Abstract
Human total brain size is consistently reported to be ~8-10% larger in males, although consensus on regionally-specific differences is weak. Here, in the largest longitudinal pediatric neuroimaging study reported to date (829 scans from 387 subjects, ages 3 to 27 years), we demonstrate the importance of examining size-by-age trajectories of brain development rather than group averages across broad age ranges when assessing sexual dimorphism. Using magnetic resonance imaging (MRI) we found robust male/female differences in the shapes of trajectories with total cerebral volume peaking at age 10.5 in females and 14.5 in males. White matter increases throughout this 24 year period with males having a steeper rate of increase during adolescence. Both cortical and subcortical gray matter trajectories follow an inverted U shaped path with peak sizes 1 to 2 years earlier in females. These sexually dimorphic trajectories confirm the importance of longitudinal data in studies of brain development and underline the need to consider sex matching in studies of brain development.
Introduction
The degree to which sexual dimorphism extends to human brain anatomy has been the subject of many investigations, with most reporting total brain size to be ~8-10% larger in males (Goldstein et al., 2001). However, the literature is notably inconsistent as to which subcomponents of the brain differ after accounting for the total brain size difference. Discrepant findings in sexual dimorphism studies may be partially accounted for by subject age, with some studies combining subjects across several decades. There is a particular paucity of data on sexual dimorphism of human brain anatomy between 4 and 22 years of age, a time of emerging sex differences in behavior and cognition (Cairns et al., 1985; Gouchie and Kimura, 1991; Johnson and Meade, 1987). Large individual variation in brain morphometry makes accurate characterization of developmental trajectories difficult with cross-sectional studies (Giedd et al., 1996c; Kraemer et al., 2000).
We compared trajectories of male and female brain development using longitudinal MRI data from healthy children and adolescents. Measures included gray and white volumes for the total cerebrum, frontal, temporal, parietal, and occipital lobes, volumes of the caudate nucleus and lateral ventricles, and corpus callosal area. Trajectories were compared by shape and by volume at the mean age.
Methods
Subject Selection
Subjects were healthy singleton or twin participants in an ongoing study at the Child Psychiatry Branch of the National Institute of Mental Health which began in 1989. Singleton subjects were recruited from the local community, twin subjects were recruited nationally. Only one subject from each family was included in the study; one member of each twin pair was chosen at random.
Healthy controls were screened via previously published criteria (Giedd et al., 1996a) which included an initial telephone interview as well as parent and teacher rating versions of the Child Behavior Checklist (Achenbach and Edelbrock, 1983), a physical and neurological assessment, and neuropsychological testing. Socioeconomic status was assessed using the Hollingshead scale(Hollingshead, 1975). Those with a psychiatric diagnosis or first-degree relatives with psychiatric diagnoses, head injury, or any known learning, developmental, or medical conditions likely to have affected brain development were not accepted into the study. Exclusion criteria related to pregnancy and birth events included gestational age of < 30 weeks; very low birth weight (< 3 lbs 4 oz.), any known exposure to psychotropic medications during pregnancy, and significant perinatal complications. Longitudinal samples were acquired at approximately 2-year intervals.
Study population characteristics
387 subjects had at least one scan (209 males), 228 had 2 scans (134 males), 125 had 3 scans (84 males), 66 had 4 scans (41 males), 19 had 5 scans (9 males), 3 had 6 scans (1 male), and 1 female had 7 scans for a total of 829 scans (475 from males). See Tables 1 and 2 for details of demographic characteristics of the sample.
Table 1.
Demographic characteristics
| Group | Number | IQ (s.d.) | SES*(s.d.) | Ethnicity** | Handedness | ||
|---|---|---|---|---|---|---|---|
| Female | 178 | 111.83 (12.1) | 42.83 (19.20) | 6 | A | 151 | R |
| 18 | B | 12 | L | ||||
| 9 | H | 13 | Mixed | ||||
| 141 | W | 2 | Unk. | ||||
| 4 | O | ||||||
|
| |||||||
| Male | 209 | 114.32 (13.24) | 39.7 (18.15) | 6 | A | 187 | R |
| 13 | B | 12 | L | ||||
| 4 | H | 9 | Mixed | ||||
| 180 | W | 1 | Unk. | ||||
| 6 | O | ||||||
|
| |||||||
| Total | 387 | 113.20 (12.79) | 41.13 (18.68) | 12 | A | 338 | R |
| 31 | B | 24 | L | ||||
| 13 | H | 22 | Mixed | ||||
| 321 | W | 3 | Unk. | ||||
| 10 | O | ||||||
Socioeconomic status (SES) assessed using the Hollingshead scale(REF), which ranges from 20 (highest SES) to 134 (lowest SES).
Ethnic groups: A = Asian, B = Black, H = Hispanic, W = White, O = Other
Table 2.
