Abstract
Purpose
This study was conducted to assess the feasibility of a pulmonary formulation of tissue plasminogen activator (tPA) for nebulization into the airway by measuring protein stability, biologic activity, particle size, and estimating human lung distribution.
Methods
Formulations were derived by varying the surfactant and protein concentrations. Protein stability and recovery of each nebulized tPA formulation were assessed by ultraviolet spectroscopy. Formulations that met protein stability feasibility criteria were assessed for biologic and fibrinolytic activities. Biologic activity was determined by their ability to inhibit superoxide anion production by human neutrophils. Fibrinolytic activity was assessed by the cleavage of plasminogen to plasmin. Aerodynamic properties were assessed using a cascade impactor, and an estimation of human airway deposition was made via a human lung replica.
Results
Twenty-seven tPA formulations were initially assessed, 15 of which met protein stability criteria. Subsequently, three of these formulations maintained biologic and fibrinolytic activities. These formulations exhibited particle sizes of 2.4–3.1 μm, and had respirable doses ≥65%. A formulation of 1 mg mL−1 tPA and 0.1% Tween 80 exhibited a 45% deposition in the lower airways of a human lung replica.
Conclusions
A suitable pulmonary tPA formulation was identified that, following nebulization, maintained protein stability as well as biologic and fibrinolytic activities, and resulted in an optimal respirable dose and human airway deposition. This formulation may be applicable in the treatment of lung diseases, such as acute respiratory distress syndrome by permitting targeted pulmonary delivery of a therapeutic protein to the lungs.
Keywords: acute respiratory distress syndrome, inflammation, nebulization, pulmonary delivery, tissue plasminogen activator
INTRODUCTION
Tissue plasminogen activator (tPA) is an endogenous serine protease that cleaves plasminogen to plasmin. This activity has been exploited clinically, as recombinant tPA is used to dissolve the thrombosis associated with myocardial infarction (MI) and stroke. We have previously shown that exogenously administered human tPA suppressed activator induced neutrophil superoxide anion (O2•−) production and possessed antiinflammatory activity in an animal model of acute respiratory distress syndrome (ARDS) (1,2). Furthermore, the antiinflammatory effect was independent of the fibrinolytic activity of tPA (2). Due to the unique properties of tPA activity, formulation of this protein for pulmonary delivery could prove beneficial in the treatment of ARDS.
Given the high mortality associated with ARDS, effective treatment could have a significant impact on the outcome of these patients (3–5). Therefore, direct administration into the airways would be the most viable route of administration of tPA. However, before this approach can be employed, it is essential to determine the feasibility of tPA formulated for direct pulmonary delivery. Evidence exists for the benefit of pulmonary delivery and this strategy would target both the antiinflammatory and fibrinolytic properties of nebulized tPA into the lungs (6–9).
Therefore, this study sought to determine the feasibility of a tPA formulation for pulmonary delivery by utilizing a high-throughput strategy with prospectively determined feasibility criteria for protein stability, recovery, and activity following nebulization. In addition, respirable dose was measured using a cascade impactor, and assessment of pulmonary deposition efficiency of a nebulized protein formulation was determined by using a human lung replica.
MATERIALS AND METHODS
Preparation and Nebulization of tPA Formulations
Human recombinant tPA was prepared at a concentration of 1.25 mg mL−1 (tPA, alteplase, Activase®; Genentech, South San Francisco, CA, USA). A range (0–0.5%) of surfactant (pharmaceutical grade Tween 80; Pierce Biotechnology, Rockford, IL, USA) and protein concentrations (0–1.0 mg mL−1) were used in the preparation of 27 tPA formulation candidates. The formulations were prepared fresh and nebulization (5 mL) was performed for 15 min using a Micromist nebulizer (Hudson Respiratory Care, Temecula, CA, USA) with a Pulmo Aide compressor (DeVilbiss, Somerset, PA, USA). Each atomized formulation was collected by condensation.
Assessment of Protein Stability
Immediately preceding and following nebulization, protein stability was assessed by UV spectroscopy (HP8453 spectrophotometer; Agilent Technologies, Palo Alto, CA, USA). To obtain an accurate concentration of tPA, light scattering interference at 280 nm was subtracted by linear regression extrapolation at 340, 370, and 400 nm. Aggregation in the samples was expressed as an aggregation index (AI), which was calculated using the equation , where LS280 is the absorbance at 280 nm determined by linear regression extrapolation through 340, 370, and 400 nm.
Conformational stability of tPA was determined by measuring nebulization-induced changes in A/B ratio obtained from the second derivative UV spectrum (350 and 280 nm), which was used to assess perturbations in the tertiary structure of the protein (10,11). The A/B ratio is the peak-to-valley intensities corresponding to the tyrosine and tryptophan peaks, respectively, in second derivative UV absorbance spectra (11). Protein concentration was determined preceding and following nebulization by using the adjusted 280-nm absorbance value and an extinction coefficient of 1.9 for a 1 mg mL−1 solution of tPA. Percent protein recovery was calculated by measuring the concentration of tPA in the pre- and postnebulized samples and was expressed as a percentage of the prenebulized concentration. The original tPA formulation was nebulized and analyzed for comparison. The prospectively defined feasibility criteria required for a formulation to be eligible for further analysis were an aggregation index (AI) ≤ 10 (a clear solution with few, if any, particulates), an A/B ratio <10% different from native tPA, and a protein recovery ≥40% (with a coefficient of variation ≤10%) (12).
