER3 and PTGER4 (Hirata et al. 1991, Sugimoto et al. 1992, Funk et al. 1993, Honda et al. 1993, Watabe et al. 1993), with multiple isoforms of the PTGER3 receptor subtype (reviewed by Coleman et al. (1994)). While PTGER1 and PTGER3 receptors mediate increases in intracellular calcium concentrations, PTGER2 and PTGER4 receptors mediate stimulation of intracellular cAMP accumulation (Narumiya et al. 1999). Since PTGER receptors have different affinities for synthetic PGE2 analogues, agonists and antagonists with differing degrees of preference or selectivity for each PTGER receptor subtype can be used to delineate the role for particular PTGER receptors in a given cellular response (Coleman et al. 1994). Using RT-PCR and preferential receptor agonists/antagonists, we and other researchers have established that human granulosa–lutein cells express PTGER1, PTGER2 and PTGER4 receptors, and that the PTGER1 and PTGER2 receptors are able to elicit increases in intracellular calcium and cAMP respectively (Harris et al. 2001, Narko et al. 2001). However, the exact PG receptors through which PGE2 exerts its effects on progesterone synthesis and cortisol–cortisone interconversion by 11βHSD1 have not been previously examined in ovarian cells. Hence, the aim of the current study was to examine the potential roles for PTGER1 and PTGER2 receptors in mediating the effects of PGE2 on steroidogenesis and cortisol metabolism using human granulosa–lutein cells as our in vitro ovarian model.
Materials and Methods
Isolation and culture of human granulosa cells
Human granulosa cells were isolated from follicular aspirates of women undergoing controlled ovarian hyperstimulation for assisted conception at the Lister Private Hospital (Chelsea, London, UK) as previously described by Webley et al. (1988). Follicular aspirates were collected with informed patient consent in accordance with the Declaration of Helsinki and as approved by the local ethics committee.
Following isolation on 60% (v/v) Percoll, human granulosa–lutein cells were cultured for 48 h in 1:1 Dulbecco's modified Eagle's medium (DMEM):Ham's F12 medium supplemented with 10% (v/v) fetal calf serum (Invitrogen Life Technologies), 2 mM l-glutamine (Life Technologies), penicillin (87 000 IU/l; Sigma–Aldrich) and streptomycin (87 mg/l; Sigma–Aldrich) in an atmosphere of 5% (v/v) CO2 in air. Cells were cultured either in 250 μl volumes at a density of 1×105 viable cells/well for assessing regulation of progesterone production and cAMP accumulation or in 1 ml volumes at a density of 5×104 viable cells/well in 24-well plates for measurement of 11βHSD1 activities. For all experiments, cell viability, assessed by exclusion of 0·4% (v/v) trypan blue dye, was consistently >85%.
Concentration-dependent effects of PGE2 on progesterone production and cAMP accumulation in the absence or presence of SC19220 and AH6809
In order to examine whether PTGER2 receptors were involved in mediating the steroidogenic response to PGE2, cells were transferred to serum-free 1:1 DMEM:Ham's F12 medium supplemented with 1 μM meclofenamic acid (Sigma–Aldrich) to suppress intrinsic PG synthesis ( Jonas et al. 2006). Cells were challenged for 24 h with a range of PGE2 concentrations (0–3000 nM; Cayman Chemical Company, MI, USA) in the absence and presence of either 10 μM SC19220 or 10 μM AH6809 (Alexis Biochemicals, Nottingham, UK). SC19220 and AH6809 were prepared to a stock concentration of 10 mM in dimethylsulphoxide (DMSO), the final concentration of which was diluted in all wells to 0·1% by volume.
After the 24-h treatment period, culture plates were frozen at −20 °C. Samples were subsequently thawed and progesterone and cAMP concentrations were measured using RIAs previously described by Pallikaros et al. (1995) and Steiner et al. (1972) respectively. The progesterone RIA had a working range of 0·5–8·0 nM with inter- and intra-assay coefficients of variation of 9 and 14% respectively. The cAMP RIA included the acetylation step proposed by Harper & Brooker (1975), which gave the assay a working range of 0·2–1·3 nM with inter- and intra-assay coefficients of variation of 9 and 5% respectively.
Effects of SC19220 and AH6809 on 11βHSD1 activities in response to PGE2 and butaprost in human granulosa–lutein cells
As in the assessment of the effects on progesterone production and cAMP accumulation, cells were pre-incubated for 48 h in serum-supplemented 1:1 DMEM:Ham's F12 medium. Following this, the cells were transferred to serum-free 1:1 DMEM:Ham's F12 medium, supplemented with 1 μM meclofenamic acid and containing PGE2 or butaprost (Cayman Chemical Company; 0–1000 nM of each), each in the absence or presence of 10 μM SC19220 or AH6809 for 4 h. To overcome potential confounding effects of progesterone on 11βHSD activities (Thurston et al. 2003b), these experiments were performed in the presence of 100 μM aminoglutethimide, having confirmed that this effectively suppresses progesterone output from human granulosa–lutein cells (Fowkes et al. 2001) without affecting cAMP accumulation. During the 4 h over which cells were exposed to PGE2/butaprost±SC19220/AH6809, 11βHSD1 activities were assessed using the radiometric conversion assay as previously described by Michael et al. (1995). In brief, intact cells in primary culture were co-incubated with 100 nM [1,2,6,7-3H]-cortisol (Amersham Biosciences), diluted to a specific activity of 0·5 μCi/100 pmol using non-radiolabelled cortisol (Sigma–Aldrich). At the end of the 4-h treatment period, spent culture medium was transferred to screw cap borosilicate tubes in which steroids were extracted by the addition of two volumes of chloroform (Merck). After evaporation to dryness under nitrogen gas at 45 °C, the steroid residues were suspended in 25 μl ethyl acetate containing 1 mM cortisol and 1 mM cortisone (Sigma–Aldrich) before being resolved by thin layer chromatography (TLC) on silica 60 TLC plates (Merck), developed in an atmosphere of 92:8 chloroform:95% (v/v) ethanol. [3H]-cortisol and [3H]-cortisone were quantified using a Bioscan 200 TLC radiochromatogramme scanner (Lablogic, Sheffield, UK) and the oxidative activities of 11βHSD1 were calculated as net pmoles cortisol oxidised to cortisone over 4 h (Michael et al. 1995).