Numbers of longitudinal scans per subject
| Longitudinal MRI
scan |
Female | Male | Total | ||||
|---|---|---|---|---|---|---|---|
| Number
of scans |
Mean age at
scan (s.d). |
Number
of scans |
Mean age at
scan (s.d). |
Number
of scans |
Mean age at
scan (s.d). |
||
| 1st | All subjects | 178 | 11.8 (5.1) | 209 | 11.3 (4.4) | 387 | 11.5 (4.7) |
| Singleton | 112 | 120 | 232 | ||||
| Twin | 66 | 89 | 155 | ||||
| 2nd | All subjects | 94 | 12.6 (3.6) | 134 | 13.3 (4.0) | 228 | 13.0 (3.9) |
| Singleton | 57 | 70 | 127 | ||||
| Twin | 37 | 64 | 101 | ||||
| 3rd | All subjects | 44 | 14.5 (3.7) | 84 | 15.4 (3.6) | 125 | 15.1 (3.7) |
| Singleton | 33 | 40 | 73 | ||||
| Twin | 11 | 41 | 52 | ||||
| 4th | All subjects | 25 | 16.9 (3.7) | 41 | 17.6 (3.4) | 66 | 17.4 (3.5) |
| Singleton | 24 | 25 | 49 | ||||
| Twin | 1 | 16 | 17 | ||||
| 5th | All subjects | 10 | 20.0 (4.5) | 9 | 20.2 (4.6) | 19 | 20.1 (4.4) |
| Singleton | 9 | 8 | 17 | ||||
| Twin | 1 | 1 | 2 | ||||
| 6th | All subjects | 2 | 23.3 (1.0) | 1 | 22.8 (na) | 3 | 23.1 (0.8) |
| Singleton | 2 | 1 | 3 | ||||
| Twin | 0 | 0 | 0 | ||||
| 7th | All subjects | 1 | 24.6 (na) | 0 | na | 1 | 24.6 (na) |
| Singleton | 1 | 0 | 1 | ||||
| Twin | 0 | 0 | 0 | ||||
| All scans combined | 354 | 13.0 (4.9) | 475 | 13.3 (4.7) | 829 | 13.2 (4.7) | |
| Singleton | 238 | 264 | 502 | ||||
| Twin | 116 | 211 | 327 | ||||
Approximately 40% of the scans in this study were obtained from individuals who were twins (see Table 2). Although twins in general have a higher rate of perinatal adverse events, a cross-sectional analysis found no difference between twins and singletons in these measures in the age range of our sample population.
MRI Acquisition/Analysis
All images were acquired with the same General Electric 1.5 Tesla Signa scanner located at the NIH Clinical Center in Bethesda, Maryland. A three-dimensional spoiled gradient recalled echo sequence in the steady state sequence, designed to optimize discrimination between gray matter, white matter and CSF, was used to acquire 124 contiguous 1.5 mm thick slices in the axial plane (TE/TR = 5/24 msec; flip angle = 45 degrees, NEX=1, FOV = 24cm, acquisition time 9.9 min). The images were collected in a 192 × 256 acquisition matrix and were 0-filled in k space to yield an image of 256 × 256 pixels, resulting in an effective voxel resolution of 0.9375 × 0.9375.0 × 1.5 mm. Additional details of the scanning protocol (eg, standardized head alignment) are described by Giedd et al(Giedd et al., 1996b). A Fast Spin Echo/Proton Density weighted imaging sequence was also acquired for clinical evaluation.
Brain regions were quantified using an automated technique developed at the Montreal Neurological Institute(Evans, 2005). The native MRI scans were corrected for non-uniformity artifacts(Sled et al., 1998) and registered into standardized stereotaxic space using a linear transformation(Collins et al., 1994). The registered and corrected volumes were segmented into white matter, gray matter, cerebro-spinal fluid and background using a neural net classifier(Cocosco et al., 2003; Zijdenbos et al., 2002). Anatomical regions were defined using ANIMAL (Automated Nonlinear Image Matching and Anatomical Labelling), an image registration and labelling method based on image intensity features(Collins et al., 1995). In this method segmented images undergo nonlinear registration to a probabilistic atlas, thus obtaining an anatomical label for each voxel; the labelled images are then translated back into native space using a reverse transformation to quantify volumes of specific brain regions(Collins et al., 1999). Output measures included the midsagittal area of the corpus callosum, volumes of the caudate, and lateral ventricles, and gray and white matter volumes of the total cerebrum, frontal lobes, parietal lobes, temporal, and occipital lobes. A validation study comparing this method with manual segmentation found volumetric differences to be less than 10% and volumetric overlap to be greater than 85%(Collins et al., 1995). An independent validation study of caudate volumes comparing a sample of manually defined caudate volumes from 263 pediatric subjects from this laboratory with automated measures found them to be highly correlated (Spearman’s rho > .72, p < .01 for left, right, and total caudate volumes).
