Abstract
Local delivery of drugs to the inner ear is increasingly being used in both clinical and experimental studies. Although direct injection of drugs into perilymph appears to be the most promising way of administering drugs quantitatively, no studies have yet demonstrated the pharmacokinetics in perilymph following direct injections. In this study, we have investigated the retention of substance in perilymph following a single injection into the basal turn of scala tympani (ST). The substance injected was a marker, trimethylphenylammonium (TMPA) that can be detected in low concentrations with ion-selective microelectrodes. Perilymph pharmacokinetics of TMPA was assessed using sequential apical sampling to obtain perilymph for analysis. The amount of TMPA retained in perilymph was compared for different injection and sampling protocols. TMPA concentrations measured in fluid samples were close to those predicted by simulations when the injection pipette was sealed into the bony wall of ST but were systematically lower when the injection pipette was inserted through the round window membrane (RWM). In the latter condition it was estimated that over 60% of the injected TMPA was lost due to leakage of perilymph around the injection pipette at a rate estimated to be 0.09 μL/min. The effects of leakage during and after injections through the RWM were dramatically reduced when the round window niche was filled with 1% sodium hyaluronate gel before penetrating the RWM with the injection pipette. The findings demonstrate that in order to perform quantitative drug injections into perilymph, even small rates of fluid leakage at the injection site must be controlled.
Keywords: drug delivery, round window membrane, perilymph, pharmacokinetics, inner ear
Introduction
At present, there is considerable interest in methods for local delivery of drugs to the inner ear. Although many substances can be applied to the round window membrane (RWM) and from there diffuse into perilymph, large or anionic molecules do not easily permeate the RWM (Goycoolea and Lundman, 1997). In addition, pharmacokinetic studies suggest that there is considerable variation in the permeability of the RWM in different animals, resulting in highly variable drug levels in perilymph (Chelikh et al., 2003, Hahn et al., 2006, Salt et al., 2006, Mynatt et al, 2006, Plontke et al, 2007). To overcome these problems, an alternative approach is to inject the drug or other substance directly into the inner ear fluids. In animals, this has been performed as single injections (Stover et al., 1999, Wareing et al., 1999, Stover et al., 2000, Praetorius et al., 2007) or over a period of days with the use of mini-osmotic pumps (e.g. Brown et al., 1993, Miller et al., 1997, Nair et al., 1997, Wareing et al., 1999, Wise et al., 2005) or with custom injection devices (Chen et al., 2005). In none of these studies was the concentration of substance achieved in the perilymph reported. Substance distribution has been quantified in plastic models with dimensions approximating the human ST (Paasche et al., 2006). In some studies delivering drugs through cochleostomies, substantial damage to hearing at frequencies near the cochleostomy site was reported (Carvalho and Lalwani, 1999) while minimal functional losses have been found with other direct injection techniques (Chen et al., 2005). The present study is focused on developing methods for applying drugs directly into the ear with minimal trauma and leakage. TMPA was injected into perilymph by different techniques and the amount of TMPA retained in the cochlea and its spatial distribution along the cochlea was measured by sampling perilymph and measuring TMPA concentrations in the fluid samples.
Methods
i) Animal Preparation
Injections into perilymph were performed as acute experiments in 24 NIH strain pigmented guinea pigs, anesthetized with an initial dose of 100 mg/kg sodium thiobutabarbital (Inactin, Sigma, St. Louis, MO). Anesthetic was supplemented as required through a cannula in the external jugular vein. Pancuronium bromide (Pavulon, Baxter, Irvine, CA) was given as a muscle relaxant and animals were artificially ventilated via a tracheal cannula. End-tidal CO2 was maintained near 38 mm Hg (5%). Heart rate and vascular pO2 were monitored with a Surgivet pulse-oximeter (Waukesha, WI). Rectal temperature was maintained at 38 °C with a thermistor-controlled heating blanket. Animals were held in a head-holder and the auditory bulla was exposed by a ventral approach, allowing access to the cochlea. The experimental protocols for this study were approved by the Animal Studies Committee of Washington University (Approval number 20040209).
ii) Injections of TMPA into Perilymph
A 2 mM solution of TMPA in a background of artificial perilymph was injected into ST perilymph of the basal turn using a 10 μL gas tight syringe (#1701, Hamilton, Reno, NV), mounted on an Ultrapump (UMP2, WPI, Sarasota, FL) held in a micromanipulator. An electrode-mounting adaptor (MPH6S-1.0, WPI, Sarasota, FL) was attached with cyanoacrylate glue to the tip of the syringe, allowing 1 mm OD (outer diameter) glass injection pipettes to be mounted on the syringe. The pipettes were pulled with an electrode puller and beveled at a 45° angle (EG-40 pipette grinder, Narishige, Tokyo, Japan) to obtain a tip OD of approximately 20 μm. The injections were performed at a rate of 100 nl/min and lasted 20 min, resulting in a total injected volume of 2 μL. The artificial perilymph contained (in mM): NaCl: 127.5; KCl: 3.5; NaHCO3: 25; MgCl2: 1.2; CaCl2: 1.3; NaH2PO4; 0.75. TMPA injections followed one of three protocols:
1) Injection through the wall of scala tympani (“Wall”)
Sealing an injection pipette into scala tympani was accomplished by first removing the mucosa from the bone and then thinning the bone at the site to be perforated by shaving with a blade. A thin layer of cyanoacrylate adhesive was applied to the dry, thinned bone, followed by a layer of two-part silicone (Kwik-Cast, WPI, Sarasota, FL). The silicone was spread away from the site to be perforated, forming a shallow ring around the site. A fenestration (approximately 50 μm diameter) was made through the adhesives and the bone with a fine pick after which the glass injection pipette was inserted. Fluid emerging around the pipette formed a bead on the silicone surface that was removed with a wick an instant before cyanoacrylate was applied to the insertion site. In this manner, a verifiable, fluid-tight seal could be achieved. The distance of the fenestration from the lip of the round window (RW), was measured along the external wall of the scala using a calibrated eyepiece reticule in the operating microscope. The average distance was 1.93 mm (SD 0.23, n=6).
