Abstract
Objective: Few epidemiologic studies evaluate the relative contribution of different risk factors on sleep problems. The aim of the present study was to assess demographics, comorbid characteristics, and health outcomes in patients with sleep disorders.
Method: A population-based cohort study with nested case-control analysis was conducted in adults using the U.K. General Practice Research Database. Information was collected for 12,437 patients with a new sleep disorder diagnosis during the year 1996 and 18,350 age- and sex-matched controls. Logistic regression analysis was used to compute odds ratios (OR) and 95% confidence intervals (CI).
Results: The incidence of a new sleep disorder diagnosis was 12.5 per 1000 person-years. There was a clear association of sleep disorders with smoking and excessive alcohol consumption; prior psychiatric disorders, including stress (OR = 3.6, 95% CI = 2.9 to 4.4) and depression (OR = 3.1, 95% CI = 2.8 to 3.3); prior circulatory diseases, including heart failure (OR = 1.8, 95% CI = 1.4 to 2.2) and coronary heart disease (OR = 1.4, 95% CI = 1.2 to 1.6); and prior gastrointestinal diseases, including gastroesopha-geal reflux disease (OR = 1.4, 95% CI = 1.2 to 1.7) and irritable bowel syndrome (OR = 1.5, 95% CI = 1.2 to 1.9). Use of hypnotics and anti-depressants was increased in the year after diagnosis. Relative 1-year mortality risk was 3-fold higher in the sleep disorder group than in controls, with a noticeably higher proportion of deaths due to suicide.
Conclusion: The fact that sleep disorders were associated with several morbidities, most strongly with psychiatric disorders as well as with increased mortality, underscores the importance of sleep problems as indicators of health status.
Sleep problems are common in the general population. One of the earliest epidemiologic studies on sleep disorders was conducted in Los Angeles in the late 1970s: a survey of over 1000 households that were representative of the United States general population revealed a prevalence of 32.2% for current insomnia.1 In addition, 7.1% of respondents reported excessive sleep, either past or current.1 A national survey in the United States—the 2002 Sleep in America poll—aimed to assess sleep habits and their relation to daytime performance among a random sample of 1000 adults.2 Overall, 27% of respondents characterized their sleep quality as less than good in the past year. More than one third of all respondents reported that, at least a few days a month, they were so sleepy during the day that it interfered with their daily activities. In a health survey conducted in 8580 people in the United Kingdom, insomnia was reported by 37% of respondents.3 A recent review of epidemiologic studies on insomnia suggests that about 33% of the population suffers from symptoms of insomnia.4 If frequency and severity cutoffs are included, the prevalence is about 16% to 21% and 10% to 28%, respectively.4 It has been estimated that, in total, 70 million Americans suffer from a sleep problem, adding about $15.9 billion per year to the national health care bill.5
Among the existing epidemiologic studies, few have examined the frequency of occurrence of new sleep disorder cases. A study6 conducted in young adults in Michigan between 1989 and 1992 obtained a 3.5-year incidence for new insomnia of 14.8% in women and 10.6% in men. The lifetime prevalence of insomnia was 16.6%, and that of hypersomnia, 8.2%. A 3-year follow-up study7 in individuals aged 65 years and older reported chronic difficulty falling asleep or early morning arousal in 15% of the participants who had no symptoms of insomnia at baseline.
The management of sleep disorders remains a complex challenge. The category of sleep disorder encompasses over 70 diagnoses that cross the boundaries of many therapeutic areas. Some sleep disorders, such as sleep apnea, are relatively well characterized and understood. The challenge is how to manage the majority of patients with less well-characterized sleep problems. The task of the physician when patients present with sleep problems is to try to decide whether there is a significant medical disorder underlying their sleep disturbance or whether the problems are due to some relatively minor situational disturbance: for example, worry, extraneous noise, excessive caffeine intake, or repeated international air travel. Data evaluating potential risk factors for and complications of sleep disorders are limited: while a number of studies have shown that a particular disease disturbs sleep, few have evaluated the relative contribution of different potential risk factors. Epidemiologic studies providing data on the diagnosis of sleep problems are especially scarce.4
The aim of this retrospective study was to determine the incidence of sleep disorder diagnosis in primary care, to describe the demographic and comorbid characteristics of patients with sleep disorders, and to compare subsequent treatment patterns and mortality over a 1-year follow-up period.