Measurement of Biologic and Fibrinolytic Activities
The biologic activity of tPA formulations that met protein stability feasibility criteria was determined by the ability of each formulation to inhibit phorbol myristate acetate (PMA)- induced human neutrophil production of O2•−, as measured by horse heart ferricytochrome c reduction (2). This study complied with the tenets of the Declaration of Helsinki promulgated in 1964, and was approved by the Colorado Multi-Institutional Review Board; written informed consent was obtained from all study participants. Neutrophils were isolated from whole blood (60 mL) obtained via venipuncture of a healthy medication-free adult. The whole blood was anticoagulated with 3.8% sodium citrate and separated over a sodium diatrizoale–Dextran 500 gradient (Polymorphprep; Greiner Bio-One Longwood, FL, USA) as previously described (13). Briefly, the polymorphonuclear layer was resuspended in RPMI 1640 (37°C) and washed with Krebs–Ringers phosphate dextrose (KRPD, pH 7.4) buffer. Hypotonic hemolysis of red blood cells was performed and cells were resuspended to a density of 5 × 106 cells mL−1 in KRPD. Viability was assessed by Trypan blue exclusion. Isolated human neutrophils (200 μL, 5 × 106 cells mL−1) were plated into a 96-well microtiter plate. Formulation or native tPA were added to a final concentration of 100 μg mL−1, cytochrome c was added to all wells (120 nM), and the plate was incubated for 60 min at 37°C. Phorbol myristate acetate (PMA; Sigma, St. Louis, MO, USA) was added (1.25 μg mL−1) and the plate was incubated again (60 min, 37° was measured as cytochrome c C). The production of O2•− reduced (550 nm) every 2 min for 1 h. The kinetic disposition of each treatment was compared.
Formulations of tPA that retained antiinflammatory activity were assessed for fibrinolytic activity by measuring the cleavage of human plasminogen to plasmin following nebulization. Native tPA or tPA formulations (100 μg mL−1) were incubated with human plasminogen (375 μg mL−1; Enzyme Research Laboratories, South Bend, IN, USA) for 5 h (37°C) at a reaction volume of 50 μL. Following incubation, protein bands were separated by 10% SDS-PAGE and gels were visualized by Coomasie blue stain.
The rate of cytochrome c reduction for each experiment was determined from the slope of the time vs. cytochrome c (mM) reduced curve using an extinction coefficient of 21.1 mM−1 cm−1 for cytochrome c (14). The mean (±SEM) rate of cytochrome c (mM min−1) reduced for each formulation was determined and compared with the mean rate of cytochrome c reduction for PMA control treatment using analysis of variance (ANOVA). Post-hoc analyses were performed using a Fischer’s PLSD test for cytochrome c reduction and a p value ≤ 0.05 was considered significant. Data analysis was performed using the statistical software StatView (Cary, NC, USA).
The feasibility criteria for maintenance of biologic activity of the tPA formulations was an inhibition of O2•− generation by at least 50% compared to PMA control treatment. Furthermore, for a formulation to meet biologic feasibility, treatment of neutrophils with the tPA formulation alone could not induce neutrophil O2•− production. It was also required that tPA formulations retain fibrinolytic activity following nebulization.
Determination of Aerodynamic Parameters by Cascade Impactor
Aerodynamic parameters of formulations that met biologic and fibrinolytic feasibility criteria were measured using a seven-stage nonviable Thermo-Electron cascade impactor (Waltham, MA) operated at 60 L min−1. Each drug formulation was nebulized into the cascade impactor for 15 min after which the cascade impactor was disassembled and each stage plate was weighed. The mass of deposited formulation was determined. Effective cutoff diameter (ECD), which is the smallest particle size capable of depositing on a given stage and the cumulative percent less than size range, was determined by comparing the deposited mass of all stages below a particular size limit to the total mass collected on all stages (15). Aerodynamic properties of each formulation nebulized into the impactor were plotted on a log-probability graph. From linear regression, the mass mean diameter (MMD) and geometric standard deviation (σg) were calculated. The MMD was calculated at the 50th percentile acquired from the regression line and the σg was determined using the equation (15). The respirable dose (percent of particles ≤ 5 μm) was extrapolated from the regression line of the log-probability plots (15,16). Each formulation was analyzed in triplicate. A formulation was considered feasible if the respirable dose was at least 10% (15).
Airway Deposition Into a Human Lung Replica
To estimate lung deposition efficiency, the most feasible tPA formulation was nebulized into a replica of the human airway. The human lung replica is a useful tool for the estimation of airway deposition because it mimics the aerodynamic characteristics of the human airway and allows for a more precise characterization of the lower airway to the fourth bronchial generation (17). It also permits a better estimation of oral and throat deposition compared with the cascade impactor (15,16). However, drug deposition at the alveolar level is not assessed by this model; rather, an estimation of deep lung deposition is made by measuring the amount of drug that passes through the replica without depositing.
The human airway replica and oral cavity was made from Dow-E silicone rubber as previously described (17,18). Briefly, the oral cavity of the cast was obtained from a human volunteer, whereas the remainder of the airway cast was produced from a human cadaver. Wax was poured into the production airway mold and was allowed to solidify; then the cast was removed. To minimize the effect of electrostatic deposition, an electrically conductive silicone rubber compound (KE-4576, one-component RTV; GemTech, Grapevine, TX, USA) was applied to the wax mold. Multiple identical rubber casts were made from the production mold allowing multiple deposition determinations. During nebulization, airflow distribution in each terminal airway of the cast was measured using a hot-wire anemometer probe (Model 8470; TSI, Inc., St. Paul, MN, USA). The overall airflow of the model was set at 30 L min−1. For each of the three casts, tPA formulation (10 mg) was nebulized into the cast. The drug penetrating the terminal branches of the cast was collected in each of the ten impingers. Following nebulization, the airway cast was cut into sections, as shown in Fig. 1, and the protein was eluted from each section. Protein concentration in the eluate was determined by bicinchoninic acid protein assay (Pierce Biotechnology, Rockford, IL, USA). The total amount (mg) of tPA formulation that deposited in regions B1–5 and T1–5 of the human airway replica was represented as a percentage of the total tPA formulation nebulized into the airway cast (total protein deposited in all regions of the cast). This value indicated the deposition efficiency of the formulation in the lower regions of the human airways (regions below the 4th generation of the airway cast). Feasibility criterion used in the human lung replica dictated that at least 10% of the formulation must deposit in the lower airways (regions below the 4th generation of the cast) (Fig. 1).
Fig. 1.