Statistical analyses
All experimental data are presented as the mean±s.e.m. for up to six independent experiments, where each experimental replicate was performed using cells from individual patients and each experimental condition was repeated either in quadruplicate or in triplicate within an experiment. Due to differences in absolute levels of progesterone production, cAMP accumulation and enzyme activities between patients, the results for each experiment were standardised and presented as a percentage of basal control. However, all statistical analyses were performed using absolute data.
In all experiments, a one-way ANOVA with repeated measures was performed, followed by Bonferroni's multiple comparison as the post hoc test. All statistical evaluations were performed using GraphPad Prism version 3.02 (GraphPad Software Inc., San Diego, CA, USA) and significance was assessed in all experiments as a probability value of P<0·05.
Results
Effects of SC19220 and AH6809 on progesterone production and 11βHSD1 activity
Before use, both PTGER receptor antagonists were tested for possible direct effects on basal progesterone production and 11βHSD1 activity in human granulosa–lutein cells. Under conditions in which intrinsic PG synthesis had been suppressed with 1 μM meclofenamic acid, incubation for 24 h with the preferential PTGER1 receptor antagonist SC19220 (10 μM) and the PTGER1/PTGER2 receptor antagonist AH6809 (10 μM) had no significant effect on basal progesterone production (over 24 h) or the oxidative activity of 11βHSD1 (over 4 h; Table 1).
Table 1.
Effects of SC19220 and AH6809 on basal progesterone production and 11β-hydroxysteroid dehydrogenase (11βHSD1) activity. Values are the mean±s.e.m. for three independent experiments with quadruplicate determinations in each experiment (one-way ANOVA)
| Progesterone (pmol/105 cells) | 11βHSD1 activity (pmol cortisone/4 h .5×104cells) | |
|---|---|---|
| Control | 2351·1±1028·3 | 3·7±1·2 |
| +SC19220 | 2041·6±913·6 | 3·8±1·1 |
| +AH6809 | 2712·5±1318·1 | 2·5±0·4 |
| ANOVA | P=0·161 | P=0·158 |
Progesterone concentrations in culture medium and 11βHSD1 activities in human granulosa–lutein cells were each assessed following a 24- or 4-h incubation respectively with 10 μM SC19220 or 10 μM AH6809.
Concentration-dependent effects of PGE2 on progesterone production in the absence or presence of SC19220 and AH6809
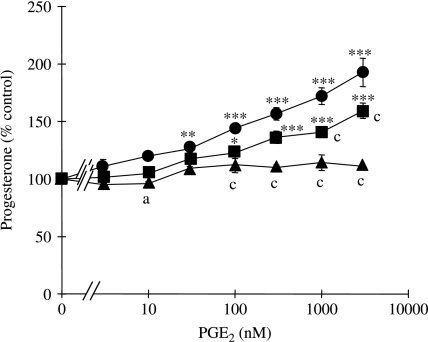
Treatment with PGE2 for 24 h resulted in a concentration-dependent increase in progesterone synthesis (Fig. 1). At PGE2 concentrations ≥30 nM, progesterone production increased progressively by up to 91±6% (at 3000 nM PGE2) relative to the control rate of progesterone output in the absence of PGE2 (P<0·01). However, co-treatment with SC19220 attenuated the responses to both 1000 and 3000 nM PGE2 by 56±4% (P<0·001) and 35±3% (P<0·001) respectively, while co-treatment with AH6809 completely abolished the ability of all concentrations of PGE2 to stimulate progesterone output.
Figure 1.
Effects of SC19220 and AH6809 on the progesterone responses to PGE2 by human granulosa–lutein cells. Progesterone concentrations in medium collected after a 24-h incubation in the presence of 0–3000 nM PGE2, either in the absence (circle) or in the presence of 10 μM SC19220 (square) or 10 μM AH6809 (triangle). Values are the mean±s.e.m. for three independent experiments with quadruplicate determinations in each experiment. *P<0·05, **P<0·01, ***P<0·001 relative to 0 nM PGE2 in the absence or presence of the PTGER receptor antagonist, as appropriate. aP<0·05, cP<0·001 relative to cells treated with the same PGE2 concentration in the absence of PTGER receptor antagonists (one-way ANOVA plus Bonferroni's post hoc multiple comparison test).
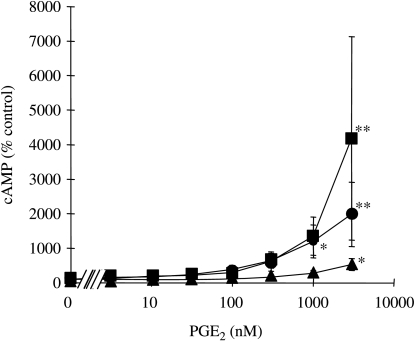
Incubation for 24 h with PGE2 evoked a concentration-dependent increase in cAMP accumulation which only achieved significance at the supraphysiological concentrations of 1000 and 3000 nM PGE2 (Fig. 2). At the highest tested concentration of 3000 nM, PGE2 increased cAMP accumulation by 18·7±6·8-fold (P<0·01) in the absence of PTGER receptor antagonists. In cells co-treated with SC19220, this maximal concentration of PGE2 increased cAMP accumulation by 40·3±27·8-fold (P<0·01), whereas co-treatment with AH6809 suppressed the cAMP response to 3000 nM PGE2 to just 4·1±1·2-fold (P<0·05; Fig. 2).
Figure 2.
Effects of SC19220 and AH6809 on the cAMP responses to PGE2 by human granulosa–lutein cells. Concentrations of cAMP after a 24-h incubation in the presence of 0–3000 nM PGE2, either in the absence (circle) or in the presence of 10 μM SC19220 (square) or 10 μM AH6809 (triangle). Values are the mean±s.e.m. for three independent experiments with quadruplicate determinations in each experiment. *P<0·05, **P<0·01 relative to 0 nM PGE2 in the absence or presence of the PTGER receptor antagonists, as appropriate (one-way ANOVA plus Bonferroni's post hoc multiple comparison test).