Statistical Analysis
Mixed model regression, which accounts for missing data, irregular intervals between measurements, and within-person correlation was used to examine the developmental trajectories (Diggle et al., 1994). For a given structure, the ith individual’s jth measurement was modeled as Volumeij = intercept + di + B1(Age - mean Age) + B2(Age - mean Age)2 + B3(Age - mean Age)3 + eij. In the equation above, di is a normally distributed random effect that models within-person dependence. The ei term represents the usual normally distributed residual error. The B1, B2, and B3 coefficients show how volume changes with age. The intercept and B terms were modeled as fixed effects. We allowed the intercept and B terms to vary by sex group, producing two growth curves with different height and shape characteristics. F tests were used to determine whether cubic, quadratic, linear, or constant growth models best fit the data. F tests were used to determine if the diagnostic curves differed in shape, and t tests were used to determine if the groups’ curves differed in height at the average age. Fixed effects (slopes and intercepts) were used to generate fitted values used for graphing purposes. Total brain volume was calculated as the sum of volumes of gray matter, white matter, and lateral ventricles. Values were compared both with and without adjustment for total brain volume at the same age. Two False Discovery Rate procedures (Benjamini and Hochberg, 1995) were used to correct for multiple comparisons: 1) for the 14 structures/28 comparisons (14 height and 14 shape) without TCV in the model and 2) for the 13 structures/26 comparisons (13 height and 13 shape) with TCV in the model. We used a q (the percentage of false positives among all rejected hypotheses) threshold set at 0.05. The p values reported are uncorrected. Mixed model statistics were performed using SPSS 11.0.1.
Results
Robust sex differences in developmental trajectories were noted for nearly all structures with peak gray matter volumes generally occurring earlier for females. A representative scatterplot showing raw data for total brain volume and modelled developmental trajectories are presented in Figures 1-3. Summary of relevant statistical analysis is presented in Tables 3 and 4.
Figure 1.
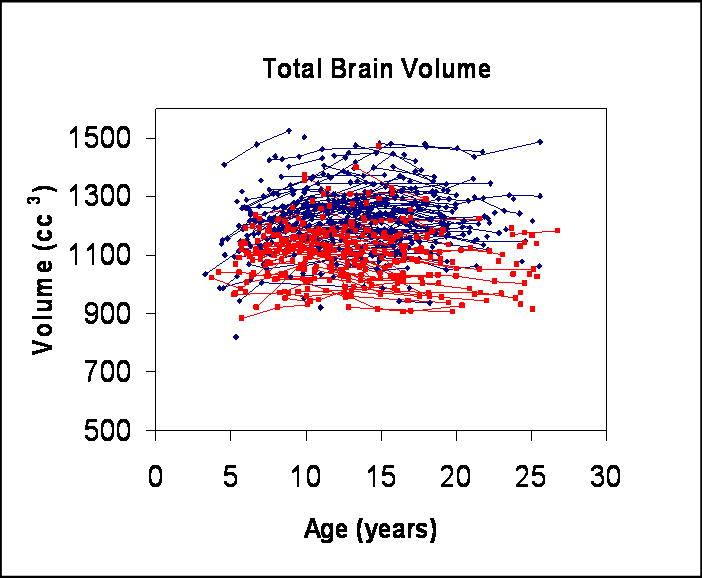
Scatterplot of longitudinal measurements of total brain volume for males (N = 475 scans, shown in dark blue) and females (N = 354 scans, shown in red).
Figure 3.
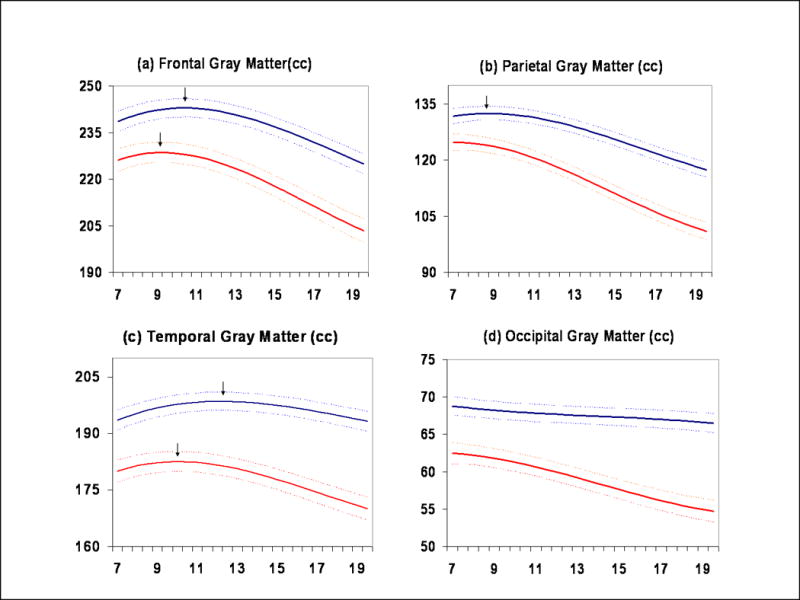
Gray matter subdivisions. (a) Frontal lobe, (b) Parietal lobe, (c) Temporal lobe, (d) Occipital lobe
Table 3.