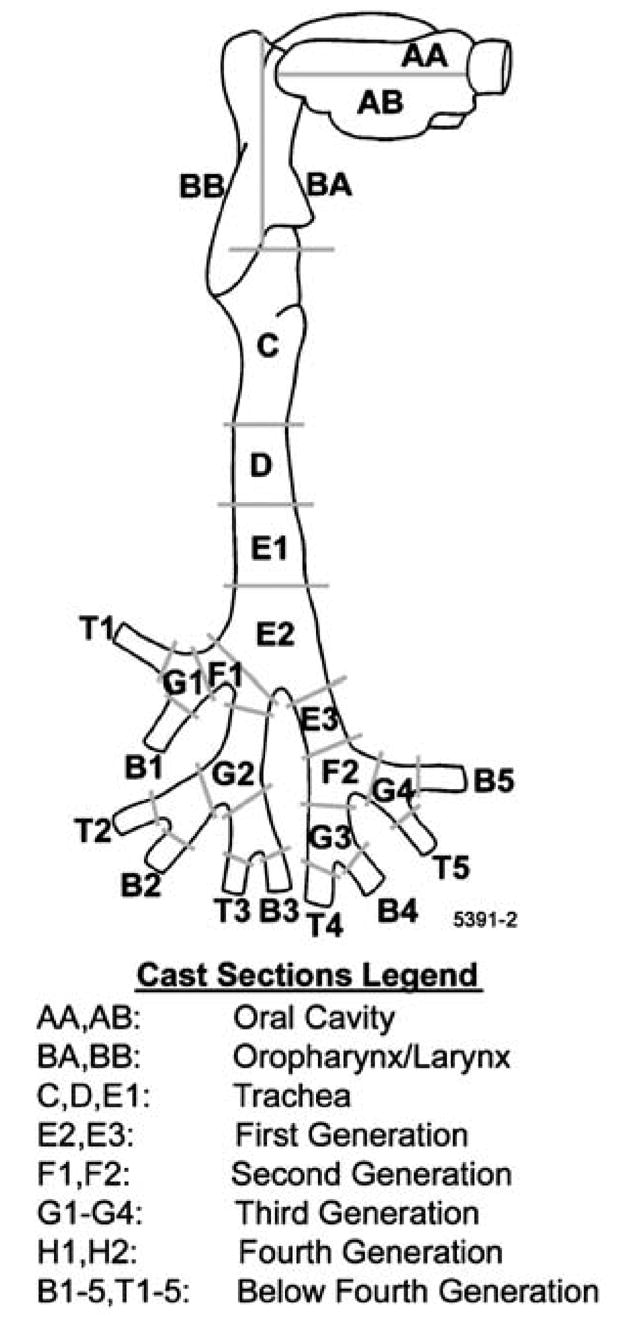
Schematic representation of the human lung replica into which tPA formulation was nebulized. The labels represent physiologic sections of the airway. Impingers were connected to the terminal outlets of the cast (sections B1–5 and T1–5) to collect any formulation that did not deposit in the cast. The gray cut lines represent approximate points at which the lung replica was sectioned.
RESULTS
Protein Stability
Twenty-seven formulations were initially screened for protein stability. The tPA concentration ranged from 0 to 1 mg mL−1 and Tween 80 concentration ranged from 0 to 0.5%. Protein recovery and stability varied upon nebulization, based on the tPA and surfactant concentrations of the formulations. As the surfactant concentration increased (with an optimal concentration of 0.1%) and as protein concentration increased (with an optimal concentration of 1 mg mL−1), the stability of the nebulized protein was enhanced (Fig. 2). There were 15 formulations that met the feasibility criteria of protein recovery and stability. They had a tPA concentration of 0.5–1.0 mg mL−1, a surfactant concentration of 0–0.5%, and exhibited an AI of less than 10 (Table I). Formulation #27 was selected for further analysis despite an AI of 14.1 due to exceptional recovery and stability characteristics. Formulations that failed to meet the stability criteria typically had low initial protein concentrations (≤0.25 mg mL−1) or low Tween 80 concentrations (≤0.1%). All assessments were made from three individual measurements before and after nebulization.
Fig. 2.
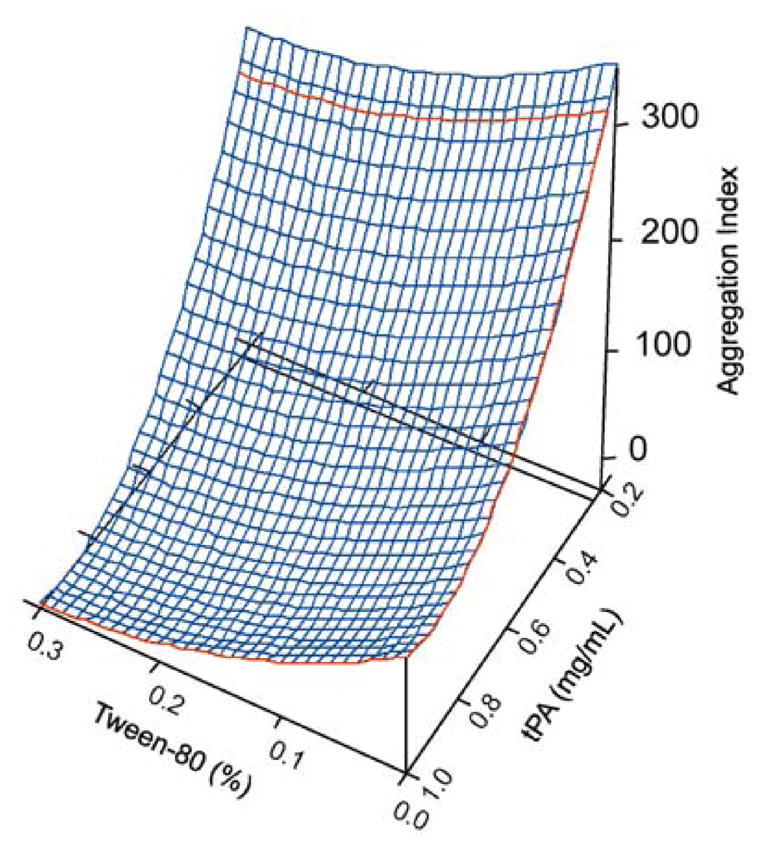
The correlation of tPA and Tween 80 concentration to aggregation index. As Tween 80 concentration increased, protection of the protein against aggregation was improved, demonstrated by a lower AI value. Furthermore, formulations with a higher tPA concentration (>0.25 mg mL−1) showed enhanced stability.