Effects of SC19220 and AH6809 on 11βHSD1 activities in response to PGE2 and butaprost in human granulosa–lutein cells
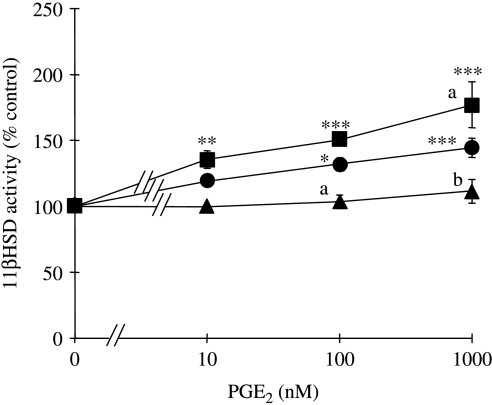
Consistent with our recent report ( Jonas et al. 2006), PGE2 significantly increased 11βHSD1 activity in a concentration-dependent manner; net oxidation of cortisol to cortisone was increased by up to 43±3% at the highest tested PGE2 concentration of 1000 nM (P<0·001; Fig. 3). Co-treatment with SC19220 further enhanced 11βHSD1 activities at each of the tested concentrations of PGE2. At the highest tested concentration of 1000 nM PGE2, co-treatment with SC19220 increased the 11βHSD1 activity by a further 32·7±10·2% (P<0·05) relative to the cells treated with PGE2 in the absence of PTGER receptor antagonists. In contrast, co-treatment with AH6809 completely abolished the 11βHSD1 response to all tested concentrations of PGE2 (Fig. 3).
Figure 3.
Effects of PGE2 on 11βHSD1 activities human granulosa–lutein cells in the absence and presence of SC19220 and AH6809. 11βHSD1 activities following treatment of cells with 0–1000 nM PGE2 either in the absence (circle) or in the presence of 10 μM SC19220 (square) or 10 μM AH6809 (triangle). Values are the mean±s.e.m. for three independent experiments with triplicate determinations in each experiment. *P<0·05, **P<0·01, ***P<0·001 relative to 0 nM PGE2 in absence or presence of the PTGER receptor antagonists, as appropriate. aP<0·05, bP<0·01 relative to cells treated with the same PGE2 concentration in the absence of PTGER receptor antagonists (one-way ANOVA plus Bonferroni's post hoc multiple comparison test).
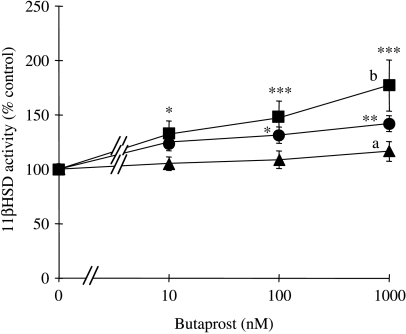
Incubation for 4 h with the preferential PTGER2 receptor agonist butaprost also elicited a concentration-dependent increase in 11βHSD1 activities (Fig. 4). At the highest tested concentration of 1000 nM, butaprost increased net cortisol oxidation by 40±3% (P<0·01). Co-treatment with SC19220 further increased 11βHSD1 activity at each tested concentration of butaprost by up to 31±1% (P<0·05) relative to cells treated with butaprost alone. In contrast, as for PGE2, co-treatment with AH6809 completely abolished the stimulation of 11βHSD1 activities by each concentration of butaprost.
Figure 4.
Effects of butaprost on 11βHSD1 activities in human granulosa–lutein cells in the absence and presence of SC19220 and AH6809. 11βHSD1 activities following treatment of cells with 0–1000 nM butaprost either in the absence (circle) or in the presence of 10 μM SC19220 (square) or 10 μM AH6809 (triangle). Values are the mean±s.e.m. for three independent experiments with triplicate determinations in each experiment. *P<0·05, **P<0·01, ***P<0·001 relative to 0 nM butaprost in the absence or presence of the PTGER receptor antagonists, as appropriate. aP<0·05, bP<0·01 relative to cells treated with the same butaprost concentration in the absence of PTGER receptor antagonists (one-way ANOVA plus Bonferroni's post hoc multiple comparison test).
Discussion
This study has investigated the potential participation of PTGER1 and PTGER2 receptors in mediating the effects of PGE2 on progesterone production and glucocorticoid metabolism in human granulosa–lutein cells. Using preferential pharmacological agonists and antagonists for the PTGER1 and PTGER2 receptor subtypes, the combined data implicate PTGER2 receptors as mediators of the stimulation of progesterone synthesis and 11βHSD1 activity by PGE2 in this human ovarian cell model in vitro.
The ability of PGE2 to stimulate progesterone synthesis in human and non-human primate luteal cells, and in the widely used model of human granulosa–lutein cells, is well documented (Hahlin et al. 1988, Fehrenbach et al. 1999, Vaananen et al. 2001). Given the reported ability of PGE2 to increase intracellular cAMP concentrations in these cells (Michael et al. 1993) and the established role for cAMP as a second messenger that stimulates steroidogenesis (Cooke 1999), it has widely been assumed that the progesterone response to PGE2 is mediated via this cyclic nucleotide. We have previously demonstrated that both progesterone production and cAMP accumulation in human granulosa–lutein cells can be stimulated by the mixed PTGER1/PTGER2 receptor agonist, dimethyl-PGE2 and by the preferential PTGER2 receptor agonist, butaprost (Harris et al. 2001). The cAMP responses to dimethyl-PGE2 and butaprost were completely abolished by co-treatment with the PTGER1/PTGER2 receptor antagonist AH6809, but not the selective PTGER1 receptor antagonist SC19220, implicating the PTGER2 receptor in the cAMP response to these pharmacological agonists (Harris et al. 2001). Interestingly, the cAMP response to PGE2 was only partially suppressed (by around 60%) with AH6809, and we did not assess the ability of these compounds to interfere with the steroidogenic responses to PGE2 or its functional analogues.