METHOD
Data Source
Data for this study were extracted from the General Practice Research Database (GPRD). The GPRD is the world's largest longitudinal primary care database and contains information entered by about 1500 primary care physicians, covering a population of about 3 million individuals who are broadly representative of the U.K. population.8,9 Participant physicians hold the complete medical records of all individuals registered with them, including demographics, diagnoses (recorded using Oxford Medical Information Systems [OXMIS] and Read codes), prescriptions, and referrals. This information is anonymized and sent to the Medicines and Healthcare products Regulatory Agency (MHRA), which is in charge of quality control and data management for use in research projects. Data from the GPRD have been validated in several studies.10,11
Study Cohort
All patients aged 20 to 79 years registered in the GPRD database with a recorded diagnosis of a sleep disorder during the year 1996 were identified and pooled. Codes for sleep disorder covered specific diagnoses, such as “insomnia” and “hypersomnia,” as well as less specific diagnoses, such as “sleep disturbance” (Table 1). Patients with sleep apnea and sleep-related respiratory failure were not included (Table 2). Only patients registered with a general practitioner for at least 2 years and having at least 1 entry in the GPRD in the previous 3 years were included. Patients with a consultation for any sleep disorder during the 2 years before the start of the study (January 1, 1996) were excluded. Patients with cancer before 1996 were excluded, as were women who were pregnant during the year before or year after the start date (final study cohort: N = 12,437). The date of diagnosis of the sleep disorder was assigned as the index date. We also identified an age- and sex-matched control group without a diagnosed sleep disorder or related diagnosis (N = 18,350) from the same source cohort using the same exclusion criteria as the sleep disorder group and assigned a random day during 1996 for use as the index date.
Table 1.
Sleep Disorder Codes Used to Define the Study Cohort
Table 2.
Codes for Sleep-Related Symptoms Not Used to Ascertain the Study Cohort
Data Collection
Demographic data and information on risk factors (smoking status, body mass index, and alcohol intake) were collected from computer records. We also collected information on morbidity and the number of consultations, admissions, and referrals in the 12 months before the index date; prescription medication use in the 12 months before and 12 months after the index date; and mortality in the 12 months after the index date. Cause of death was ascertained after manual review of patient profiles of deceased patients in both groups.
Statistical Analysis
The incidence of a sleep disorder diagnosis was calculated as the number of patients with a new diagnosis of sleep disorder divided by the total number of patient-years in each age and sex stratum. Logistic regression analysis was used to compute odds ratios (OR) and 95% confidence intervals (CI) for patients' demographic characteristics, health care use, comorbid conditions, and drug prescriptions. All risk estimates were adjusted for age and sex and for the number of visits to the physician in the previous year. We estimated 1-year mortality in both groups.
RESULTS
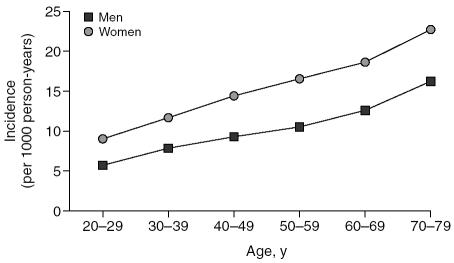
We identified 12,437 patients with a recorded code for a sleep disorder in the study year who had been free of diagnosed sleep disorders in the previous 2 years. More than half (61%) were women. The mean age at diagnosis was 51.7 years (standard deviation [SD] = 15.7) in men and 53.3 years (SD = 15.3) in women. The overall incidence for a new diagnosis of sleep disorder in U.K. primary care settings was 12.5 per 1000 person-years. The incidence was greater in women (15.4 per 1000 person-years) than in men (9.7 per 1000 person-years). It increased with increasing age throughout adulthood and old age in both men and women, though the rate of increase with age was greater among women than men (Figure 1). In the 20- to 29-year age group, the incidence of sleep disorders per 1000 person-years was 5.6 for men and 8.9 for women. In the 70- to 79-year age group the incidence per 1000 person-years increased to 16.3 for men and 22.7 for women.