Table I.
tPA Pulmonary Formulations Which Met Protein Stability and Recovery Feasibility Criteria
| Formulation no. | Prenebulized protein concentration(mg mL−1) | Postnebulized protein concentration (mg mL−1) | Tween 80 (%) | Protein recovery (%) | Aggregation index (AI) of nebulized samples | Difference in A/B ratio of pre- and postnebulized samples |
|---|---|---|---|---|---|---|
| 1 | 0.945 | 0.708 | 0.05 | 76.5 | 2.8 | 0.15 |
| 2 | 0.597 | 0.528 | 0.05 | 87.8 | 3.0 | 0.26 |
| 3 | 0.964 | 0.894 | 0.18 | 79.4 | 4.4 | 0.21 |
| 4 | 0.967 | 0.837 | 0.30 | 88.8 | 3.5 | 0.10 |
| 10 | 0.695 | 0.440 | 0.30 | 60.7 | 7.6 | 0.09 |
| 15 | 0.672 | 0.291 | 0.00 | 43.3 | 1.6 | 0.04 |
| 16 | 0.458 | 0.184 | 0.00 | 40.2 | 7.0 | N.D.a |
| 17 | 0.738 | 0.745 | 0.30 | 100.9 | 3.2 | 0.14 |
| 18 | 0.966 | 0.786 | 0.15 | 81.4 | 2.9 | 0.04 |
| 19 | 0.937 | 0.796 | 0.30 | 85.0 | 0.6 | 0.12 |
| 23 | 0.721 | 0.616 | 0.50 | 85.4 | 5.0 | 0.04 |
| 24 | 0.989 | 0.774 | 0.40 | 78.3 | 2.7 | 0.02 |
| 25 | 0.981 | 0.745 | 0.50 | 75.9 | 3.7 | 0.01 |
| 26 | 0.918 | 0.412 | 0.00 | 44.9 | 2.3 | 0.06 |
| 27 | 0.983 | 0.716 | 0.10 | 72.8 | 14.1 | 0.06 |
Values for protein recovery, Tween 80 concentration, aggregation index, and A/B ratio are represented. All values are the mean of at least three separate determinations.
Value not determined.
Biological Activity
Biological activities of the formulations that met the protein stability feasibility criteria were assessed. Three of the nebulized formulations (#1, #25, and #27) maintained the ability to reduce the rate of PMA-induced neutrophil O2•− production (Fig. 3). The mean (±SEM) rates of cytochrome c reduction (mM min−1) in PMA-stimulated neutrophils were 5.95 × 10−5 ± 2.51 × 10−5, 1.86 × 10−4 ± 5.15 × 10−5, and 1.58 × 10−4 ± 4.84 × 10−5 (p = 2.92 × 10−6, 0.022, and 0.007) for formulations #1, #25, and #27, respectively. By comparison, the rate of PMA-induced O2•− production was 3.75 × 10−4 ± 3.92 × 10−5 mM min−1. Therefore, inhibition of •− production by human neutrophils was reduced by 84.1, O2 50.4, and 57.9% for formulations #1, #25, and #27, respectively. In addition, exposure of neutrophils to these formulations in the absence of PMA did not prompt O2•− production. The remainder of the tested formulations either failed to inhibit PMA-induced neutrophil O2•− production by at least 50% or activated the neutrophils independent of PMA administration.
Fig. 3.
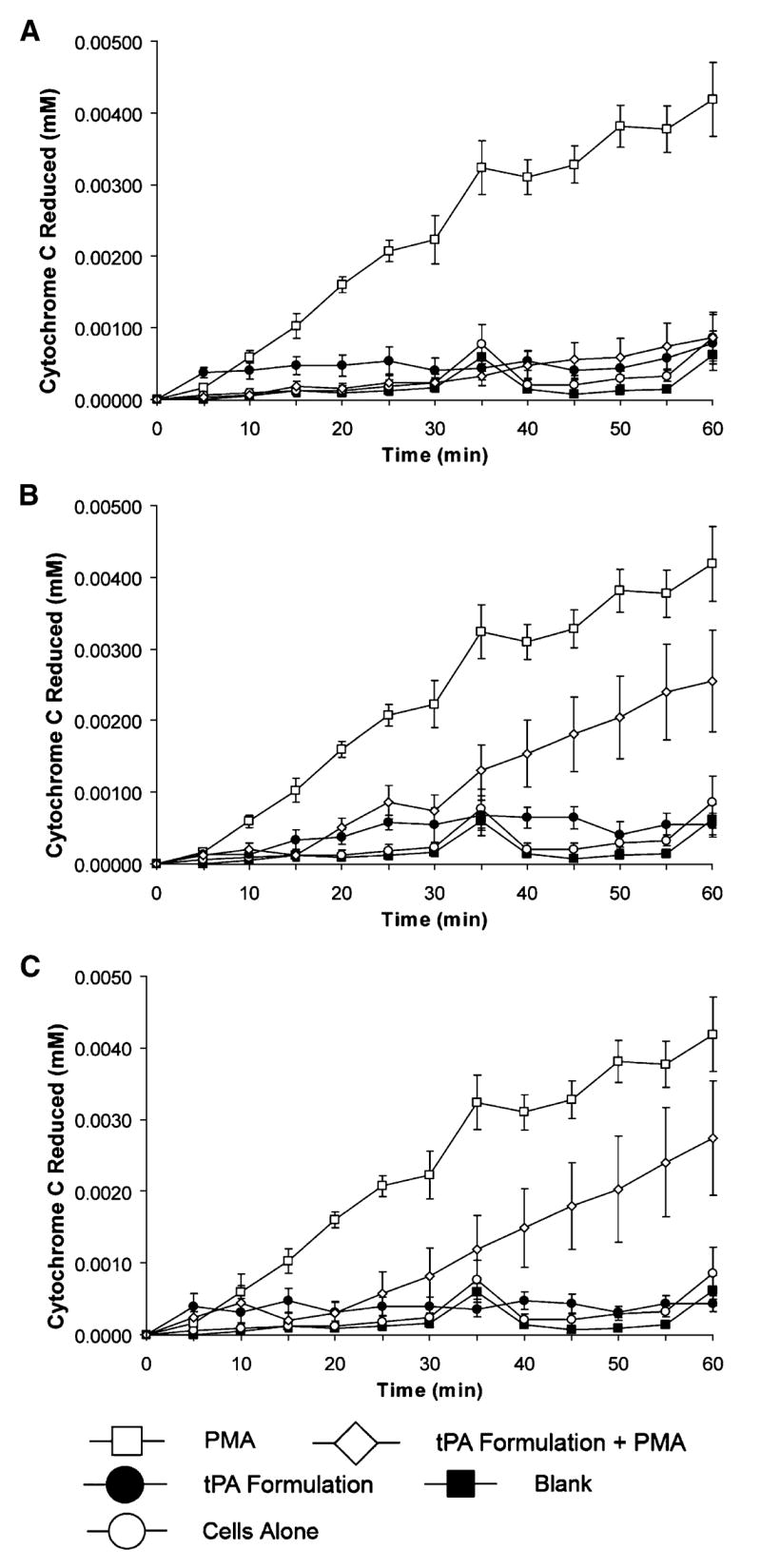
Inhibition of PMA-induced human neutrophil superoxide anion (O2•−) production by three different nebulized formulations of tPA as measured by the rate of cytochrome c reduction (mM min−1). production Formulations #1 (A), #25 (B), and #27 (C) reduced O2•− by 84, 50, and 58%, respectively. Data represent the mean (±SEM) of results from at least seven separate experiments.