In the present study, when cells were co-treated with SC19220, the progesterone response to PGE2 was partially inhibited but not completely abolished. This suggests that the PTGER1 receptors participate in the stimulation of steroidogenesis by PGE2, but do not mediate the full steroidogenic response. We have now confirmed that co-treatment with AH6809 could completely prevent the steroidogenic response to PGE2 at all tested concentrations, implicating an AH6809-sensitive receptor in the steroidogenic response to PGE2.
The concentrations of PGE2 (and butaprost) used for the studies reported herein were selected based on previous investigations of human granulosa–lutein cells, which routinely use PGs at supraphysiological concentrations of 500–5000 nM. It is noteworthy that, at the lower tested concentrations of 3 and 10 nM, PGE2 failed to stimulate steroidogenesis, even though Abramovitz et al. (2000) have previously reported that PGE2 concentrations of <30 nM should be sufficient to stimulate all four PTGER receptor subtypes. Moreover, although PGE2 and butaprost were able to stimulate progesterone synthesis (and cortisol metabolism) at concentrations of 30 nM and above, at these concentrations, PGE2 would be expected to saturate all four classes of PTGER receptor and possibly to activate non-PTGER receptors (i.e. PTGFR and D series prostaglandin (PTGDR) receptors), particularly at the conventional concentrations of 300, 1000 and 3000 nM (Abramovitz et al. 2000). Since PTGFR receptors are known to inhibit (rather than stimulate) progesterone production (Sugimoto et al. 1997, Tsai et al. 1998, 2001), it seems highly unlikely that the steroidogenic response to PGE2 (even at concentrations >30 nM) is mediated via the PTGFR receptor. However, we cannot yet exclude participation of the PTGDR receptor in the stimulation of steroidogenesis at high concentrations of PGE2, given that AH6809 has also been reported to function as a weak PTGDR antagonist when used at a concentration of 10 μM (Keery & Lumley 1988, Woodward et al. 1995).
In terms of effects on cAMP accumulation, we found that although PGE2 increased cAMP accumulation in a concentration-dependent manner, this response only achieved statistical significance at the higher concentrations of 1000 and 3000 nM PGE2, which would be expected to bind all PTGER maximally, and possibly to activate PTGDR and PTGFR receptors as well (Abramovitz et al. 2000). Co-treatment of cells with AH6809 suppressed the PGE2-induced increase in the cAMP accumulation, but did not completely abolish the cAMP response to PGE2, indicating that the residual effect of high PGE2 concentrations on cAMP may be mediated through an AH6809-insensitive receptor. SC19220, on the other hand, did not suppress the responsiveness of the cells to PGE2. If anything, co-treatment with the PTGER1 antagonist SC19220 enhanced the increase of cAMP in response to PGE2. These observations raise two possibilities. First, inhibition of PTGER1 receptor binding by SC19220 may have facilitated increased binding of PGE2 to other prostanoid receptors which are capable of stimulating cAMP accumulation (e.g. PTGER2, PTGER4 and/or PTGDR receptors). Secondly, co-stimulation of the PTGER1 receptor would be expected to activate the calcium–protein kinase C signalling pathway (Narumiya et al. 1999), which can inhibit the stimulation of cAMP production (Abayasekara et al. 1993a,b). Based on these observations, prostanoid receptors which are sensitive to AH6809 but insensitive to SC19220 (e.g. PTGER2 and/or PTGDR) are implicated in the ability of PGE2 to stimulate cAMP accumulation in human granulosa–lutein cells. In light of our previous report (Harris et al. 2001), we would suggest that while the ability of dimethyl-PGE2 and butaprost to elevate intracellular cAMP concentrations is mediated solely via a PG receptor that can be antagonised with AH6809, consistent with a role for the PTGER2 receptor in eliciting a cAMP response, at least some of the second messenger response to PGE2 is mediated via AH6809-resistant receptors, which may include PTGER4 receptors and possibly even PTGDR receptors. We also note that, in the present study, the residual cAMP responses to the higher concentrations of PGE2 in cells co-treated with AH6809 were not accompanied by any significant increases in progesterone synthesis, showing that second messengers other than cAMP may be required for PGE2 to stimulate steroidogenesis.
Turning to the regulation of cortisol–cortisone metabolism by PGE2, we have previously established that luteinizing human granulosa cells only express 11βHSD1 with no detectable expression of 11βHSD2 protein or mRNA, even when the PCR is used to increase the sensitivity of detection (Michael et al. 1997, Thurston et al. 2003a). Although the 11βHSD1 enzyme is intrinsically bidirectional, in most tissues this enzyme acts predominantly as an 11-ketosteroid reductase to regenerate cortisol from cortisone (Seckl & Walker 2001, Seckl 2004, Draper & Stewart 2005). However, we have previously found that 11βHSD1 acts predominantly, if not exclusively, to catalyse the oxidative inactivation of cortisol in human granulosa–lutein cells (Michael et al. 1997, Thurston et al. 2003a), bovine granulosa and luteal cells (Thurston et al. 2007) and porcine granulosa cells (Sunak et al. 2007). 11βHSD1 has also been reported to act predominantly as a 11β-dehydrogenase in rat testis Leydig cells (Gao et al. 1997, Ge & Hardy 2000) suggesting that the major action of the 11βHSD1 enzyme may be fundamentally different in the steroidogenic cells of the ovary and testis (Michael et al. 2003, Ge et al. 2005). Recent studies have established that the predominant direction of 11βHSD1 is dependent on the ratio of the reduced form of nicotinamide adenine dinucleotide phosphate to its oxidised form (NADPH: NADP+) within the lumen of the smooth endoplasmic reticulum (Draper et al. 2003, Atanasov et al. 2004, Banhegyi et al. 2004, Bujalska et al. 2005, McCormick et al. 2006) such that changes in this ratio in steroidogenic gonadal cells may favour the oxidative activity of 11βHSD1 in these cells (Michael et al. 2003, Ge et al. 2005).