Figure 1.
The Incidence of Sleep Disorders, Calculated According to Age and Sex
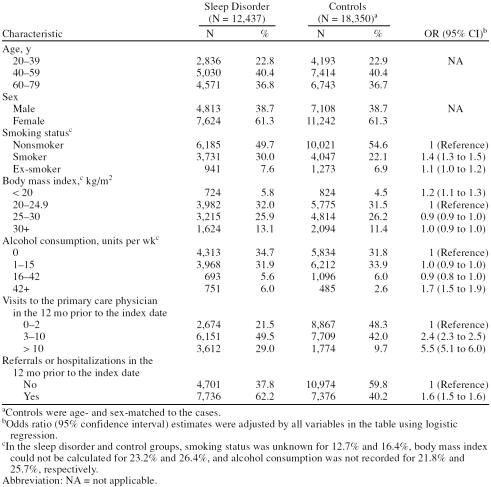
Demographic characteristics of patients with a sleep disorder diagnosis and the control group are presented in Table 3. Smoking (OR = 1.4, 95% CI = 1.3 to 1.5) and excessive alcohol consumption (more than 42 units per week) (OR = 1.7, 95% CI = 1.5 to 1.9) were associated with an increased risk of sleep disorders. In contrast, body mass index did not correlate with an increased risk of sleep disorders. In the 12 months before the index date, patients in the sleep disorder group were more likely than those in the control group to have consulted a primary care physician more than twice. Patients with sleep disorders were also more likely than controls to have been referred or hospitalized in the year prior to the index date.
Table 3.
Demographic and Clinical Characteristics of the Sleep Disorder and Control Groups and Their Association With a New Sleep Disorder Diagnosis
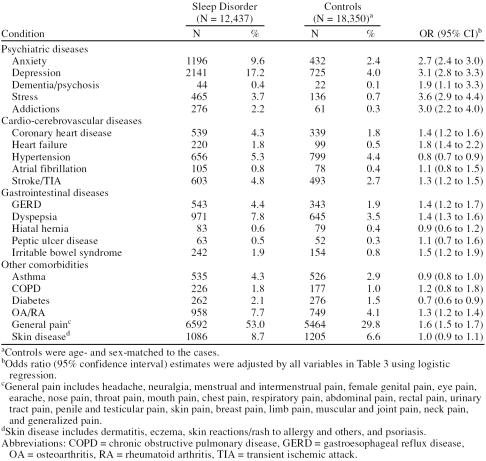
Diagnoses of comorbid conditions were collated for the 12 months before the index date according to anatomical systems. Diagnoses were more common in the sleep disorder group than in the control group in all 11 categories examined. Differences were most marked for psychiatric disorders (26% vs. 6%), followed by central nervous system disorders (4% vs. 2%), peripheral nervous system disorders (4% vs. 2%), and digestive diseases (30% vs. 15%). Other morbidities more commonly diagnosed in the group with sleep disorders than in the control group were circulatory (16% vs. 9%), respiratory (37% vs. 25%), skin (22% vs. 14%), endocrine (7% vs. 4%), genitourinary (18% vs. 11%), musculoskeletal/ connective (16% vs. 9%), and eye (12% vs. 9%). Diagnoses were also collated for the 12-month period after the index date using the same categories, with mostly very similar results as for the 12 months before the index date (results not shown).