Fibrinolytic Activity
The enzymatic activity of formulations #1, #25, and #27 was assessed by the ability of each tPA formulation to cleave human plasminogen to the active product plasmin. The Genentech tPA formulation was used in an unaltered form as a reference for typical recombinant tPA fibrinolytic activity. Following the 5-h reaction period, each of the three formulations cleaved plasminogen comparable to that of native tPA (Fig. 4). These data show that nebulization did not impair the enzymatic activity of the protein compared with native, nonnebulized tPA.
Fig. 4.
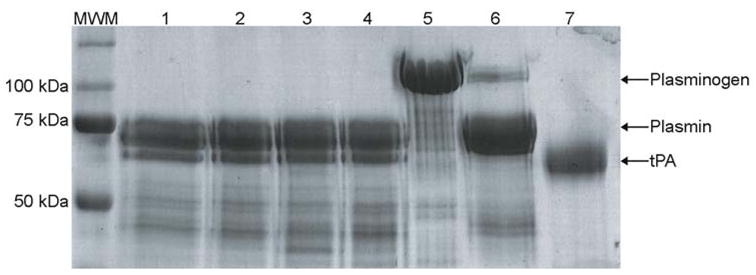
Representative SDS-PAGE gel showing the fibrinolytic activity of three nebulized formulations of tPA. Exposure of formulations #1 (lane 1), #25 (lane 2), #27 (lane 3), and unmodified and non–nebulized tPA control (lane 4; 100 μg mL−1) to plasminogen (375 μg mL−1) resulted in plasmin formation. Plasminogen (lane 5), plasmin (lane 6), human tPA (lane 7), and molecular weight marker (MWM) were included for protein identification and size determination. The enzymatic data were typical of at least four separate experiments.
Aerodynamic Properties Determination
Formulations #1, #25, and #27 were evaluated for their aerodynamic properties using a Thermo Electron cascade impactor system. Each of the three formulations were nebulized into the cascade impactor, and particle size distribution was determined on three separate occasions. The MMD of the nebulized formulations was 2.4, 3.1, and 2.8 μm for formulations #1, #25, and #27, respectively. These values represent the average particle size of the nebulized formulation where half of the particle sizes are larger than this value and half of the particle sizes are smaller than this size value. σg was also calculated from the linear regression of the particle size distribution with values of 2.7, 1.9, and 2.5 μm for formulations #1, #25, and #27, respectively (Fig. 5). σg describes the distribution of particle sizes about the MMD. A σg greater than 1.22 is characteristic of most aerosols and illustrates a heterogenous mixture of particle sizes. This is also described by the log-probability distributions of the individual stage mass balances (Fig. 5). A linear correlation between ECD and cumulative percent less than size range represents an evenly distributed range of particle sizes of the nebulized formulations. Respirable dose was also calculated as a function of particle distribution (≤5 μm) based on the linear regression of the log-probability plot. Nebulization of all three formulations resulted in a respirable dose of ≥65%. Based on particle size distribution and respirable dose, all three formulations nebulized into the cascade impactor met feasibility criteria.
Fig. 5.