In the present study, the stimulation of the net oxidation of cortisol in human granulosa–lutein cells by PGE2 showed good agreement with our recently published data (Jonas et al. 2006). The fact that this stimulation could be reproduced using the preferential PTGER2 receptor agonist butaprost, combined with the fact that AH6809 could completely suppress the effects of PGE2 and butaprost on cortisol oxidation, suggests that the stimulation of the oxidative activity of 11βHSD1 is mediated via the PTGER2 receptor. In contrast, co-treatment with SC19220 enhanced the 11βHSD1 response at each of the tested concentrations of PGE2, suggesting that the co-activation of PTGER1 receptors limits the stimulation of cortisol–cortisone metabolism by PGE2. PTGER1 receptors are known to act via calcium as a second messenger, and we have confirmed that in human granulosa–lutein cells, SC19220 can antagonise the ability of PGE2 and its dimethyl derivative to increase the intracellular calcium concentration (Harris et al. 2001). Given that studies of placental biochemistry have shown that calcium can mediate the inhibition of 11βHSD1 activity in response to PGs and leukotriene B4 (Hardy et al. 1999, 2001), we would propose that activation of the PTGER1 receptor–calcium signalling pathway by PGE2 limits the ability of this PG to stimulate 11βHSD1 in human granulosa–lutein cells via the PTGER2–cAMP pathway.
In comparing the three responses considered in this study, we note that at the lower concentrations tested (<1000 nM), which are likely to fall within the physiological range and selectively to activate only the PTGER receptor subtypes, PGE2 can stimulate both progesterone synthesis and cortisol metabolism without exerting a significant effect on the intracellular cAMP concentration. This suggests that either the effects of 10–300 nM PGE2 on steroid synthesis and metabolism are mediated via local increases in cAMP within specific subcellular microenvironments (not reflected by changes in the total intracellular cAMP concentration) or these effects are mediated via a second messenger other than cAMP.
In summary, we have presented data which implicate PTGER2 receptors in the stimulation of progesterone synthesis, cAMP accumulation and cortisol metabolism via 11βHSD by PGE2 in human granulosa–lutein cells. Further studies are necessary to elucidate potential roles for other prostanoid receptors, such as PTGDR receptors, in the responses at the upper concentrations of PGE2.
Acknowledgements
We wish to thank the staff at the Lister Private Hospital assisted conception unit (Chelsea, London, UK) for providing the human granulosa–lutein cells used for this study. This work was supported by a Wellcome Trust Grant No. 056630. For this publication, the contemporary HUGO nomenclature has been used for the prostanoid receptors, where PTGER1, PTGER2, PTGER3, PTGER4, PTGDR and PTGFR receptors have been previously described as EP1, EP2, EP3, EP4, DP and FP receptors respectively. According to the HUGO nomenclature, the hydroxysteroid dehydrogenase enzymes type 1 11βHSD/11βHSD1 and type 2 11βHSD/11βHSD2 have been designated as HSD11B1 and HSD11B2 respectively; however, for this publication, the conventional (pre-HUGO) nomenclature has been used for the 11βHSD enzymes. The authors declare that there is no conflict of interest that would prejudice the impartiality of this scientific work.
References
- Abayasekara DRE, Jones PM, Persaud SJ, Michael AE, Flint APF. Prostaglandin F2α activates protein kinase C in human ovarian cells. Molecular and Cellular Endocrinology. 1993a;91:51–57. doi: 10.1016/0303-7207(93)90254-h. [DOI] [PubMed] [Google Scholar]
- Abayasekara DRE, Michael AE, Webley GE, Flint APF. Mode of action of prostaglandin F2α in human luteinized granulose cells: role of protein kinase C. Molecular and Cellular Endocrinology. 1993b;97:81–91. doi: 10.1016/0303-7207(93)90213-4. [DOI] [PubMed] [Google Scholar]
- Abramovitz M, Adam M, Boie Y, Carriere MC, Denis D, Godbout C, Lamontagne S, Rochette C, Sawyer N, Tremblay NM, et al. The ustilisation of recombinant prostanoid receptors to determine the affinities and selectivities of prostaglandins and related analogues. Biochimica et Biophysica Acta. 2000;1483:285–293. doi: 10.1016/s1388-1981(99)00164-x. [DOI] [PubMed] [Google Scholar]
- Alfaidy N, Xiong ZG, Myatt L, Lye SJ, Macdonald JF, Challis JRG. Prostaglandin F2α potentiates cortisol production by stimulating 11β-hydroxysteroid dehydrogenase 1: a novel feedback loop that may contribute to human labor. Journal of Clinical Endocrinology and Metabolism. 2001;86:5585–5592. doi: 10.1210/jcem.86.11.7995. [DOI] [PubMed] [Google Scholar]
- Atanasov AG, Nashev LG, Schweizer RAS, Frick C, Odermatt A. Hexose-6-phosphate dehydrogenase determines the reaction direction of 11β-hydroxysteroid dehydrogenases type 1 as an oxoreductase. FEBS Letters. 2004;571:129–133. doi: 10.1016/j.febslet.2004.06.065. [DOI] [PubMed] [Google Scholar]