Because an analysis of all comorbidities was beyond the scope of the current study, we focused on some of the more frequently encountered diagnoses that were noticeably more common in the group with sleep disorders than in the control group: logistic regression analyses were performed to assess the risk of psychiatric, cardio-cerebrovascular, and gastrointestinal diseases in patients with sleep disorders and in the control group (Table 4). The risk of asthma, chronic obstructive pulmonary disease, diabetes, osteoarthritis/rheumatoid arthritis, general pain, and skin disease was also assessed. All risk estimates were adjusted for age, sex, body mass index, smoking status, alcohol use, and number of primary care consultations in the 12 months before the index date. Among all diagnoses studied, stress was most strongly associated with a sleep disorder (OR = 3.6, 95% CI = 2.9 to 4.4). Depression, addictions, anxiety, and dementia/psychoses were also significantly associated with sleep disorders. Among patients with prior gastrointestinal diseases, those with gastroesophageal reflux disease (GERD) (OR = 1.4, 95% CI = 1.2 to 1.7), dyspepsia (OR = 1.4, 95% CI = 1.3 to 1.6), or irritable bowel syndrome (IBS) (OR = 1.5, 95% CI = 1.2 to 1.9) were at increased risk of sleep problems. Similarly, prior circulatory conditions, such as heart failure (OR = 1.8, 95% CI = 1.4 to 2.2), coronary heart disease (OR = 1.4, 95% CI = 1.2 to 1.6), and a recent ce-rebrovascular event (OR = 1.3, 95% CI = 1.2 to 1.5) were associated with an increased incidence of a sleep disorder diagnosis. In contrast, no statistically significant associations were observed between sleep disorders and conditions such as atrial fibrillation, hiatal hernia, and skin diseases, among others. Furthermore, patients with hypertension (OR = 0.8, 95% CI = 0.7 to 0.9) or diabetes (OR = 0.7, 95% CI = 0.6 to 0.9) were less likely than controls to consult a primary care physician for sleep problems.
Table 4.
Morbidity in the 12 Months Prior to the Index Date and Its Association With a New Sleep Disorder Diagnosis
Among patients with sleep disorders, we ascertained changes in treatment pattern before and after the diagnosis. We observed that the proportion of patients in the sleep disorder cohort who used hypnotics increased more than 3-fold after the diagnosis, from 18.5% in the year before the diagnosis to 71.3% in the year after. A similar pattern, though less marked, was seen for antidepressants (42.5% after diagnosis vs. 28.6% before diagnosis). Prescriptions for anxiolytics (13.0% after diagnosis vs. 11.1% before diagnosis) and antipsychotics (13.5% after diagnosis vs. 11.7% before diagnosis) were also increased slightly following a diagnosis of sleep disorder. In contrast, the overall number of prescriptions for gastrointestinal medications remained broadly similar before and after a sleep disorder diagnosis, except for proton pump inhibitors, for which prescriptions increased slightly (9.4% after diagnosis vs. 8.2% before diagnosis). Prescriptions for all other drugs examined, including anti-hypertensives, antiarrhythmics, aspirin, nonsteroidal anti-inflammatory drugs, paracetamol, and oral steroids, remained broadly similar.
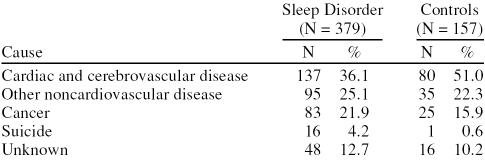
In the 12 months after a sleep disorder diagnosis, there were 379 deaths in the sleep disorder group compared with 157 deaths after the index date in the control group. The death rate per 100 person-years was 2.9 (95% CI = 2.6 to 3.2) in the sleep disorder group compared with 0.8 (95% CI = 0.7 to 0.9) in the control group. The 1-year mortality risk was approximately 3-fold higher in the sleep disorder group than it was in the control group when adjusted for age, sex, and primary care consultations in the previous year (relative risk: 2.9, 95% CI = 2.4 to 3.5). We found a noticeably higher proportion of deaths due to suicide in the sleep disorder group than in the control group (4.2% vs. 0.6%), mostly in patients with associated psychiatric diseases. There was also a higher proportion of deaths due to cancer in patients with sleep disorders (Table 5).
Table 5.