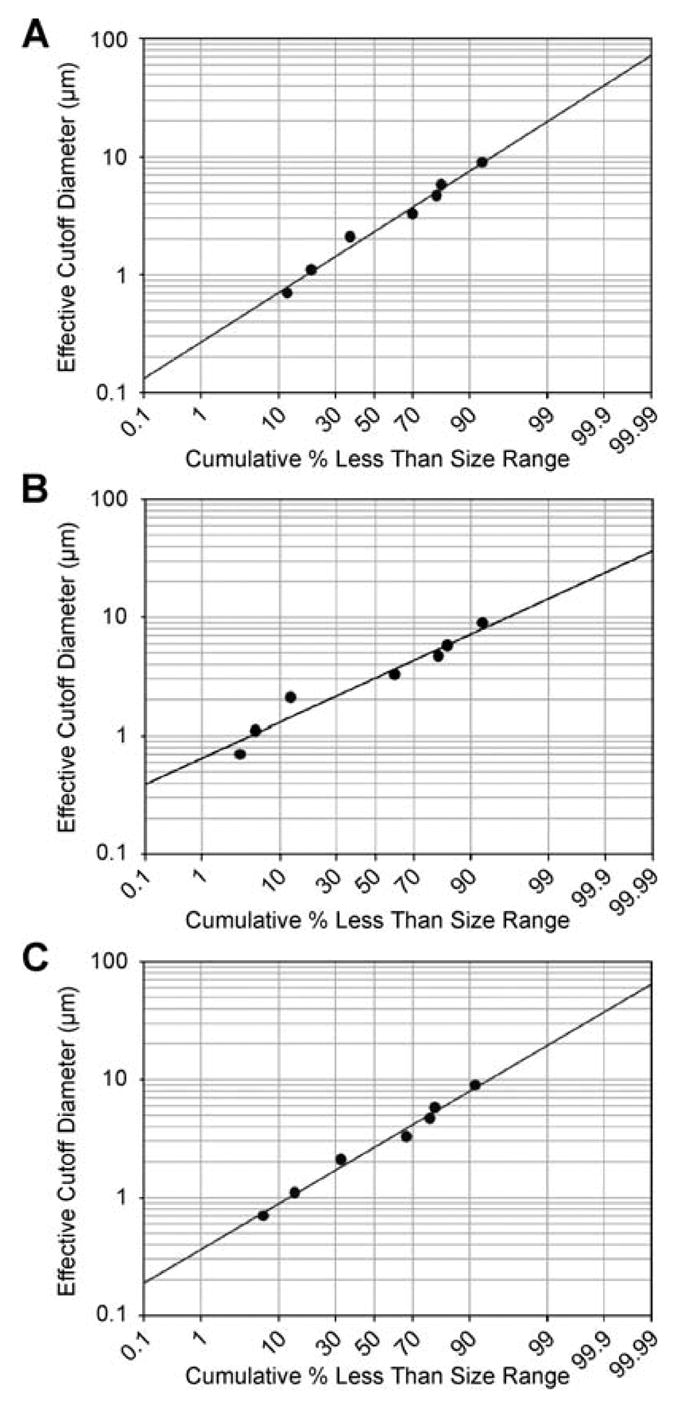
Plots of formulations (A) #1, (B) #25, and (C) #27 representing the distribution of nebulized particles in the cascade impactor. Nebulized pulmonary formulations having a normally distributed range of particle sizes will follow a linear relationship when the mass balances of the cascade impactor stages are plotted on a log-probability graph. All three nebulized formulations showed linear relationships (R2 = 0.9385, 0.9166, and 0.9352 for formulations #1, #25, and #27, respectively).
Deposition Efficiency in an Human Airway Replica
To assess the lung deposition efficiency of nebulized tPA formulation, a four-generation replica of a human lung was used. Because formulation #27 exhibited the optimal performance in the biological assays and aerodynamic characterization, it was chosen for testing in the lung cast model. The protein concentrations from each cast section were divided into appropriate physiologic sections (Fig. 1). The feasibility goal of ≥10% lower airway deposition required that at least 1 mg of the tPA formulation deposit in the regions B1–5 and T1–5. A large portion of the tPA deposited in the oral, larynx, and trachea regions (33.3% for these three sections) (Fig. 6). The intermediate airways (cast generations 1–4) showed less than 5% formulation deposition. On average, 18% of the formulation did not enter the cast and was trapped in the tee that joined the nebulizer to the oral region of the cast or remained in the nebulizer reservoir. Greater than 45% of the 10-mg nebulized dose deposited in the lower airways (below 4th generation). This demonstrated that formulation #27 met the feasibility criterion for pulmonary deposition in the human lung replica.
Fig. 6.
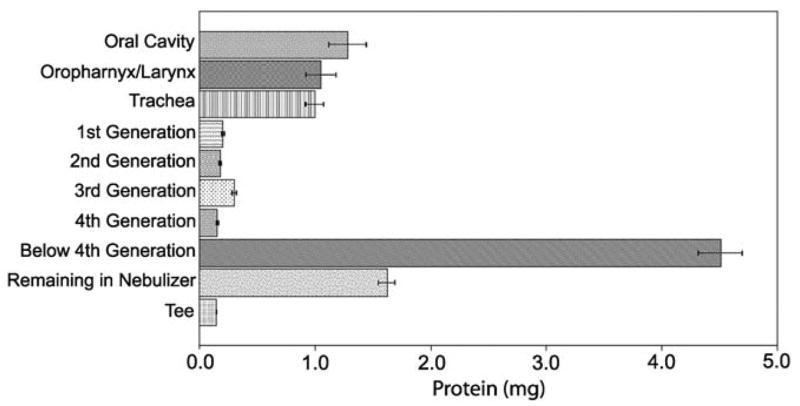
Lung deposition efficiency of nebulized formulation #27 in a human lung replica. Protein concentrations represented are the results of triplicate determinations of formulation nebulized into three separate lung casts. The “Below 4th Generation” section (comprising cast sections T1–5 and B1–5) showed a protein deposition of 4.5 ± 0.19 mg yielding a lower airway deposition of 45%.
DISCUSSION
The results of this study demonstrate the feasibility of tPA formulated for pulmonary delivery by showing that nebulization of the protein generated a respirable aerosol that maintained biologic and fibrinolytic activities necessary for therapeutic administration. Clinically, tPA has been delivered systemically for treatment of MI and stroke (19,20). We have demonstrated that tPA possesses antiinflammatory properties in addition to the well-characterized fibrinolytic activity (2,21). Taken together, the antiinflammatory and fibrinolytic activities of tPA could prove beneficial in the treatment of ARDS because the condition is associated with both an acute inflammatory response and fibrin deposition (22–24). The data presented in this study support the feasibility of formulating tPA for nebulization and pulmonary delivery. Although there have been anecdotal reports of pulmonary delivered plasminogen activator, this work is the first report to demonstrate the feasibility of a pulmonary formulation using a systematic and scientific approach (25,26).
Nebulization was chosen as the delivery method for pulmonary tPA. Nebulization has been used extensively for the delivery of pharmaceuticals in the treatment of cystic fibrosis, asthma, and diabetes (27–29). One of the advantages of nebulization is that it is routinely available in the clinical setting. Another advantage of nebulization is that it permits targeted lung delivery of the antiinflammatory and fibrinolytic activities of tPA that, as evidenced by this work, was not compromised by the process of nebulization. Although nebulization of a protein formulation has traditionally proven challenging due to loss of protein by aggregation and disruption of protein function, the prospectively defined feasibility criteria in this study were met. This was accomplished by enhancing the structural protection of the protein using varying concentrations of Tween 80, which resulted in the identification of several feasible pulmonary formulations of tPA.