- Banhegyi G, Benedetti A, Fulceri R, Senesi S. Co-operativity between 11β-hydroxysteroid dehydrogenases type 1 and hexose-6-phosphate dehydrogenase in the lumen of the endoplasmic reticulum. Journal of Biological Chemistry. 2004;279:27017–27021. doi: 10.1074/jbc.M404159200. [DOI] [PubMed] [Google Scholar]
- Bujalska IJ, Draper N, Michailidou Z, Tomlinson JW, White PC, Chapman KE, Walker EA, Stewart PM. Hexose-6-phosphate dehydrogenase confers oxo-reductase activity upon 11β-hydroxysteroid dehydrogenase type 1. Journal of Molecular Endocrinology. 2005;34:675–684. doi: 10.1677/jme.1.01718. [DOI] [PubMed] [Google Scholar]
- Challis JR, Lye SJ, Gibb W. Prostaglandins and parturition. Annals of the New York Academy of Science. 1997;26:254–267. doi: 10.1111/j.1749-6632.1997.tb48546.x. [DOI] [PubMed] [Google Scholar]
- Challis JRG, Matthews SG, Gibb W, Lye SJ. Endocrine and paracrine regulation of birth at term and preterm. Endocrine Reviews. 2000;21:514–550. doi: 10.1210/edrv.21.5.0407. [DOI] [PubMed] [Google Scholar]
- Chen WY, Yang JG, Li PS. Effect of dexamethasone on the expression of p34(cdc2) and cyclin B1 in pig oocytes in vitro. Molecular Reproduction and Development. 2000;56:74–79. doi: 10.1002/(SICI)1098-2795(200005)56:1<74::AID-MRD9>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Coleman RA, Smith WL, Narumiya S. International union of pharmacology classification of prostanoid receptors: properties, distribution, and structure of the receptors and their subtypes. Pharmacological Reviews. 1994;46:205–229. [PubMed] [Google Scholar]
- Cooke BA. Signal transduction involving cyclic AMP-dependent and cyclic AMP-independent mechanisms in the control of steroidogenesis. Molecular and Cellular Endocrinology. 1999;151:25–35. doi: 10.1016/s0303-7207(98)00255-x. [DOI] [PubMed] [Google Scholar]
- Draper N, Stewart PM. 11β-hydroxysteroid dehydrogenase and the pre-receptor regulation of corticosteroid hormone action. Journal of Endocrinology. 2005;186:251–271. doi: 10.1677/joe.1.06019. [DOI] [PubMed] [Google Scholar]
- Draper N, Walker EA, Bujalska IJ, Tomlinson JW, Chalder SM, Arlt W, Lavery GG, Bedendo O, Ray DW, Laing I, et al. Mutations in the genes encoding 11β-hydroxysteroid dehydrogenase type 1 and hexose-6-phosphate dehydrogenase interact to cause cortisone reductase deficiency. Nature Genetics. 2003;34:434–439. doi: 10.1038/ng1214. [DOI] [PubMed] [Google Scholar]
- Fehrenbach A, Hodges JK, Einspanier A. Direct effects of the prostaglandins E2 and F2α on progesterone release by the corpus luteum of the marmoset monkey (Callithrix jacchus) studied by in vitro microdialysis. Journal of Endocrinology. 1999;161:433–443. doi: 10.1677/joe.0.1610433. [DOI] [PubMed] [Google Scholar]
- Fowkes RC, Chandras C, Chin EC, Okolo S, Abayasekara DRE, Michael AE. Relationship between the production of prostaglandins and progesterone by luteinizing human granulosa cells. Journal of Endocrinology. 2001;171:455–462. doi: 10.1677/joe.0.1710455. [DOI] [PubMed] [Google Scholar]
- Funk CD, Furci L, FitzGerald GA, Grugorczyk R, Rochette C, Bayne MA, Abramovitz M, Adam M, Metters KM. Cloning and expression of a cDNA for the human prostaglandin E receptor EP1 subtype. Journal of Biological Chemistry. 1993;268:26767–26772. [PubMed] [Google Scholar]
- Gao HB, Ge RS, Lakshmi V, Marandici A, Hardy MP. Hormonal regulation of oxidative and reductive activities of 11β-hydroxysteroid dehydrogenase in rat Leydig cells. Endocrinology. 1997;138:156–161. doi: 10.1210/endo.138.1.4837. [DOI] [PubMed] [Google Scholar]
- Ge RS, Hardy MP. Initial predominance of the oxidative activity of type I 11β-hydroxysteroid dehydrogenase in primary rat Leydig cells and transfected cell lines. Journal of Andrology. 2000;21:303–310. [PubMed] [Google Scholar]
- Ge RS, Dong Q, Niu EM, Sottas CM, Hardy DO, Catterall JF, Latif SA, Morris DJ, Hardy MP. 11β-hydroxysteroid dehydrogenase 2 in rat leydig cells: its role in blunting glucocorticoid action at physiological levels of substrate. Endocrinology. 2005;146:2657–2664. doi: 10.1210/en.2005-0046. [DOI] [PubMed] [Google Scholar]
- Greeley MS, Calder DR, Taylor MH, Hols H, Wallace RA. Oocyte maturation in mummichog (Fundulus heterclitus): effects of steroids on germinal vesicle breakdown of intact follicles. General and Comparative Endocrinology. 1986;62:281–289. doi: 10.1016/0016-6480(86)90118-8. [DOI] [PubMed] [Google Scholar]
- Hahlin M, Dennefors B, Johanson C, Hamberger L. Luteotropic effects of prostaglandin E2 on the human corpus luteum of the menstrual cycle and early pregnancy. Journal of Clinical Endocrinology and Metabolism. 1988;66:909–914. doi: 10.1210/jcem-66-5-909. [DOI] [PubMed] [Google Scholar]
- Hardy DB, Pereria LE, Yang K. Prostaglandins and leukotriene B4 are potent inhibitors of 11β-hydroxysteroid dehydrogenase type 2 activity in human choriocarcinoma JEG-3 cells. Biology of Reproduction. 1999;61:40–45. doi: 10.1095/biolreprod61.1.40. [DOI] [PubMed] [Google Scholar]
- Hardy DB, Dixon SJ, Narayanan N, Yang K. Calcium inhibits human placental 11β-hydroxysteroid dehydrogenase type 2 activity. Biochemical and Biophysical Research Communications. 2001;283:756–761. doi: 10.1006/bbrc.2001.4851. [DOI] [PubMed] [Google Scholar]