Distribution of Causes of Death in the First Year in Sleep Disorder and Control Groups
DISCUSSION
In our study, based on a source population of approximately 1.5 million individuals in U.K. primary care meeting the study criteria, we observed an overall incidence of new diagnoses of sleep disorder of 12.5 per 1000 person-years. These results show that approximately 1% of the U.K. population have a new consultation for a sleep disorder each year. A new diagnosis of insomnia does not, however, mean that the patient has a new sleep problem; the patient is only new to medical care in this system. A telephone survey conducted by the National Sleep Foundation in conjunction with the Gallup Organization in the United States suggests that individuals who have difficulty sleeping rarely visit a physician to discuss their sleep problems.12 Consequently, the incidence of diagnosis of sleep disorders is likely to underestimate the true incidence of sleep problems in the general population.
We detected an increase in incidence of sleep disorder diagnoses with increasing age: in women, the incidence was 2.5-fold higher in the 70- to 79-year age group than the 20- to 29-year age group, and in men it was 2.9-fold higher. Women were more likely than men to have a sleep disorder diagnosed, with the incidence being approximately 1.5-fold higher in women than in men in all age groups. Several prevalence studies13–15 have observed a similar trend in the distribution of sleep disorders, reporting an increase in prevalence with increasing age and a higher prevalence in women than in men. Interestingly, previous studies have shown that there is no or only a slight increase in the prevalence of sleep problems in healthy elderly,4 which suggests that the increase in sleep problems with age is secondary to an increase in other morbidities.
As part of a nested case-control analysis, we compared the demographic characteristics of patients with a new consultation for a sleep disorder with those in a control group consisting of an age- and sex-matched sample of patients without a sleep disorder. Patients with a sleep disorder were more likely to be smokers or to consume large quantities of alcohol (more than 42 units per week). Alcohol is a central nervous system depressant that is often used as a sleeping aid in the general population.12 However, it also causes disruption in the second half of the sleep period and, while prolonged use of alcohol at bedtime loses its effects on sleep onset, the effect on sleep disruption remains.4 Several previous sleep prevalence studies4,12 have also observed an association of alcohol dependence and smoking with sleep problems. We did not detect an association between sleep disorders and body mass index in our study. By contrast, 2 previous studies16,17 have reported an association between sleep problems and obesity in adults over the age of 60 years; this may be secondary to the effects of a sedentary lifestyle on both sleep and body weight in older adults.18
Patients with sleep disorders were more likely than controls to have other comorbid conditions. It should be noted, however, that because the patients self-present with sleep complaints, the role of comorbid disorders is likely to be particularly important and they may be more prevalent in this population than they would be in the general population. In the 12 months before the index date, diagnoses were more common in the sleep disorder group than in the control group in all 11 anatomical system categories examined. Similar results were obtained for the 12 months after the index date, suggesting that at least some patients with sleep disorders have serious underlying health problems. These problems are likely to have contributed to the approximately 3-fold higher 1-year mortality risk that we observed in the group with sleep disorders compared with that of the control group.