Parameters for developmental trajectories
| Anatomical
Structure |
Best
fitting model |
Sex | Intercept (s.e.) | Age
coefficient β1 (s.e.) |
Age
coefficient β2 (s.e.) |
Age
coefficient β3 (s.e.) |
|---|---|---|---|---|---|---|
| Total cerebral
volume |
Cubic | M | 1241.116 (7.04) | 2.206 (0.64) | -0.911 (0.07) | 0.055 (0.01) |
| F | 1118.202 (7.70) | -5.452 (0.79) | -0.658 (0.09) | 0.070 (0.01) | ||
|
| ||||||
| Total gray | Cubic | M | 765.176 (4.44) | -4.165 (0.53) | -0.563 (0.06) | 0.040 (0.01) |
| F | 694.095 (4.89) | -8.932 (0.65) | -0.470 (0.07) | 0.062 (0.01) | ||
|
| ||||||
| Total white | Cubic | M | 464.477 (3.17) | 5.995 (0.33) | -0.341 (0.04) | 0.015 (0.01) |
| F | 414.069 (3.48) | 3.334 (0.40) | -0.190 (0.05) | 0.007 (0.01) | ||
|
| ||||||
| Frontal gray | Cubic | M | 240.397 (1.49) | -1.630 (0.18) | -0.223 (0.02) | 0.0144 (0.00) |
| F | 222.85 (1.65) | -2.572 (0.22) | -0.207 (0.03) | 0.020 (0.00) | ||
|
| ||||||
| Frontal white | Quadratic | M | 175.908 (1.28) | 2.638 (0.081) | 2.638 (0.08) | n.a. |
| F | 160.653 (1.41) | 1.768 (0.10) | -0.072 (0.01) | n.a. | ||
|
| ||||||
| Parietal gray | Cubic | M | 128.605 (0.90) | -1.548 (0.12) | -0.103 (0.01) | 0.011 (0.01) |
| F | 115.644 (1.00) | -2.466 (0.14) | -0.070 (0.02) | 0.015 (0.00) | ||
|
| ||||||
| Parietal white | Quadratic | M | 90.181 (0.71) | 1.224 (0.06) | -0.045 (0.01) | n.a. |
| F | 80.942 (0.78) | 0.937 (0.08) | -0.042 (0.01) | n.a. | ||
|
| ||||||
| Temporal gray | Cubic | M | 198.354 (1.22) | -0.285 (0.14) | -0.129 (0.02) | 0.007 (0.00) |
| F | 180.370 (1.34) | -1.227 (0.17) | -0.137 (0.02) | 0.011 (0.00) | ||
|
| ||||||
| Temporal white | Quadratic | M | 99.964 (0.73) | 1.353 (0.07) | -0.067 (0.01) | 0.001 (0.00) |
| F | 90.068 (0.80) | 0.879 (0.09) | -0.061 (0.01) | 0.002 (0.00) | ||
|
| ||||||
| Occipital gray | Cubic | M | 67.511 (0.58) | -0.128 (0.07) | 0.003 (0.01) | -0.001 (0.00) |
| F | 59.012 (0.64) | -0.778 (0.08) | -0.011 (0.01) | 0.004 (0.00) | ||
|
| ||||||
| Occipital white | Quadratic | M | 44.707 (0.39) | 0.827 (0.05) | -0.015 (0.01) | n.a. |
| F | 39.396 (0.43) | 0.463 (0.06) | -0.023 (0.01) | n.a. | ||
|
| ||||||
| Corpus callosum | Linear | M | 54.185 (5.11) | 3.87 (0.39) | n.a. | n.a. |
| F | 53.640 (0.56) | 3.45 (0.48) | n.a. | n.a. | ||
|
| ||||||
| Caudate nucleus | Cubic | M | 10.054 (0.07) | 0.007 (0.01) | -0.004 (0.00) | 0.000 (0.00) |
| F | 9.659 (0.08) | -0.032 (0.01) | -0.005 (0.00) | 0.00 (0.00) | ||
|
| ||||||
| Lateral ventricles | Quadratic | M | 11.431 (0.40) | 0.249 (0.03) | -0.005 (0.00) | n.a. |
| F | 10.199 (0.43) | 0.219 (0.03) | -0.007 (0.00) | n.a. | ||
Intercept is the predicted value at the average age of the sample (13.1 years). s.e. = standard error.
Table 4.