- Harlow CR, Jenkins JM, Winston RML. Increased follicular fluid total and free cortisol levels during the luteinizing hormone surge. Fertility and Sterility. 1997;68:48–53. doi: 10.1016/s0015-0282(97)81474-4. [DOI] [PubMed] [Google Scholar]
- Harper JF, Brooker G. Femtomole sensitive radioimmunoassay for cyclic AMP and cyclic GMP after 2’0 acetylation by acetic anhydride in aqueous solution. Journal of Cyclic Nucleotide Research. 1975;1:207–218. [PubMed] [Google Scholar]
- Harris TE, Squires PE, Michael AE, Lopez-Bernal A, Abayasekara DRE. Human granulosa-lutein cells express functional EP1 and EP2 prostaglandin receptors. Biochemical and Biophysical Research Communications. 2001;285:1089–1094. doi: 10.1006/bbrc.2001.5301. [DOI] [PubMed] [Google Scholar]
- Hirata M, Hayashi Y, Ushikubi F, Yokota Y, Kageyama R, Nakanishi S, Narumiya S. Cloning and expression of cDNA for a human thromboxane A2 receptor. Nature. 1991;349:617–620. doi: 10.1038/349617a0. [DOI] [PubMed] [Google Scholar]
- Honda A, Sugimoto Y, Namba T, Watabe A, Irie A, Negishi M, Narumiya S, Ichikawa A. Cloning and expression of a cDNA for mouse prostaglandin E receptor EP2 subtype. Journal of Biological Chemistry. 1993;268:7759–7762. [PubMed] [Google Scholar]
- Jonas KC, Chandras C, Abayasekara DRE, Michael AE. Role for prostaglandins in the regulation of type 1 11β-hydroxysteroid dehydrogenase in human granulosa-lutein cells. Endocrinology. 2006;147:5865–5872. doi: 10.1210/en.2006-0723. [DOI] [PubMed] [Google Scholar]
- Keery RJ, Lumley P. AH6809, a prostaglandin DP-receptor blocking drug on human platelets. British Journal of Pharmacology. 1988;94:745–754. doi: 10.1111/j.1476-5381.1988.tb11584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López Bernal A, Anderson ABM, Turnbull AC. Cortisol:cortisone interconversion by human decidua in relation to parturition. Effect of tissue manipulation on 11β-hydroxysteroid dehydrogenase activity. Journal of Endocrinology. 1982a;93:141–149. doi: 10.1677/joe.0.0930141. [DOI] [PubMed] [Google Scholar]
- López Bernal A, Anderson ABM, Turnbull AC. The lack of influence of parturition on human placental 11β-hydroxysteroid dehydrogenase activity. Journal of Clinical Endocrinology and Metabolism. 1982b;54:1251–1254. doi: 10.1210/jcem-54-6-1251. [DOI] [PubMed] [Google Scholar]
- McCormick KL, Wang X, Mick GJ. Evidence that the 11β-hydroxysteroid dehydrogenase (11βHSD1) is regulated by pentose pathway flux. studies in rat adipocytes and microsomes. Journal of Biological Chemistry. 2006;281:341–347. doi: 10.1074/jbc.M506026200. [DOI] [PubMed] [Google Scholar]
- Michael AE, Abayasekara DRE, Webley GE. The luteotrophic actions of prostaglandin E2 and F2α are differentially mediated via cyclic AMP and protein kinase C. Journal of Endocrinology. 1993;138:291–298. doi: 10.1677/joe.0.1380291. [DOI] [PubMed] [Google Scholar]
- Michael AE, Abayasekara DRE, Webley GE. Cellular mechanisms of luteolysis. Molecular and Cellular Endocrinology. 1994;99:R1–R9. doi: 10.1016/0303-7207(94)90158-9. [DOI] [PubMed] [Google Scholar]
- Michael AE, Gregory L, Piercy EC, Walker SM, Shaw RW, Cooke BA. Ovarian 11β-hydroxysteroid dehydrogenase activity is inversely related to the outcome of in vitro fertilization-embryo transfer treatment cycles. Fertility and Sterility. 1995;64:590–598. [PubMed] [Google Scholar]
- Michael AE, Evagelatou M, Norgate DP, Clarke RJ, Antoniw JW, Stedman B, Brennan A, Welsby R, Bujalska I, Stewart PM. Isoforms of 11β-hydroxysteroid dehydrogenase in human granulosa-lutein cells. Molecular and Cellular Endocrinology. 1997;132:43–52. doi: 10.1016/s0303-7207(97)00118-4. [DOI] [PubMed] [Google Scholar]
- Michael AE, Thurston LM, Rae MT. Glucocorticoid metabolism and reproduction: a tale of two enzymes. Reproduction. 2003;126:425–441. doi: 10.1530/rep.0.1260425. [DOI] [PubMed] [Google Scholar]
- Narko K, Saukkonen K, Ketola I, Butzow R, Heikinheimo M, Ristimaki A. Regulated expression of prostaglandin E2 receptors EP2 and EP4 in human ovarian granulosa cells. Journal Clinical Endocrinology and Metabolism. 2001;86:1765–1768. doi: 10.1210/jcem.86.4.7535. [DOI] [PubMed] [Google Scholar]
- Narumiya S, Sugimoto Y, Ushikubi F. Prostanoid receptors: structures, properties and functions. Physiological Reviews. 1999;79:1193–1226. doi: 10.1152/physrev.1999.79.4.1193. [DOI] [PubMed] [Google Scholar]
- Olofsson J, Leung PCK. Autocrine/paracrine role of prostaglandins in corpus luteum function. Molecular and Cellular Endocrinology. 1994;100:87–91. doi: 10.1016/0303-7207(94)90284-4. [DOI] [PubMed] [Google Scholar]
- Pallikaros Z, Schulster D, Baldwin SA, Helliwell RJA, Michael AE, Cooke BA. Characterization of site-directed antibodies to the LH receptor in functionally active gonadal cells and their differential effects on LH-stimulated signal transduction in Leydig tumor (MA-10) cells. Molecular and Cellular Endocrinology. 1995;114:57–68. doi: 10.1016/0303-7207(95)03642-k. [DOI] [PubMed] [Google Scholar]
- Richardson MC. Hormonal control of ovarian luteal cells. Oxford Reviews in Reproductive Biology. 1986;8:321–378. [PubMed] [Google Scholar]