2) Injection through the round window membrane with no Healon (“RW”)
A fine-diameter (20 μm), beveled injection pipette was advanced slowly while viewing with the operating microscope until it contacted the RWM. The membrane could be seen to be indented by the pipette. The pipette was then advanced further until the membrane “popped back”, confirming that the pipette tip had entered ST.
3) Injection through the round window membrane with Healon (“RW-Healon”)
The RW niche was filled with 1% sodium hyaluronate gel (Healon, Advanced Medical Optics, Santa Ana, CA) to an extent that the gel formed a flat meniscus at the opening of the niche. This provided a clear, undistorted view of the RWM. A fine-diameter, beveled injection pipette was advanced through the gel under visual observation until it was seen to “pop” through the membrane. Insertion sites close to the margin of the RWM were avoided as it was difficult to judge whether the membrane had been perforated. In all experiments, only a single pipette insertion was performed (i.e. it was not acceptable to withdraw the pipette and repeat the insertion).
iii) Fluid Sampling from the Cochlear Apex
It has been shown that sequentially-obtained samples from the cochlear apex can be interpreted in terms of the amount of drug in perilymph of ST and can be used to calculate the drug gradient along ST prior to sampling (Mynatt et al., 2006). The method is based on the rationale that if all the fluid from the apex is collected with no fluid loss to the middle ear then the sample composition, and, more importantly, the sites of origin of the samples from the cochlea can be determined. To collect fluid without loss to the middle ear, a silicone cup was constructed at the apex, as described elsewhere (Salt et al., 2006). At the time of perilymph sampling, the cochlear apex was perforated with a fine pick and the fluid that emerged into the silicone cup was collected into hand-held glass capillary tubes, marked at 1 μL volume. Ten samples, each nominally 1 μL in volume, were collected from each animal. The exact volume of each sample was determined by measuring the sample length in the capillary under a calibrated dissecting microscope. The mean sample volume was 1.06 μL (SD 0.07, n=240). The exact timing of each sample was documented with an audio recording made on the computer with Audacity software (http://audacity.sourceforge.net/). The mean time to collect each sample was 39 sec (SD 25, n=240). One of two sampling protocols were used in this study:
1) Sampling 30 min after the start of injection (“Sample30”)
Under this protocol, following the 20 min injection of TMPA, the injection pipette remained in place in the RWM for the remainder of the experiment, including during fluid sampling from the apex. Fluid sampling commenced 10 min after the injection was complete.
2) Sampling 40 min after the start of injection (“Sample40”)
In this case, the injection pipette was removed 5 min after the TMPA injection was complete and fluid sampling from the apex commenced 15 min after the injection pipette was withdrawn. This protocol was intended to represent an injection protocol that could be used for the one-time delivery of drugs to the cochlea in a manner that permitted long-term recovery of the animal to occur. In such experiments, the injection pipette must be removed after the drug application, potentially leaving an open perforation site through which fluid could leak. This contrasts with delivery systems (such as mini-osmotic pumps) that use indwelling catheters sealed into the scala.
Each group of experiments was designated as the TMPA injection procedure (Wall, RW or RW+Healon) combined with the sampling protocol (Sample30 or Sample40). The number of animals (in parentheses) for each group were Wall:Sample30 (6); RW:Sample30 (4); RW+Healon:Sample30 (4); RW:Sample40 (4); RW+Healon:Sample40 (6).
iv) Analysis of TMPA Content of Samples
After the volume of each sample had been determined, it was diluted in 25 μL of artificial perilymph. The TMPA levels of diluted samples and of similar volumes of known standards (0, 2, 20 μM TMPA) were measured in small wells in a Teflon block using double-barreled TMPA-selective microelectrodes. Ion-selective electrodes were made by pulling double-barreled glass pipettes and storing them in a cabinet at 70% humidity, 40 °C overnight. One barrel of the electrode was silanized by exposure to dimethyldichlorosilane vapor (Sigma, St. Louis, MO) after which it was baked at 140 °C for 1 hour. Electrode tips were beveled to produce diameters of 2-4 μm. The ion barrel was filled with 500 mM KCl and the reference barrel was filled with 500 mM NaCl. A small column of TMPA-selective ion exchanger was drawn into the tip by suction. The ion exchanger consisted of 5% potassium tetrakis (4-chlorophenyl)borate (Fluka AG, Switzerland) in 2-nitrophenyloctylether (Sigma, St. Louis, MO) which was pre-equilibrated with aqueous TMPA solution prior to use. Electrodes were first calibrated in standards containing 0, 2, 20, 200 and 2000 μM TMPA in a background of artificial perilymph at room temperature, after which they were used to measure samples and standards in the Teflon block, as described above. The procedure involved measuring the standards and all the samples twice and then measuring the standards again for a third time. Readings in standards were used to correct for influences of electrode drift and sample evaporation.