Sex differences of developmental trajectories
| Structure | Male-female comparisons | Comparisons adjusted for total
brain volume |
||||||
|---|---|---|---|---|---|---|---|---|
| Height Differences | Shape
Differences |
Height
Differences |
Shape
Differences |
|||||
| p values | F | p values | F | p values | F | p values | F | |
| Total cerebral volume | 0.00 | 138.85 | 0.00 | 30.44 | n.a. | n.a. | ||
| Total gray matter | 0.00 | 115.79 | 0.00 | 14.38 | 0.17 | 1.91 | 0.23 | 1.44 |
| Total white matter | 0.00 | 114.51 | 0.00 | 23.55 | 0.16 | 1.95 | 0.01 | 2.11 |
| Frontal gray matter | 0.00 | 62.47 | 0.00 | 4.48 | 0.00 | 20.65 | 0.00 | 6.84 |
| Frontal white matter | 0.00 | 64.07 | 0.00 | 27.97 | 0.37 | 0.82 | 0.00 | 4.55 |
| Parietal gray matter | 0.00 | 93.47 | 0.00 | 11.85 | 0.14 | 2.14 | 0.28 | 1.3 |
| Parietal white matter | 0.00 | 76.67 | 0.00 | 10.74 | 0.22 | 1.59 | 0.04 | 2.81 |
| Temporal gray matter | 0.00 | 98.07 | 0.00 | 9.02 | 0.91 | 0.01 | 0.03 | 3.62 |
| Temporal white matter | 0.00 | 83.08 | 0.00 | 17.33 | 0.23 | 1.42 | 0.32 | 1.16 |
| Occipital gray matter | 0.00 | 96.66 | 0.00 | 13.38 | 0.02 | 5.31 | 0.01 | 4.34 |
| Occipital white matter | 0.00 | 85.70 | 0.00 | 15.26 | 0.00 | 12.4 | 0.05 | 2.67 |
| Caudate nucleus | 0.00 | 14.79 | 0.00 | 8.41 | 0.06 | 4.04 | 0.71 | 1.04 |
| Corpus callosum area | 0.47 | 0.51 | 0.76 | 0.27 | 0.02 | 9.82 | 0.09 | 2.89 |
| Lateral ventricles | 0.03 | 4.82 | 0.48 | 0.73 | 0.01 | 6.92 | 0.64 | 0.44 |
Numbers in bold type are statistically significant following Bonferonni correction for multiple comparisons.
Consistent with previous investigations (Giedd et al., 1999), mean total cerebral volume was approximately 10% larger in males. Total cerebral volume peaked at 10.5 years in females and 14.5 years in males (Figure 2a). There was a greater decline in total cerebral size in females during the second decade. Lateral ventricle volumes were larger in males but the shape of the trajectory was not significantly different in males and females.
Figure 2.
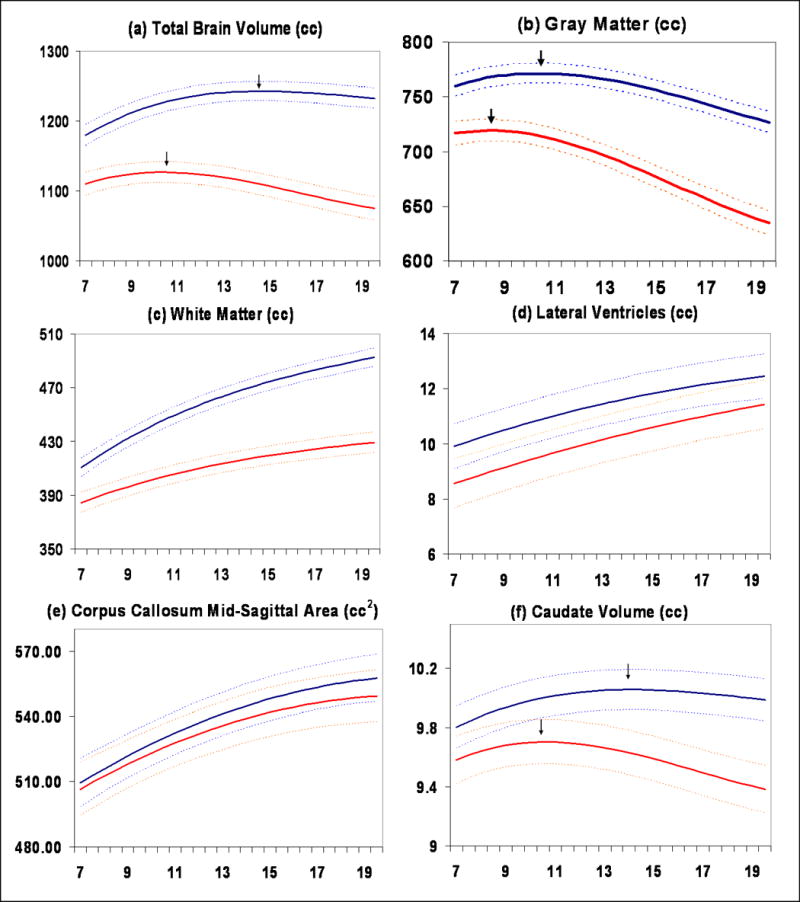
Mean volume by age in years for males (N = 475 scans) and females (N = 354 scans). Middle lines in each set of three lines represent mean values, and upper and lower lines represent upper and lower 95% confidence intervals. All curves differed significantly in height and shape with the exception of lateral ventricles, in which only height was different, and mid-sagittal area of the corpus callosum, in which neither height nor shape were different. (a) Total brain volume, (b) Gray matter volume, (c) White matter volume, (d) Lateral ventricle volume, (e) Mid-sagittal area of the corpus callosum, (f) Caudate volume
Both cortical and subcortical gray matter volumes exhibited an inverted U shaped trajectory. Total gray matter peaked at age 8.5 in females and 10.5 in males (Figure 2b). The caudate nucleus, a subcortical gray matter structure, peaked at age 10.5 in females and 14 in males (Figure 2f). The shapes of cortical gray matter trajectories were lobar specific (Figure 3a-d), although the pattern of earlier peak size in females was consistent. For instance, in the frontal lobe peak gray matter volume occurred at age 9.5 for females and 10.5 for males, in the parietal lobe at age 7.5 in females and 9 in males, and in the temporal lobe at 10 for females and 11 for males. The shape of the developmental trajectories for the caudate was more similar to that of gray matter in the frontal lobe than in the parietal or temporal lobes. Male brains consistently showed a higher rate of change throughout childhood and adolescence, although rates started to converge as subjects reached their late teens and early twenties (see Figure 5).