The strongest associations were observed between sleep disorders and prior psychiatric disorders. Patients with stress, depression, addictions, and dementia were up to 3.6 times more likely than controls to have a new consultation for a sleep disorder. This trend is also reflected in the increased prescribing of hypnotics and antidepressants that we observed in the year after diagnosis in patients with sleep disorders. The role of psychological factors, particularly depression, in sleep disorders is well documented. Insomnia, sleeplessness, excessive sleepiness, and fatigue are common symptoms of depressed patients.19 Current sleep disturbance may also be a predictor of future depression.6,20 Anxiety and stress have also been linked with sleep disorder,21,22 as have dementias.23
The proportion of deaths due to suicide was 7-fold higher in the sleep disorder group than in the control group in the year after the index date, mainly due to suicides in patients with associated psychiatric diseases. Of the 379 deaths in the sleep disorder group, 16 (4.2%) were due to suicide compared with 1 (0.6%) of 157 deaths in the control group. Psychiatric disorders such as depression are associated with suicidal behavior.24 A study conducted in 2 large samples of insomniac patients in the United States reported a 4-fold higher rate of attempted suicide in patients with sleep problems than in controls.25 A recent review of studies on alcoholism and suicidal behavior also showed that excessive alcohol use (observed in the current study to be associated with sleep disorders) is also associated with a considerable risk of suicide.26
In addition to prior psychiatric disorders, we also observed significant associations of sleep disorders with prior gastrointestinal diseases, such as GERD, dyspepsia, and IBS. The association of sleep disorders with GERD may be reflected in the slight increase in prescription of proton pump inhibitors in the year after diagnosis in patients with sleep disorders. GERD, particularly nighttime heartburn, is generally acknowledged as an important cause of sleep disorders.27–29 A nationwide U.S. telephone survey on behalf of the American Gastroenterological Association conducted among adults experiencing heartburn at least once a week found that 75% of those with nighttime heartburn (79% of participants) reported that symptoms affected their sleep.30 Moreover, nighttime GERD may be associated with an increased risk of serious esophageal and respiratory complications due to longer esophageal acid clearance and acid-mucosal contact times during nocturnal hours.27 The association of sleep disorders with IBS and dyspepsia observed in our study may help to resolve previously reported inconsistencies, with one study seeing a significant association with IBS (but not dyspepsia)31 and another observing an association with dyspepsia (but not IBS).32
We also observed significant associations of sleep disorders with prior circulatory diseases, including coronary heart disease, heart failure, and stroke/transient ischemic attack. Several studies have linked coronary heart disease to sleep disorders that are secondary to obstructive sleep apnea (reviewed in Ancoli-Israel33). However, patients with sleep apnea and sleep-related respiratory failure were not included in the present study. In line with our observations, a review of several epidemiologic studies that excluded patients with sleep apnea reported an explicit association between the remaining sleep complaints and coronary heart disease.34 Higher quality studies showed risk ratios of 1.5 to 3.9 between trouble falling asleep and coronary events. The authors concluded that it was not possible to ascertain whether there was a direct causal link between sleep disorders and coronary heart disease or whether the 2 morbidities simply formed part of a larger syndrome with common risk factors.
Our study has several strengths. Most importantly, the very large base population of approximately 1.5 million primary care patients makes the observed associations generally applicable, as well as increases statistical precision and minimizes the opportunity for bias. In addition, because sleep disorders were identified by physician diagnoses rather than by patient self-reports, our findings are applicable to primary care. Most of the published literature examines 1 or other selected cause of sleep problems rather than the totality of sleep disorders. In our study based on the U.K. GPRD, we aimed to capture all sleep problems, from the very specific to the mild, unspecific sleep disturbances. We also examined a wide range of morbidities for their associations with sleep disorders. This allowed us to assess the relative importance that specific diseases had with respect to sleep disorders as a whole.
Our study also had corresponding limitations. The basis of diagnosis, including symptomatology, was unknown. Due to the time constraints of modern-day medicine, we do not know much about the quality and consistency of the diagnoses. Data on the success (or otherwise) of different treatment approaches were also not available. Lastly, because there was not a structured assessment of all possible associated factors, some may have been missed.
In conclusion, our study underscores the importance of a sleep complaint as an indicator of the health status of the patient. However, although sleep problems are very sensitive to the general well-being of the patient, they are not very specific regarding the associated morbidities. Sleep disorders are associated with several morbidities, mainly psychiatric disorders but also gastrointestinal and circulatory diseases, and therapeutic options are available for many patients with these potential underlying causes of sleep disorders. The impact of treatment on outcomes and the overall long-term outcomes of patients with sleep disorders warrant further research.
Footnotes
The study was supported by a research grant from AstraZeneca. Drs. Anja Becher and Chris Winchester from Oxford PharmaGenesis provided editorial assistance on behalf of AstraZeneca.
Drs. Wallander and Johansson are employees of AstraZeneca R & D. Drs. Ruigómez and García Rodríguez work for CEIFE, which has received research grants from AstraZeneca. Dr. Jones has held consultancies with and has received grant/research support and honoraria from AstraZeneca, Reckitt Benckiser, Altana, and Pfizer.