v) Interpretation of Sequential Sample Data
The concentrations of the ten samples obtained in each experiment were interpreted using a finite-element cochlear fluids simulation program, available for download at http://oto.wustl.edu/cochlea/, and modified to incorporate algorithms for fluid sampling from the cochlear apex. The program was used to simulate drug spread during and following injection, and during sequential sampling from the apex. The simulation of sampling took into account the fluid flows associated with the specific volume and time of each sample. The diffusion coefficient used for TMPA was 1.01 × 10-9 m2/s. Model parameters were adjusted until the sample concentrations predicted by the model best fit the measured values. Fit was optimized by minimizing the sums of squares of differences between calculated and measured sample values. Statistical significance between experimental groups was assessed by t-tests for normally-distributed data or by Mann-Whitney rank sum tests for non normally-distributed data (Sigmastat, Sysat, San Jose, Ca).
Results
The TMPA concentrations in perilymph achieved by injections from a pipette sealed into the bony wall of the basal turn are summarized in Figure 1. By summing the TMPA content and volume of the 10 samples in each experiment, relative to the amount of TMPA injected it was calculated that 37.9% (SD 9.9, n=6) of the injected TMPA was recovered in the samples. The same injection performed by inserting the pipette through the RWM resulted in considerably lower TMPA levels, as shown in the upper panel of Figure 2. In 3 of the 4 experiments, very low TMPA levels were present and the calculated variation was large. The mean amount of TMPA recovered, calculated by summing the 10 samples from each experiment was 11.9% (SD 10.8, n=4). The lower concentrations were thought to be primarily the result of perilymph leakage around the injection pipette. In an attempt to control this leakage, identical experiments were performed with the RW niche filled with Healon before inserting the injection pipette through the RWM. Measured TMPA levels were considerably higher in this group, as shown in the lower panel of Figure 2. The mean amount of TMPA recovered in this group was 33.8% (SD 5.0, n=4), which was significantly greater than the experiments performed in the absence of Healon (Mann Whitney rank sum test, p= 0.029). The finding that Healon gel could control perilymph leakage during drug injections through the RWM was encouraging but in these experiments the pipette remained in place during sampling. In order to assess whether Healon could control leakage after the pipette was withdrawn, leaving a perforation in the RWM, the pipette was withdrawn 5 min after the injection ended and sampling was performed 15 minutes later. The measured TMPA levels for this protocol, with and without Healon in the RW niche, are shown in Figure 3. In the absence of Healon, although TMPA was retained in two animals, the other two showed very low TMPA levels, resulting in a large calculated variation. The mean TMPA recovery was 12.1% (SD 12.6, n=4). In experiments with Healon in the RW niche, no animals were found with very low TMPA levels, resulting in a higher mean level, lower variation, and a mean TMPA recovery of 17.4% (SD 9.6, n=6). This was higher than in the absence of Healon, but the differences in TMPA recovered were not statistically significant.
Figure 1.

TMPA concentrations in 10 samples obtained sequentially from the apex in 6 animals following TMPA injection into the basal turn of scala tympani through a pipette sealed into the bony wall of the scala. Dotted lines represent individual experiments, with the mean and standard deviation shown as solid lines. The highest concentrations seen in samples 3 and 4 are in accordance with those samples originating from the basal part of the cochlea, where the TMPA injection occurred.
Figure 2.

TMPA concentration in 10 sequential samples from the apex following TMPA injection through the round window (RW) membrane in 4 animals without Healon in the RW niche (upper panel) or in 4 animals with Healon in the RW niche (lower panel). A more consistent, higher concentration of TMPA is achieved (i.e. higher curves) when healon is present in the RW niche. The low curves seen when no Healon was used is consistent with a fluid leak around the injection pipette washing away the injected TMPA.
Figure 3.