Figure 5.
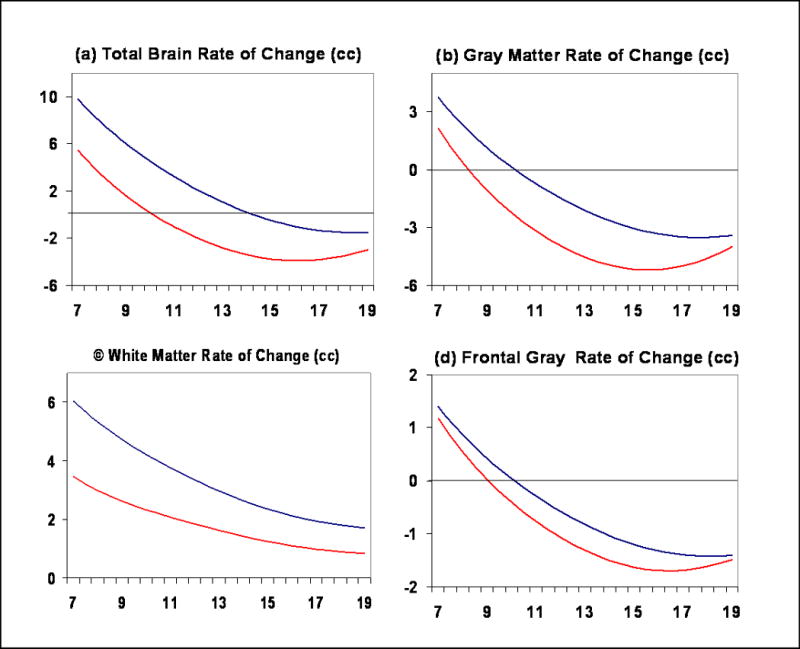
Change in brain volume (cc) every six months for (a) Total brain volume, (b) Gray matter, (c) White matter, (d) Frontal lobe gray matter. Positive values represent increasing volume and negative values loss of volume.
Total white matter volume increased with age in both sexes. The trajectories diverged as males increased more rapidly during adolescence (Figure 2c). This pattern is consistent across white matter of the frontal, temporal, parietal, and occipital lobes (Figure 3a-d).
The developmental trajectory of the midsagittal corpus callosum area was not different in shape or height between males and females (Figure 1e). Similarly to what is seen for gray matter, the rate of increase was higher in males than in females, although the more rapid rate of increase in males continued longer in white matter.
In order to determine whether differences in individual regions were driven by the overall larger brain volume in males, we also compared the shape and height of trajectories after covarying for total brain volume (See Table 4). Developmental trajectories in the frontal lobe were significantly different between males and females even after removing effects of differing overall brain size. The trajectories for gray matter were different both in height and shape, with more frontal gray matter in females than males, while for frontal lobe white matter the shape of the trajectory was different but the height was not. The shape of the trajectory for occipital gray matter was different, although height was not, while the converse was true for occipital white matter, in which males continued to have larger white matter volumes throughout development. Trajectories for the temporal lobes, parietal lobes, and caudate were no longer significantly different. Height differences persisted for the lateral ventricles, with larger ventricles still seen in males. The area of the corpus callosum was larger in females, with no differences in shape.
Discussion
Here we have demonstrated the importance of considering trajectories rather than group averages across broad age spans in investigations of brain sexual dimorphism. The finding that sex differences were age-dependent may partially account for discrepant findings of sexual dimorphism in the literature.
Previous work comparing brain growth patterns between males and females has been sparse and limited by small sample sizes or cross-sectional designs. In a cross-sectional sample of 118 healthy children and adolescents growth rates were faster in males than females for total gray and white matter volumes and the area of the corpus callosum (De Bellis et al., 2001). In this study, however, a linear model was used to describe changes with age, limiting the ability to detect more complex interactions.
The one currently available longitudinal study is based on an earlier sample from the same population reported here(Giedd et al., 1999). This study was the first to show that brain development follows a nonlinear trajectory, but did not find significant interactions of sex with age. Additionally, the age at which peak volume is reached is somewhat different between the two studies. For example, frontal gray matter volume peaks in the earlier study at 11 years in females and 12.1 in males, while in the current study frontal gray matter peaks at 10.5 and 11.5 for females and males, respectively. The differences in findings are likely related to the pronounced increase in sample size and numbers of longitudinal scans in the current study (145 children and adolescents contributing 243 scans in the previous study, versus 387 subjects contributing 829 scans in the current report), and to the difference in models for longitudinal changes; the previous study was constrained to linear and quadratic models, while many of the structures in the present report are best described by a cubic model. These differences highlight the need for large longitudinal samples to describe the complex developmental changes occurring over this age range.