REFERENCES
- Bixler EO, Kales A, and Soldatos CR. et al. Prevalence of sleep disorders in the Los Angeles metropolitan area. Am J Psychiatry. 1979 136:1257–1262. [DOI] [PubMed] [Google Scholar]
- National Sleep Foundation. 2002 Sleep in America Poll. Available at: http://www.sleepfoundation.org/site/c.huIXKjM0IxF/b.2417355/k.143E/2002_Sleep_in_America_Poll.htm. Accessed Apr 12, 2007. [Google Scholar]
- Stewart R, Besset A, and Bebbington P. et al. Insomnia comorbidity and impact and hypnotic use by age group in a national survey population aged 16 to 74 years. Sleep. 2006 29:1391–1397. [DOI] [PubMed] [Google Scholar]
- Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev. 2002;6:97–111. doi: 10.1053/smrv.2002.0186. [DOI] [PubMed] [Google Scholar]
- National Center on Sleep Disorders Research. 1994. Available at: http://www.nhlbi.nih.gov/health/prof/sleep/sleep.txt. Accessed Feb 27, 2006. [Google Scholar]
- Breslau N, Roth T, and Rosenthal L. et al. Sleep disturbance and psychiatric disorders: a longitudinal epidemiological study of young adults. Biol Psychiatry. 1996 39:411–418. [DOI] [PubMed] [Google Scholar]
- Foley DJ, Monjan AA, and Izmirlian G. et al. Incidence and remission of insomnia among elderly adults in a biracial cohort. Sleep. 1999 22suppl 2. S373–S378. [PubMed] [Google Scholar]
- García Rodríguez LA, Pérez Gutthann S. Use of the UK General Practice Research Database for pharmacoepidemiology. Br J Clin Pharmacol. 1998;45:419–425. doi: 10.1046/j.1365-2125.1998.00701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Office for National Statistics. Key Health Statistics from General Practice 1996: Analyses of Morbidity and Treatment Data, Including Time Trends, England and Wales. Series MB6 No. 1. London, England: Office for National Statistics. 1998 [Google Scholar]
- Jick H, Jick SS, Derby LE. Validation of information recorded on general practitioner based computerised data resource in the United Kingdom. BMJ. 1991;302:766–768. doi: 10.1136/bmj.302.6779.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jick SS, Kaye JA, and Vasilakis-Scaramozza C. et al. Validity of the general practice research database. Pharmacotherapy. 2003 23:686–689. [DOI] [PubMed] [Google Scholar]
- Ancoli-Israel S, Roth T. Characteristics of insomnia in the United States: results of the 1991 National Sleep Foundation Survey, 1. Sleep. 1999 22suppl 2. S347–S353. [PubMed] [Google Scholar]
- Klink M, Quan SF. Prevalence of reported sleep disturbances in a general adult population and their relationship to obstructive airways diseases. Chest. 1987;91:540–546. doi: 10.1378/chest.91.4.540. [DOI] [PubMed] [Google Scholar]
- Buscemi N, Vandermeer B, and Friesen C. et al. Manifestations and management of chronic insomnia in adults. Evid Rep Technol Assess (Summ). 2005 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman AB, Enright PL, and Manolio TA. et al. Sleep disturbance, psychosocial correlates, and cardiovascular disease in 5201 older adults: the Cardiovascular Health Study. J Am Geriatr Soc. 1997 45:1–7. [DOI] [PubMed] [Google Scholar]
- Ohayon MM, Vecchierini MF. Normative sleep data, cognitive function and daily living activities in older adults in the community. Sleep. 2005;28:981–989. [PubMed] [Google Scholar]
- Foley D, Ancoli-Israel S, and Britz P. et al. Sleep disturbances and chronic disease in older adults: results of the 2003 National Sleep Foundation Sleep in America Survey. J Psychosom Res. 2004 56:497–502. [DOI] [PubMed] [Google Scholar]