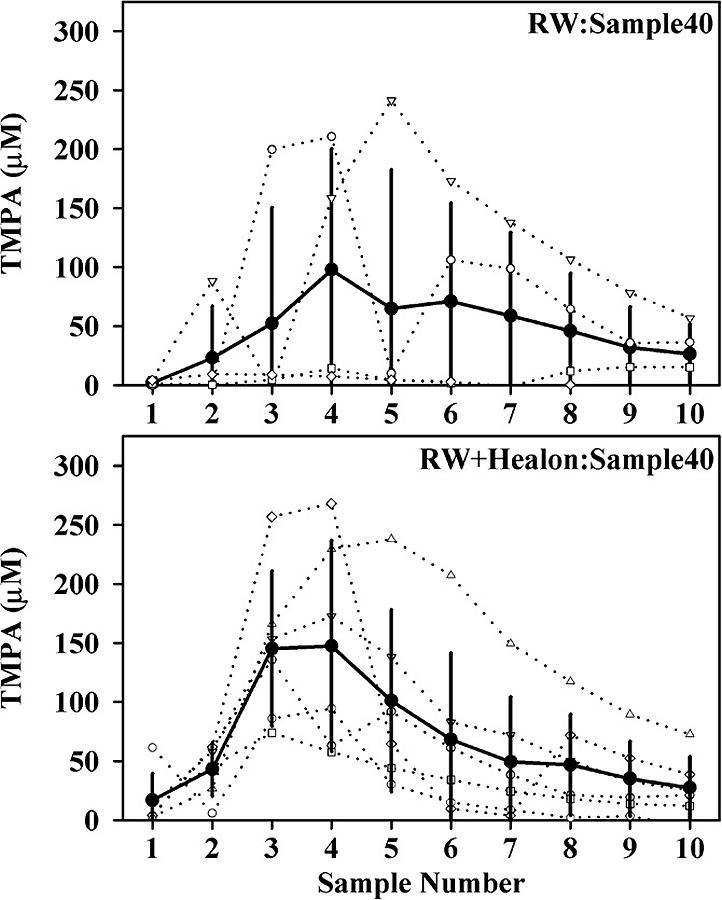
TMPA concentration of apical samples following TMPA injection through the round window (RW) membrane when the injection pipette was removed from the cochlea. Concentrations are shown for 4 experiments without Healon in the RW niche (upper panel) and in 6 experiments with Healon in the RW niche (lower panel). The incidence of low TMPA recovery was reduced with Healon present.
The sample curves from the three injection protocols were interpreted with the finite-element cochlear fluids simulator. In the absence of fluid leaks, the three parameters that dominate the distribution of TMPA during the application period were the rate of TMPA clearance from ST, the rate of perilymph flow along ST, and the accessibility of TMPA into fluid spaces parallel to ST, such as the spiral ligament and modiolus. For individual experiments, with injection through the wall of scala tympani (Wall:Sample30) model parameters were adjusted to best fit the experimental samples. The average parameters derived were: ST clearance half-time=38 min; Perilymph flow=0.027 μL/min; Access to parallel compartments 24% of free diffusion. The upper panel of Figure 4 shows the mean measured sample concentrations (solid circles; Wall:Sample30-Expt, taken from Figure 1) and the calculated samples based on these averaged parameters (open circles; Wall:Sample30-Model). The calculated curves give a reasonable approximation of the experimental data. Each of the individual experiments using RW application with Healon in the niche was modeled similarly. The results are compared in the upper panel of Figure 4 (solid squares; RW+Healon:Sample30-Expt, taken from Figure 2 and open squares; RW: Sample 30-Model). The average parameters derived for this condition were: ST clearance half-time=78 min; Perilymph flow=0.044 μL/min; Access to parallel compartments 18% of free diffusion. As the TMPA clearance half-time in this group was no shorter than for injections through the wall and clearance rates of the two groups were not significantly different (t-test, p = 0.27), there was therefore no evidence for increased leakage in this group and the model parameters derived from both groups were combined to represent the best estimate of pharmacokinetic parameters for TMPA in the absence of fluid leak. All subsequent simulations in which leaks were superimposed on TMPA dispersal used these mean parameters, derived from 10 experiments, which were: ST clearance half-time=49 min; perilymph flow=0.032 μL/min; access to parallel compartments 21% of free diffusion. The lower panel of Figure 4 shows the calculated sample concentrations for the three major injection/sampling protocols without any leaks superimposed using the mean parameter set. The differences between the three curves arise directly from the differences of injection locations and sampling times that must be considered in the interpretation of sample data. For the two RW injection protocols, sample concentrations are expected to be lower than for injections through the wall due to the more basal injection site. For the curve calculated with sampling at 40 min after RW injection (RW:Sample40), initial samples are higher than for sampling at 30 min (RW:Sample30), as the TMPA has additional time to diffuse further up the cochlea, but the peak is lower as more TMPA is lost by partitioning to other compartments. The predicted overall recovery of TMPA in the 10 samples for each protocol in the absence of fluid leakage were Wall:Sample30 (41.5%); RW:Sample30 (30.6%) and RW:Sample40 (28.2%).
Figure 4.

Upper panel: Mean experimental (solid) and calculated (open) sample concentrations for the two experimental protocols in which fluid leaks from the cochlea were not apparent (see text). Lower Panel: Calculated sample concentrations for the three experimental protocols using identical model parameters, demonstrating that TMPA distribution and amount are predicted to vary according to the exact protocol used.