Our work also revealed the suitability of tPA pulmonary formulations for aerodynamic particle distribution and respirable dose determination. An aerosol formulation is considered optimal for lower airway deposition if it exhibits a particle size ≤5 μm (16). As determined by cascade impactor, the MMD and σg of each formulation were within the range of an ideal aerosol (MMD: 2.4–3.1 μm; σg: 1.9–2.7 μm) accessible to the lower airways. All three tPA formulations assessed by cascade impactor exhibited a heterogeneous distribution of particle sizes, indicating that the particle sizes were varied and evenly distributed. The mass of aerosol deposited within the cascade impactor indicated that the nebulization of each formulation resulted in a respirable dose of at least 65%.
Although assessment by cascade impactor is an established measure of particle size, this method does not accurately estimate the physiologic distribution of a formulation in the human lung. Therefore, we elected to utilize a human lung replica to evaluate the deposition of tPA formulation in specific lung regions. Formulation #27 was chosen for assessment in the human lung replica because it exhibited optimal structural, biologic, and particle size characteristics compared with other tPA formulations. The human lung replica offered a more accurate approximation of formulation distribution in the airway due to the differing airflow dynamics of the bronchial branches vs. the constant airflow characteristics of the cascade impactor (17,18). In general, the pulmonary delivery of medications is an inefficient process with only 10% of the nebulized drug reaching lower airways (30). However, nebulization of the tPA formulation into the human lung replica showed 45% deposition efficiency into the lower airways. This approach provided a more accurate approximation of mouth and throat deposition than the cascade impactor. The substantially greater deposition in the lung replica model could be influenced, in part, by the constant airflow of the system. Furthermore, the lung replica does not account for particles that would tend to be exhaled by normal respiration. In the human lung replica model, large particles deposit in the large airways by inertial impaction. For smaller particles (≤3 μm), the predominant mode of deposition is diffusion. Therefore, small particles are able to pass through the upper airway and will deposit in the mid- to lower airways by diffusion. Use of the lung replica for assessing the feasibility of the formulation proved that a tPA formulation could be generated that was effectively delivered into the human lung with an expectation of suitable lower airway distribution.
This study proved the feasibility of a pulmonary formulation of tPA by demonstrating that protein stability and antiinflammatory and fibrinolytic activities were maintained following nebulization. Furthermore, the aerodynamic characteristics of the formulation were conducive to delivery of protein to the lower airways of the human lungs. Nebulized tPA could prove particularly advantageous in the treatment of ARDS, because recent studies demonstrate the involvement of the coagulation system in the pathogenesis of ARDS (31,32). The fibrinolytic activity of tPA coupled with its antiinflammatory activity could alleviate fibrin deposition and inflammation in the ARDS lungs. Because the formulation of tPA for pulmonary delivery has proven feasible, we plan to extend this work to assess its efficacy and toxicity in animal models. Additionally, increasingly detailed assessments of protein structural and conformational stability will be performed to further optimize tPA for pulmonary delivery.
Acknowledgments
This work was funded by a National Institutes of Health Grant R41 HL071439-01A1.
References
- 1.Stringer KA. Tissue plasminogen activator inhibit reactive oxygen species production by macrophages. Pharmacotherapy. 2000;20(4):375–379. doi: 10.1592/phco.20.5.375.35059. [DOI] [PubMed] [Google Scholar]
- 2.Stringer KA, Lindenfeld J, Repine AJ, Cohen Z, Repine JE. Tissue plasminogen activator (tPA) inhibits human neutrophil superoxide anion production in vitro. Inflammation. 1997;21(1):27–34. doi: 10.1023/a:1027334607606. [DOI] [PubMed] [Google Scholar]
- 3.Piantadosi CA, Schwartz DA. The acute respiratory distress syndrome. Ann Intern Med. 2004;141:460–470. doi: 10.7326/0003-4819-141-6-200409210-00012. [DOI] [PubMed] [Google Scholar]
- 4.Kane C, Galanes S. Adult respiratory distress syndrome. Crit Care Nurs Q. 2004;27(4):325–335. doi: 10.1097/00002727-200410000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Udobi KF, Childs E, Touijer K. Acute respiratory distress syndrome. Am Fam Phys. 2003;67(2):315–322. [PubMed] [Google Scholar]
- 6.Yang T, Mustafa F, Bai S, Ahsan F. Pulmonary delivery of low molecular weight heparins. Pharm Res. 2004;21(11):2009–2016. doi: 10.1023/b:pham.0000048191.69098.d6. [DOI] [PubMed] [Google Scholar]
- 7.Aguiar MMG, Rodrigues JM, Jr, Cunha AS. Encapsulation of insulin–cyclodextrin complex in PLGA microspheres: a new approach for prolonged pulmonary insulin delivery. J Microencapsul. 2004;21(5):553–564. doi: 10.1080/02652040400000447. [DOI] [PubMed] [Google Scholar]