- Seckl JR, Walker BR. Minireview: 11β-hydroxysteroid dehydrogenase type 1- a tissue-specific amplifier of glucocorticoid action. Endocrinology. 2001;142:1371–1376. doi: 10.1210/endo.142.4.8114. [DOI] [PubMed] [Google Scholar]
- Seckl JR. 11β-hydroxysteroid dehydrogenases: changing glucocorticoid action. Current Opinion in Pharmacology. 2004;4:597–602. doi: 10.1016/j.coph.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Steiner AL, Wehmann RE, Parker CW, Kipnis DM. Radioimmunoassay for the measurement of cyclic nucleotides. Advances in Cyclic Nucleotide Research. 1972;2:51–61. [PubMed] [Google Scholar]
- Sugimoto Y, Namba T, Shigemoto R, Negishi M, Ichikawa A, Narumiya S. Cloning and expression of a cDNA for mouse prostaglandin E receptor EP3 subtype. Journal of Biological Chemistry. 1992;267:6463–6466. [PubMed] [Google Scholar]
- Sugimoto Y, Yamasaki A, Segi E, Tsuboi K, Aze Y, Nishimura T, Oida H, Yoshida N, Tanaka T, Katsuyama M, et al. Failure of parturition in mice lacking the prostaglandin F receptor. Science. 1997;277:681–683. doi: 10.1126/science.277.5326.681. [DOI] [PubMed] [Google Scholar]
- Sunak N, Green DF, Abeydeera LR, Thurston LM & Michael AE 2007 Implication of cortisol and 11β-hydroxysteroid dehydrogenase enzymes in the development of porcine ovarian follicles and cysts. Reproduction, 133 1149–1158. [DOI] [PubMed]
- Tetsuka M, Thomas FJ, Thomas MJ, Anderson RA, Mason JI, Hillier SG. Differential expression of messenger ribonucleic acids encoding 11β-hydroxysteroid dehydrogenase types 1 and 2 in human granulosa cells. Journal of Clinical Endocrinology and Metabolism. 1997;82:2006–2009. [PubMed] [Google Scholar]
- Tetsuka M, Haines LC, Milne M, Simpson GE, Hillier SG. Regulation of 11β-hydroxysteroid dehydrogenase type 1 gene expression by LH and interleukin-1β in cultured rat granulosa cells. Journal of Endocrinology. 1999;163:417–423. doi: 10.1677/joe.0.1630417. [DOI] [PubMed] [Google Scholar]
- Thurston LM, Chin E, Jonas KC, Bujalska IJ, Stewart PM, Abayasekara DRE, Michael AE. Expression of 11β-hydroxysteroid dehydrogenase (11βHSD) proteins in luteinizing human granulosa-lutein cells. Journal of Endocrinology. 2003a;178:127–135. doi: 10.1677/joe.0.1780127. [DOI] [PubMed] [Google Scholar]
- Thurston LM, Norgate DP, Jonas KC, Gregory L, Wood PJ, Cooke BA, Michael AE. Ovarian modulators of type 1 11β-hydroxysteroid dehydrogenase (11βHSD) activity and intra-follicular cortisol:cortisone ratios correlate with the clinical outcome of IVF. Human Reproduction. 2003b;18:1603–1612. doi: 10.1093/humrep/deg322. [DOI] [PubMed] [Google Scholar]
- Thurston LM, Abayasekara DRE, Michael AE. 11β-Hydroxysteroid dehydrogenase expression and activities in bovine granulosa cells and corpora lutea implicate corticosteroids in bovine ovarian physiology. Journal of Endocrinology. 2007;193:299–310. doi: 10.1677/joe.1.07025. [DOI] [PubMed] [Google Scholar]
- Tsai SJ, Wiltbank MC. Prostaglandin F2α regulates distinct physiological changes in early and mid-cycle bovine corpora lutea. Biology of Reproduction. 1998;58:346–352. doi: 10.1095/biolreprod58.2.346. [DOI] [PubMed] [Google Scholar]
- Tsai SJ, Wu MH, Chuang PC, Chen HM. Distinct regulation of gene expression by prostaglandin F2α (PGF2α) is associated with PGF2α resistance or susceptibility in human granulosa-luteal cells. Molecular Human Reproduction. 2001;7:415–423. doi: 10.1093/molehr/7.5.415. [DOI] [PubMed] [Google Scholar]
- Vaananen JE, Tong BL, Vaananen CC, Chan IH, Yuen BH, Leung PC. Interaction of prostaglandin F2α and prostaglandin E2 on progesterone production in human granulosa-luteal cells. Biological Signals and Receptors. 2001;10:380–388. doi: 10.1159/000046905. [DOI] [PubMed] [Google Scholar]
- Watabe A, Sugimoto Y, Honda A, Irie A, Namba T, Negishi M, Ito S, Narumiya S, Ichikawa A. Cloning and expression of cDNA for a mouse EP1 subtype of prostaglandin E receptor. Journal of Biological Chemistry. 1993;268:20175–20178. [PubMed] [Google Scholar]
- Webley GE, Luck MR, Hearn JP. Stimulation of progesterone secretion by cultured human granulosa cells with melatonin and catecholamines. Journal of Reproduction and Fertility. 1988;84:669–677. doi: 10.1530/jrf.0.0840669. [DOI] [PubMed] [Google Scholar]
- Whittle WL, Patel FA, Alfaidy N, Holloway AC, Fraser M, Gyomorey S, Lye SJ, Gibb W, Challis JR. Glucocorticoid regulation of human and ovine parturition: the relationship between fetal hypothalamic–pituitary–adrenal axis activation and intrauterine prostaglandin production. Biology of Reproduction. 2001;64:1019–1032. doi: 10.1095/biolreprod64.4.1019. [DOI] [PubMed] [Google Scholar]
- Woodward DF, Pepperl DJ, Burkey TH, Regan JW. 6-Isopropoxy-9-oxoxanthene-2-carboxylic acid (AH6809), a human EP2 receptor antagonist. Biochemical Pharmacology. 1995;50:1731–1733. doi: 10.1016/0006-2952(95)02035-7. [DOI] [PubMed] [Google Scholar]
- Yang JG, Chen WY, Li PS. Effects of glucocorticoids on maturation of pig oocytes and their subsequent fertilizing capacity in vitro. Biology of Reproduction. 1999;60:929–936. doi: 10.1095/biolreprod60.4.929. [DOI] [PubMed] [Google Scholar]