The results from RW injections, with and without Healon present in the RW niche, are compared with simulations incorporating fluid leaks at specific rates in Figure 5. In each case, curves with solid symbols are the mean experimental curve. Curves with open symbols indicate sample concentrations with the indicated rate of fluid leak (μL/min) occurring at the site of injection. In the simulations, the fluid volume leaking was replaced by an equal volume of zero concentration fluid entering at the cochlear aqueduct, with appropriate volume flow calculated between the aqueduct and the site of leakage. For the 30 min sampling protocol, the experimental data in the absence of Healon was best approximated with a fluid leakage rate of 0.09 μL/min. In the presence of Healon, the experimental data were slightly greater than, and were therefore best approximated by the zero leak condition. For the 40 min sampling protocol, in which the injection pipette was removed, the results were qualitatively similar to the 30 min sampling protocol. For the no-Healon condition the mean curve was best approximated with a fluid leak of 0.06 μL/min. It should be noted that in the simulations of this protocol we used a single leak rate for the entire period, and did not change rate at the time the injection pipette was withdrawn. In the presence of Healon with this protocol, the data were best fitted with a leak of 0.03 μL/min. It therefore remains possible that leakage was occurring in some of the experiments with this protocol, probably when the injection pipette was removed.
Figure 5.

Open symbols: Computer simulations of the ten sequential sample concentrations produced by the two round window application protocols (upper panel RW:Sample 30; lower panel: RW sample 40) in which varying amounts of fluid leak from the injection site (indicated as “no Leak” or as the rate of leakage in uL/min). The calculated curves show that even small amounts of fluid leak will markedly influence the amount of TMPA recovered in the samples. Solid symbols: Mean curves from the experimental groups for the two RW application protocols with and without Healon in the round window niche, as indicated, taken from Figures 2 and 3. It can be seen that with healon present the TMPA recovered corresponds to that expected with little or no leak present. In the absence of healon, the data are more consistent with the existence of a small fluid leak from the scala.
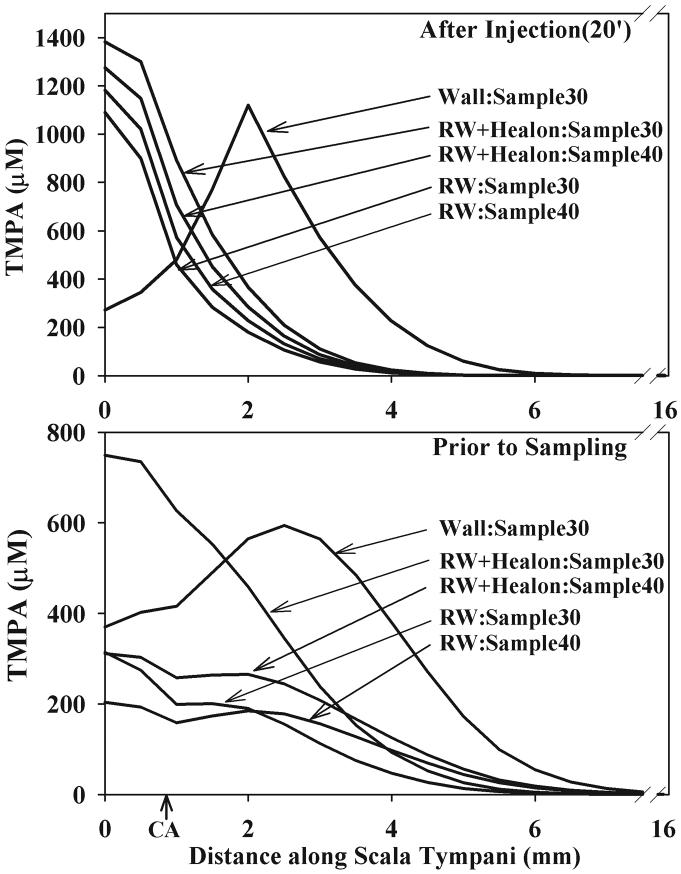
In order to relate the sample curves of the prior figures back to the concentration gradients existing along ST, Figure 6 shows the calculated concentration profiles for the volume leak parameters that best fit each experimental group. The profiles are given at the end of injection (upper panel) or immediately prior to sampling (30 or 40 min, depending on protocol). The differences in TMPA distribution following injection through the bony wall or through the RWM is apparent. It is also notable, that while we have demonstrated the influence of leaks on overall TMPA levels at the time of sampling, the influence on TMPA levels during injection is less, relative to the large changes of concentration with distance. At later times, the influence of leaks becomes cumulative and greater, while the longitudinal gradients become smaller.
Figure 6.
Calculated distribution of TMPA along scala tympani at two time points in which volume leak was adjusted to most closely match the mean sample curves for each experimental group. Note that only the basal half of scala tympani is shown, and the scaling of TMPA concentration differs in the upper and lower panels. CA: location of cochlear aqueduct entry.
Simulations of the sample data also allow the distribution of TMPA in different structures of the ear to be estimated for the different experimental conditions used here. Figure 7 shows the calculated distribution of TMPA before and after sampling for the three injection conditions performed with sampling at 30 min. The calculated amount of TMPA driven through the aqueduct into CSF during injection is greater for RW injections, as this injection site is closer to the aqueduct, but is reduced by the presence of a leak (compare “RW+Healon” and “RW” in the figure). The significance of the leak, comparing RW with and without Healon, is highly apparent. The influence of leak (with accompanying CSF entry at 0.09 μL/min) on TMPA entry into CSF during TMPA injection (at 0.1 μL/min) is underestimated by the present model as each of these flows is calculated independently with no interaction as would occur in the narrow aqueduct. As a result, the TMPA amount in CSF would be lower than shown and the amount lost to leakage would be greater if flow interactions in the aqueduct were included. It is also apparent that when samples are taken, the TMPA recovered in the samples is primarily from ST but also includes TMPA that has repartitioned into the scala from other compartments. Finally, the TMPA clearance to blood also plays an important role, but this clearance is highly solute-specific so this finding cannot be generalized to other drugs.