The proper interpretation of sex differences in brain morphometry given the overall larger brain size in males has been a much-debated issue. Several studies in adults have reported that if total brain size is taken into account, female brains have proportionately more gray matter. It is not clear to what extent these proportional differences are related to scaling issues. It has been proposed that white matter will increase more quickly than gray matter with increasing brain volume, due to the greater volume required by axons associated with a given surface area as they lengthen to cover longer distances between brain regions(Prothero, 1997). Luders et al. found that sex did not contribute significantly to explaining proportional differences between male and female adults, and that brain size itself was the strongest predictor, supporting the potential relevance of scaling factors (Luders et al., 2002). Studies across species of widely varying brain sizes have tended to confirm this empirically, finding that the relationship of gray and white matter volumes is exponential rather than linear(Prothero, 1997; Zhang and Sejnowski, 2000).
Our findings here of proportionately greater frontal gray matter in females is consistent with this, although the question remains as to which factors contribute to the larger brain size in males. Male/female brain size differences are often attributed to the greater average height of males (Dekaban and Sadowsky, 1978; Fausto-Sterling, 1992). However, this is clearly not the case for pediatric populations, where data from the Center for Disease Control’s National Center for Health Statistics indicate average height for girls is larger from ages 10 to 13.5 and cumulative mean height across the first 15 years of life for boys and girls are within 1% of each other (Kuczmarski et al., 2002). The decreasing brain volume and increasing height after age 12 further suggest a decoupling of these parameters.
The observation that gray matter volumes peak approximately one to two years earlier in females than males, corresponding to the average age difference at puberty, is suggestive that the switch from progressively increasing to decreasing gray matter volume is associated with pubertal maturation. In this study we did not have pubertal measures and could not examine this relationship directly. There has been debate whether sex hormones serve primarily to activate brain structures formed in earlier stages of development, or if they also have a direct effect on brain structure. Work in rodent models has shown that exposure to pubertal hormones during adolescence has effects on behaviour which persist after the hormones are removed, supporting a long-term effect which could be related to changes in brain structure(Schulz and Sisk, 2006; Sisk and Foster, 2004). It has also been shown that administration of hormones before adolescence does not result in the same behavioural changes as when given during adolescence (Meek et al., 1997), implying that pubertal effects are dependent on interactions with other brain developmental processes. Disentangling the effects of puberty from those associated with chronological age is a difficult task, particularly in humans where direct manipulation of endocrine exposure in healthy adolescents is not ethically feasible. An additional complication is that puberty itself is not an easily measurable process. Different physiologic systems become sexually mature at different rates, and the effect of endocrine factors during puberty is strongly influenced by the timing as well as the magnitude of fluctuating levels, making an accurate description of an adolescent’s degree of physical maturation less than straightforward. Such work is necessary, however, in order to better understand the developmental pathways responsible for the increasing risk of most psychiatric disorders during this period, and the origin of sex-specific differences in symptoms which arise during adolescence in conditions such as Major Depressive Disorder (Dahl, 2004; Kessler et al., 2005; Steinberg et al., 2006).
Differences in brain size between males and females should not be interpreted as implying any sort of functional advantage or disadvantage. Size/function relationships are complicated by the inverted U shape of developmental trajectories and by the myriad of factors contributing to structure size, including the number and size of neurons and glial cells, packing density, vascularity, and matrix composition. However, an understanding of the sexual dimorphism of brain development, and the factors that influence these trajectories, may have important implications for the field of developmental neuropsychiatry where nearly all of the disorders have different ages of onset, prevalence, and symptomatology between boys and girls.
Figure 4.