- 8.Labiris NR, Dolovich MB. Pulmonary drug delivery. Part I: physiological factors affecting therapeutic effectiveness of aerosolized medications. Br J Clin Pharmacol. 2003;56:588–599. doi: 10.1046/j.1365-2125.2003.01892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Labiris NR, Dolovich MB. Pulmonary drug delivery. Part II: the role of inhalant delivery devices and drug formulations in therapeutic effectiveness of aerosolized medications. Br J Clin Pharmacol. 2003;56:600–612. doi: 10.1046/j.1365-2125.2003.01893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mach H, Thomson JA, Middaugh CR, Lewis RV. Examination of phenylalanine microenvironments in proteins by second-derivative absorption spectroscopy. Arch Biochem Biophys. 1991;287(1):33–40. doi: 10.1016/0003-9861(91)90384-u. [DOI] [PubMed] [Google Scholar]
- 11.Mach H, Middaugh CR. Simultaneous monitoring of the environment of tryptophan, tyrosine, and phenylalanine residues in proteins by near-ultraviolet second-derivative spectroscopy. Anal Biochem. 1994;222:323–331. doi: 10.1006/abio.1994.1499. [DOI] [PubMed] [Google Scholar]
- 12.Dunn BM, Scarborough PE, Davenport R, Swietnicki W. Analysis of proteinase specificity by studies of peptide substrates. The use of UV and fluorescence spectroscopy to quantitate rates of enzymatic cleavage. Methods Mol Biol. 1994;36:225–243. doi: 10.1385/0-89603-274-4:225. [DOI] [PubMed] [Google Scholar]
- 13.Dunn JS, Freed BM, Gustafson DL, Stringer KA. Inhibition of human neutrophil reactive oxygen species production and p67phox translocation by cigarette smoke extract. Atherosclerosis. 2005;179:261–267. doi: 10.1016/j.atherosclerosis.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 14.van Gelder B, Slater EC. The extinction coefficient of cytochrome c. Biochim Biophys Acta. 1962;58:593–595. doi: 10.1016/0006-3002(62)90073-2. [DOI] [PubMed] [Google Scholar]
- 15.Swift DL. Aerosol characterization and generation. In: Moren F, Dolovich MB, Newhouse MT, Newman SP, editors. Aerosols in Medicine: Principles, Diagnosis and Therapy. Elsevier; Amsterdam: 1993. pp. 61–84. [Google Scholar]
- 16.Brain JD, Blanchard JD. Mechanisms of particle deposition and clearance. In: Moren F, Dolovich MB, Newhouse MT, Newman SP, editors. Aerosols in Medicine: Principles, Diagnosis and Therapy. Elsevier; Amsterdam: 1993. pp. 124–135. [Google Scholar]
- 17.Cheng YS, Fu CS, Yazzie D, Zhou Y. Respiratory deposition patterns of salbutamol pMDI with CFC and HFA-134a formulations in a human airway replica. J Aerosol Med. 2001;14(2):255–266. doi: 10.1089/08942680152484180. [DOI] [PubMed] [Google Scholar]
- 18.Cheng YS, Zhou Y, Chen BY. Particle deposition in a cast of human oral airways. Aerosol Sci Technol. 1999;31:286–300. [Google Scholar]
- 19.Hoang KD, Rosen P. The efficacy and safety of tissue plasminogen activator in acute ischemic strokes. J Emerg Med. 1992;10(3):345–352. doi: 10.1016/0736-4679(92)90341-p. [DOI] [PubMed] [Google Scholar]
- 20.Rutherford JD, Braunwald E. Thrombolytic therapy in acute myocardial infarction. Chest. 1990;97(4 Suppl):136S–145S. doi: 10.1378/chest.97.4_supplement.136s. [DOI] [PubMed] [Google Scholar]
- 21.Stringer KA, Dunn JS, Gustafson DL. Administration of exogenous tissue plasminogen activator reduces oedema in mice lacking the tissue plasminogen activator gene. Clin Exp Pharmacol Physiol. 2004;31(5–6):327–330. doi: 10.1111/j.1440-1681.2004.03999.x. [DOI] [PubMed] [Google Scholar]
- 22.Fulkerson WJ, MacIntyre N, Stamlet J, Crapo JD. Pathogenesis and treatment of the adult respiratory distress syndrome. Arch Intern Med. 1996;156:29–38. [PubMed] [Google Scholar]
- 23.Cooper JA, Lo SK, Malik AB. Fibrin is a determinant of neutrophil sequestration in the lung. Circ Res. 1988;63:735–741. doi: 10.1161/01.res.63.4.735. [DOI] [PubMed] [Google Scholar]
- 24.Lee WL, Downey GP. Neutrophil activation and acute lung injury. Curr Opin Crit Care. 2001;7:1–7. doi: 10.1097/00075198-200102000-00001. [DOI] [PubMed] [Google Scholar]
- 25.Munster AM, Bendstrup E, Jensen JI, Gram J. Jet and ultrasonic nebulization of single chain urokinase plasminogen activator (scu-PA) J Aerosol Med. 2000;13(4):325–333. doi: 10.1089/jam.2000.13.325. [DOI] [PubMed] [Google Scholar]
- 26.Enkhbaatar P, Murakami K, Cox R, Westphal M, Morita N, Brantley K, Burke A, Hawkins H, Schmalstieg F, Traber L, Herndon D, Traber D. Aerosolized tissue plasminogen inhibitor improves pulmonary function in sheep with burn and smoke inhalation. Shock. 2004;22(1):70–75. doi: 10.1097/01.shk.0000129201.38588.85. [DOI] [PubMed] [Google Scholar]
- 27.Cipolla DC, Gonda I, Shak S, Kovesdi I, Crystal R, Sweeney TD. Coarse spray delivery to a localized region of the pulmonary airways for gene therapy. Hum Gene Ther. 2000;11:361–371. doi: 10.1089/10430340050016085. [DOI] [PubMed] [Google Scholar]
- 28.Dolovich MB, Ahrens RC, Hess DR, Anderson P, Dhand R, Rau JL, Smaldone GC, Guyatt G. Device selection and outcomes of aerosol therapy: evidence-based guidelines. Chest. 2005;127(1):335–371. doi: 10.1378/chest.127.1.335. [DOI] [PubMed] [Google Scholar]
- 29.Cefalu WT. Evolving strategies for insulin delivery and therapy. Drugs. 2004;64(11):1149–1161. doi: 10.2165/00003495-200464110-00001. [DOI] [PubMed] [Google Scholar]
- 30.Rau JL. The inhalation of drugs: advantages and problems. Respir Care. 2005;50(3):367–382. [PubMed] [Google Scholar]
- 31.Idell S. Anticoagulants for acute respiratory distress syndrome: can they work? Am J Respir Crit Care Med. 2001;164:517–520. doi: 10.1164/ajrccm.164.4.2102095. [DOI] [PubMed] [Google Scholar]
- 32.Idell S, Koenig KB, Fiar DS, Martin TR, McLarty J, Maunder RJ. Serial abnormalities of fibrin turnover in evolving adult respiratory distress syndrome. Am J Physiol. 1991;261:L240–L248. doi: 10.1152/ajplung.1991.261.4.L240. [DOI] [PubMed] [Google Scholar]