Figure 7.
Calculated distribution of TMPA that best accounts for the sample data for the 3 protocols in which samples were taken at 30 min. The proportion of TMPA in each compartment of the model is normalized with respect to the injected total. Compartment abbreviations are ST: scala tympani, CSF: cerebrospinal fluid, Other: fluid spaces of the cochlea not included in the scalae, such as the spiral ligament and modiolus, SV: scala vestibuli, V: vestibule. This analysis shows that lower concentrations in scala tympani are seen following round window injections because the RW injection site is closer to the cochlear aqueduct so that more of the injected TMPA is driven into the CSF through the aqueduct. The pronounced effect of volume leak from the injection site is clearly demonstrated.
Discussion
Sequential sampling of fluid from the cochlear apex (Salt et al, 2006; Mynatt et al., 2006) provides data from which the amount and distribution of substances along ST can be derived. As the total ST volume of the guinea pig is approximately 4.7 μL (Thorne et al., 1999), the act of taking samples that total approximately 10 μL from the apex requires consideration that not all the fluid obtained in this way is perilymph. Through computer modeling of the apical sample collection process, we have shown that the composition of samples can be interpreted in a highly quantitative manner.
In the present study, the apical sampling method was used to quantify the reliability of a drug delivery method that may be suitable for the application of drugs in experimental animals in long-term studies. It was demonstrated that direct injections through the RWM were compromised by the presence of minuscule fluid leaks around the injection pipette. The small leak allowed over 60% of the injected substance to be washed out of the cochlea (i.e. the magnitude of the “leak” component prior to sampling for the “RW” group in Figure 7). These leakages occurred even though we used small diameter (20 μm), sharpened injection pipettes, mounted in a manipulator to minimize lateral movements that could damage the insertion site. In some experiments without Healon, marker levels were consistent with there being little or no leak, while other experiments showed very low TMPA levels, consistent with leaks. Thus, it may be possible to “get lucky” and have drug retained in the cochlea of some animals without Healon, but the risk of substantial drug leakage is considerable. Our data show that TMPA retention in the cochlea was considerably improved with the use of Healon gel in the RW niche. In none of the experiments with Healon were very low TMPA concentrations found. This is consistent with control of the fluid leakage rate by Healon.
In prior studies, intracochlear injections through the RWM have been performed with hand-held syringes and 32-gauge needles (OD approximately 200 uM) (Stover et al., 1999), syringes with 36-gauge needles mounted on a manipulator (Praetorius et al., 2007) or glass pipettes mounted on a pump (Wareing et al., 1999). In one of these studies (Stover et al., 1999), it was reported that “no perilymph leakage was visible before closure of the middle ear”. In our experiments, fluid accumulation was observed to occur in the RW niche of some animals but there were examples in which the RWM appeared dry and in which TMPA recovered in the samples was low. It therefore appears impossible to visualize the low rate of fluid leak (approximately 0.1 μL/min) required to cause loss of injected substance from ST with an operating microscope. Our data in the absence of Healon further suggest that leakage rates were not increased dramatically when the injection pipette was removed. The apparently invisible leakage around the injection pipette seems to be the major factor causing TMPA leakage and this leakage is significantly reduced when the RW niche is filled with Healon. The ability of Healon to control leaks may be lower after pipette withdrawal, but leakage rates in this condition also appear to remain low.
The TMPA injections in this study were performed into the otherwise intact cochlea, with no fluid outlet provided. The low injection rate (100 nl/min) was intended to minimize pressure trauma. We previously reported that in guinea pigs injections into the sealed cochlea at 1.5 μL/min caused approximately 650 Pa pressure increase (Salt & Rask-Andersen, 2004). By extrapolation 100 nl/min would give 44 Pa, which is comparable to the background pressure fluctuations (40 Pa) associated with respiration seen in guinea pigs (Bohmer, 1993). This injection method was intended to be atraumatic to the cochlea and potentially of value for drug injections into perilymph in long-term experiments in which the animals recover after treatment. The prolonged (20 min) injection was intended to maximize the volume delivered while minimizing both local chemical disturbances near the injection site and the amount of drug being driven into CSF through the cochlear aqueduct. In some studies, the efflux of injected substance has been shown to influence the opposite cochlea (Stover et al., 2000) while others found no influence on the opposite cochlea (Wareing et al, 1999). The total volume injected is likely to be a major factor in such observations. Hyaluronate gels applied to the middle ear space are reported to have no toxic effects (Engstrom et al., 1987, Laurent et al., 1992, Sheppard et al., 2004). Studies investigating the functional consequences of the RW injection protocol reported here are now ongoing.