- Morgan K. Daytime activity and risk factors for late-life insomnia. J Sleep Res. 2003;12:231–238. doi: 10.1046/j.1365-2869.2003.00355.x. [DOI] [PubMed] [Google Scholar]
- Doghramji K. Treatment strategies for sleep disturbance in patients with depression. J Clin Psychiatry. 2003 64suppl 14. 24–29. [PubMed] [Google Scholar]
- Livingston G, Blizard B, Mann A. Does sleep disturbance predict depression in elderly people? a study in inner London. Br J Gen Pract. 1993;43:445–448. [PMC free article] [PubMed] [Google Scholar]
- Owens JF, Matthews KA. Sleep disturbance in healthy middle-aged women. Maturitas. 1998;30:41–50. doi: 10.1016/s0378-5122(98)00039-5. [DOI] [PubMed] [Google Scholar]
- Papadimitriou GN, Linkowski P. Sleep disturbance in anxiety disorders. Int Rev Psychiatry. 2005;17:229–236. doi: 10.1080/09540260500104524. [DOI] [PubMed] [Google Scholar]
- Bliwise DL. Sleep disorders in Alzheimer's disease and other dementias. Clin Cornerstone. 2004 6suppl 1A. S16–S28. [DOI] [PubMed] [Google Scholar]
- Bertolote JM, Fleischmann A, De Leo D. Psychiatric diagnoses and suicide: revisiting the evidence. Crisis. 2004;25:147–155. doi: 10.1027/0227-5910.25.4.147. [DOI] [PubMed] [Google Scholar]
- Kales JD, Kales A, and Bixler EO. et al. Biopsychobehavioral correlates of insomnia, 5: clinical characteristics and behavioral correlates. Am J Psychiatry. 1984 141:1371–1376. [DOI] [PubMed] [Google Scholar]
- Sher L. Alcoholism and suicidal behavior: a clinical overview. Acta Psychiatr Scand. 2006;113:13–22. doi: 10.1111/j.1600-0447.2005.00643.x. [DOI] [PubMed] [Google Scholar]
- Orr WC, Heading R, and Johnson LF. et al. Review article: sleep and its relationship to gastro-oesophageal reflux. Aliment Pharmacol Ther. 2004 20suppl 9. 39–46. [DOI] [PubMed] [Google Scholar]
- Shaker R, Brunton S, and Elfant A. et al. Review article: impact of night-time reflux on lifestyle—unrecognized issues in reflux disease. Aliment Pharmacol Ther. 2004 20suppl 9. 3–13. [DOI] [PubMed] [Google Scholar]
- McGuigan JE, Belafsky PC, and Fromer L. et al. Review article: diagnosis and management of night-time reflux. Aliment Pharmacol Ther. 2004 20suppl 9. 57–72. [DOI] [PubMed] [Google Scholar]
- Shaker R, Castell DO, and Schoenfeld PS. et al. Nighttime heartburn is an under-appreciated clinical problem that impacts sleep and daytime function: the results of a Gallup survey conducted on behalf of the American Gastroenterological Association. Am J Gastroenterol. 2003 98:1487–1493. [DOI] [PubMed] [Google Scholar]
- Vege SS, Locke GR 3rd, and Weaver AL. et al. Functional gastrointestinal disorders among people with sleep disturbances: a population-based study. Mayo Clin Proc. 2004 79:1501–1506. [DOI] [PubMed] [Google Scholar]
- Fass R, Fullerton S, and Tung S. et al. Sleep disturbances in clinic patients with functional bowel disorders. Am J Gastroenterol. 2000 95:1195–2000. [DOI] [PubMed] [Google Scholar]
- Ancoli-Israel S. The impact and prevalence of chronic insomnia and other sleep disturbances associated with chronic illness. Am J Manag Care. 2006 12suppl 8. S221–S229. [PubMed] [Google Scholar]
- Schwartz S, McDowell Anderson W, and Cole SR. et al. Insomnia and heart disease: a review of epidemiologic studies. J Psychosom Res. 1999 47:313–333. [DOI] [PubMed] [Google Scholar]