While the raw sample data give an indication of TMPA levels in ST, a more detailed interpretation is provided by computer simulations of the experiments. Small differences in application protocol can affect the amount and distribution of drug in ST. It was observed that injections through the RWM typically showed lower TMPA levels than for injections though the bony wall (e.g. Figure 4). Simulations demonstrated that the lower levels with RW injection are not only caused by the greater distance between the injection and sampling sites, but also by the injection site being closer to the cochlear aqueduct, so that more of the injected TMPA was driven into the CSF (Figure 7). Simulations also permit the influence of other protocol parameters not specifically studied here, such as the total volume injected and the duration of injection, to be evaluated.
For an intracochlear drug injection to be “successful”, the applied drug should remain in the cochlea for a reasonable amount of time. When substances are injected into the cochlea, they spread along the fluid compartments by diffusion, so that when the injection ends the concentration near the injection site is expected to decline with time. The substance may also be lost to other compartments (blood, CSF, etc), each contributing to the loss from the cochlear fluids. TMPA is a small molecule that is cleared from perilymph with a half time we estimated to be 49 min. It is necessary to consider drug losses directly associated with the injection procedures when they become significant relative to clearance by other processes. Our data show that a small amount of leakage around the injection pipette dominated the loss of TMPA. If the same leakage occurred with an injected drug that was cleared more slowly from cochlear fluids, the relative influence of the fluid leakage would have been even greater. In both 30 min and 40 min sampling groups without Healon the variability between experiments was high, with some animals showing close to the expected amount of TMPA present while others showed far lower levels. The source of this variability is explained by volume leakages around the injection pipettes, since it is reduced by the presence of Healon, but it remains uncertain why in some animals the injection pipette formed a seal while in others it did not. This did not represent a “learning curve” as experiments with and without Healon were overlapped and 30 and 40 min sampling protocols were performed sequentially. There are many factors that could influence the status of the penetration site and potential rate of fluid leakage that could not be controlled in the experiment. This includes mechanical movements, such as RWM movements caused by contractions of middle ear muscles, vibrations, variation in the rate of insertion of the pipette or in the compliance of the RWM, or possibly the location on the RWM penetrated. In addition, inter-animal differences in CSF pressure could also cause differences in leakage rate. It may be technically possible to control the pipette insertion in some manner that avoids the perilymph leakage that we found in most animals but, in this study, substantial control was achieved with the use of Healon.
The observations reported here may be relevant to the practice of applying drugs locally to the inner ear of patients as they demonstrate some of the technical difficulties that must be overcome in order to apply drugs quantitatively into the inner ear. With application of drugs into the RW niche, the RWM remains intact so the potential for fluid leaks and their influence on drug concentrations is avoided. When the RWM or the bone is perforated either by an injection pipette or, as in cases of cochlear implant insertion, then fluid leaks are potentially a problem. We have shown that even minuscule flow rates, invisible when viewed through the operating microscope, are sufficient to disturb intracochlear drug levels. Consideration of the magnitude of the fenestration, how the fenestration is “patched” and what time course of leakage occurs before the fenestration heals, may all play an important role in the drug level achieved. It may be possible that disturbances of drug levels by fluid efflux from the cochlea will be less of a problem in the human compared to the guinea pig as the cochlear aqueduct is narrower in the human. Nevertheless, quantitative drug delivery into the inner ear requires that all potential sources of fluid leakage must be controlled.
Acknowledgments
The authors would like to thank Hinrich Staecker for the suggestion to control fluid leakage from the cochlea with Healon gel. This study was supported by research grant RO1 DC01368 from the National Institute on Deafness and Other Communication Disorders, National Institutes of Health.
Abbreviations
- CA
cochlear aquaduct
- CSF
cerebrospinal fluid
- OD
outer diameter
- RW
round window
- RWM
round window membrane
- ST
scala tympani
- TMPA
trimethylphenylammonium
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bohmer A. Hydrostatic pressure in the inner ear fluid compartments and its effects on inner ear function. Acta Otolaryngol. 1993;(Suppl 507):6–24. [PubMed] [Google Scholar]
- Brown JN, Miller JM, Altschuler RA, Nuttall AL. Osmotic pump implant for chronic infusion of drugs into the inner ear. Hear Res. 1993;70:167–172. doi: 10.1016/0378-5955(93)90155-t. [DOI] [PubMed] [Google Scholar]
- Carvalho GJ, Lalwani AK. The effect of cochleostomy and intracochlear infusion on auditory brain stem response threshold in the guinea pig. Am J Otol. 1999;20:87–90. [PubMed] [Google Scholar]
- Chelikh L, Teixeira M, Martin C, Sterkers O, Ferrary E, Couloigner V. High variability of perilymphatic entry of neutral molecules through the round window. Acta Otolaryngol. 2003;123:199–202. doi: 10.1080/00016480310001042. [DOI] [PubMed] [Google Scholar]
- Chen Z, Kujawa SG, McKenna MJ, Fiering JO, Mescher MJ, Borenstein JT, Swan EE, Sewell WF. Inner ear drug delivery via a reciprocating perfusion system in the guinea pig. J Control Release. 2005;110:1–19. doi: 10.1016/j.jconrel.2005.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engstrom B, Bjurstrom S, Jansson B, Engstrom H, Angelborg C. An ultrastructural and functional study of the inner ear after administration of hyaluronan into the middle ear of the guinea pig. Acta Otolaryngol Suppl. 1987;442:66–71. doi: 10.3109/00016488709102842. [DOI] [PubMed] [Google Scholar]
- Goycoolea MV, Lundman L. Round window membrane. Structure function and permeability: a review. Microsc Res Tech. 1997;36:201–211. doi: 10.1002/(SICI)1097-0029(19970201)36:3<201::AID-JEMT8>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Hahn H, Kammerer B, DiMAuro A, Salt AN, Plontke SK. Cochlear microdialysis for quantification of dexamethasone and fluorescein entry into scala tympani during round window administration. Hear Res. 2006;212:236–244. doi: 10.1016/j.heares.2005.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent C, Hellstrom S, Anniko M. Cochlear effects of hyaluronan applied on ruptured round window membrane. Acta Otolaryngol Suppl. 1992;493:63–67. [PubMed] [Google Scholar]
- Miller JM, Chi DH, O’Keeffe LJ, Kruszka P, Raphael Y, Altschuler RA. Neurotrophins can enhance spiral ganglion cell survival after inner hair cell loss. Int J Dev Neurosci. 1997;15:631–643. doi: 10.1016/s0736-5748(96)00117-7. [DOI] [PubMed] [Google Scholar]
- Mynatt R, Hale SA, Gill RM, Plontke SK, Salt AN. Demonstration of a longitudinal concentration gradient along scala tympani by sequential sampling of perilymph from the cochlear apex. J Assoc Res Otolaryngol. 2006;7:182–193. doi: 10.1007/s10162-006-0034-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair TS, Prieskorn DM, Miller JM, Mori A, Gray J, Carey TE. In vivo binding and hearing loss after intracochlear infusion of KHRI-3 antibody. Hear Res. 1997;107:93–101. doi: 10.1016/s0378-5955(97)00024-5. [DOI] [PubMed] [Google Scholar]
- Paasche G, Bogel L, Leinung M, Lenarz T, Stover T. Substance distribution in a cochlea model using different pump rates for cochlear implant drug delivery electrode prototypes. Hear Res. 2006;212:74–82. doi: 10.1016/j.heares.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Plontke SK, Mynatt R, Gill RM, Salt AN. Concentration gradient along the scala tympani following local application of gentamicin to the round window membrane. Laryngoscope. 2007 doi: 10.1097/MLG.0b013e318058a06b. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praetorius M, Baker K, Brough DE, Plinkert P, Staecker H. Pharmacodynamics of adenovector distribution within the inner ear tissues of the mouse. Hear Res. 2007;227:53–58. doi: 10.1016/j.heares.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Salt AN, Rask-Andersen H. Responses of the endolymphatic sac to perilymphatic injections and withdrawals: evidence for the presence of a one-way valve. Hear Res. 2004;191:90–100. doi: 10.1016/j.heares.2003.12.018. [DOI] [PubMed] [Google Scholar]
- Salt AN, Hale SA, Plontke SK. Perilymph sampling from the cochlear apex: A reliable method to obtain higher purity perilymph samples from scala tympani. J Neurosci Methods. 2006;153:121–129. doi: 10.1016/j.jneumeth.2005.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard WM, Wanamaker HH, Pack A, Yamamoto S, Slepecky N. Direct round window application of gentamicin with varying delivery vehicles: a comparison of ototoxicity. Otolaryngol Head Neck Surg. 2004;131:890–896. doi: 10.1016/j.otohns.2004.05.021. [DOI] [PubMed] [Google Scholar]
- Stover T, Yagi M, Raphael Y. Cochlear gene transfer: round window versus cochleostomy inoculation. Hear Res. 1999;136:124–130. doi: 10.1016/s0378-5955(99)00115-x. [DOI] [PubMed] [Google Scholar]
- Stover T, Yagi M, Raphael Y. Transduction of the contralateral ear after adenovirus-mediated cochlear gene transfer. Gene Ther. 2000;7:377–383. doi: 10.1038/sj.gt.3301108. [DOI] [PubMed] [Google Scholar]
- Thorne M, Salt AN, DeMott JE, Henson MM, Henson OW, jr., Gewalt SL. Cochlear fluid space dimensions for six species derived from reconstructions of 3-D magnetic resonance images. Laryngoscope. 1999;109:1661–1668. doi: 10.1097/00005537-199910000-00021. [DOI] [PubMed] [Google Scholar]
- Wareing M, Mhatre AN, Pettis R, Han JJ, Haut T, Pfister MH, Hong K, Zheng WW, Lalwani AK. Cationic liposome mediated transgene expression in the guinea pig cochlea. Hear Res. 1999;128:61–69. doi: 10.1016/s0378-5955(98)00196-8. [DOI] [PubMed] [Google Scholar]
- Wise AK, Richardson R, Hardman J, Clark G, O’Leary S. Resprouting and survival of guinea pig cochlear neurons in response to the administration of the neurotrophins brain-derived neurotrophic factor and neurotrophin-3. J Comp Neurol. 2005;487:147–165. doi: 10.1002/cne.20563. [DOI] [PubMed] [Google Scholar]