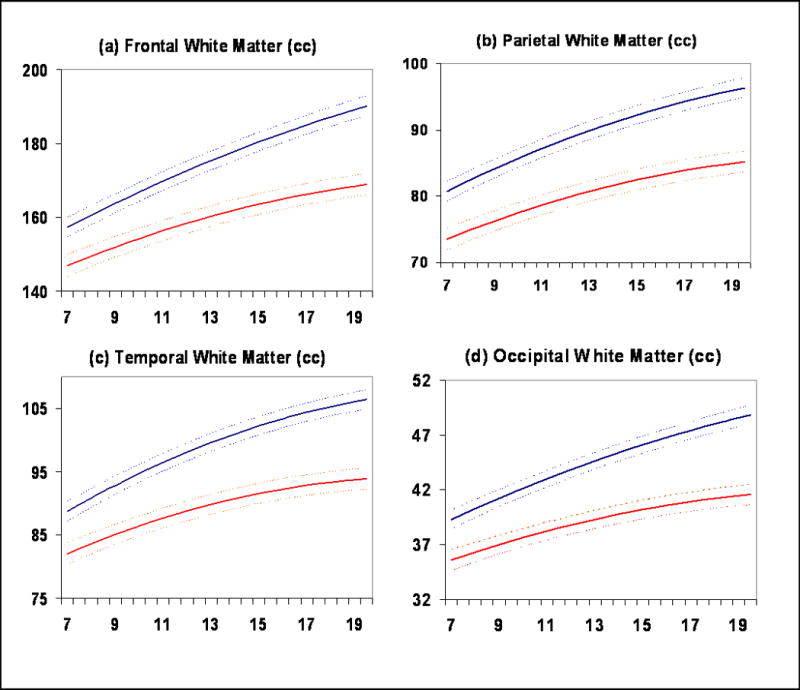
White matter subdivisions. (a) Frontal lobe, (b) Parietal lobe, (c) Temporal lobe, (d) Occipital lobe
Acknowledgments
RL and JG wrote the paper, oversaw data acquisition and analysis. JG conceived the study. NG was involved in acquiring and interpreting the data. AZ, JL, AE, JB, and CV were involved in analyzing the images. EM and GW were involved in subject recruitment and screening. DG and LC conducted the statistical analysis. PT was involved in image analysis and data interpretation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Ref
- Achenbach TM, Edelbrock CS. Manual for child behavior checklist and revised behavior profile. Department of Psychiatry, University of Vermont; Burlington, VT: 1983. [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society Series B. 1995:289–300. [Google Scholar]
- Cairns E, Malone S, Johnston J, Cammock T. Sex differences in children’s group embedded figures test performance. Person individ Diff. 1985;6:653–654. [Google Scholar]
- Cocosco CA, Zijdenbos AP, Evans AC. A fully automatic and robust brain MRI tissue classification method. Med Image Anal. 2003;7:513–527. doi: 10.1016/s1361-8415(03)00037-9. [DOI] [PubMed] [Google Scholar]
- Collins DL, Holmes CJ, Peters TM, Evans AC. Automatic 3-D model-based neuroanatomical segmentation. Human Brain Mapping. 1995;3:190–208. [Google Scholar]
- Collins DL, Neelin P, Peters TM, Evans AC. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr. 1994;18:192–205. [PubMed] [Google Scholar]
- Collins DL, Zijdenbos AP, Baare WFC, Evans AC. Proceedings of the Annual Conference on Information Processing in Medical Imaging (IPMI) Springer; Visegrad, Hungary: 1999. ANIMAL+INSECT: Improved cortical structure segmentation; pp. 210–223. [Google Scholar]
- Dahl RE. Adolescent brain development: a period of vulnerabilities and opportunities. Keynote address. Ann N Y Acad Sci. 2004;1021:1–22. doi: 10.1196/annals.1308.001. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Keshavan MS, Beers SR, Hall J, Frustaci K, Masalehdan A, Noll J, Boring AM. Sex differences in brain maturation during childhood and adolescence. Cereb Cortex. 2001;11:552–557. doi: 10.1093/cercor/11.6.552. [DOI] [PubMed] [Google Scholar]
- Dekaban AS, Sadowsky D. Changes in brain weight during the span of human life: relation of brain weights to body heights and body weights. Annals of Neurology. 1978;4:345–356. doi: 10.1002/ana.410040410. [DOI] [PubMed] [Google Scholar]
- Diggle PJ, Liang KY, Zeger SL. Analysis of longitudinal data. Oxford University Press; Oxford: 1994. [Google Scholar]
- Evans AC. Large-scale morphometric analysis of neuroanatomy and neuropathology. Anat Embryol (Berl) 2005;210:439–446. doi: 10.1007/s00429-005-0045-1. [DOI] [PubMed] [Google Scholar]
- Fausto-Sterling A. Myths of gender : biological theories about women and men. 2. BasicBooks; New York, NY: 1992. [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Snell JW, Lange N, Rajapakse JC, Casey BJ, Kozuch PL, Vaituzis AC, Vauss YC, Hamburger SD, Kaysen D, Rapoport JL. Quantitative magnetic resonance imaging of human brain development: ages 4-18. Cereb Cortex. 1996a;6:551–560. doi: 10.1093/cercor/6.4.551. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Snell JW, Lange N, Rajapakse JC, Casey BJ, Kozuch PL, Vaituzis AC, Vauss YC, Hamburger SD, Kaysen D, Rapoport JL. Quantitative magnetic resonance imaging of human brain development: ages 4-18. Cereb Cortex. 1996b;6:551–560. doi: 10.1093/cercor/6.4.551. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Snell JW, Lange N, Rajapakse JC, Kaysen D, Vaituzis AC, Vauss YC, Hamburger SD, Kozuch PL, Rapoport JL. Quantitative magnetic resonance imaging of human brain development: ages 4-18. Cerebral Cortex. 1996c;6:551–560. doi: 10.1093/cercor/6.4.551. [DOI] [PubMed] [Google Scholar]
- Goldstein JM, Seidman LJ, Horton NJ, Makris NK, Kennedy DN, Caviness VS, Jr, Faraone SV, Tsuang MT. Normal sexual dimorphism of the adult human brain assessed by in vivo magnetic resonance imaging. Cerebral Cortex. 2001;11:490–497. doi: 10.1093/cercor/11.6.490. [DOI] [PubMed] [Google Scholar]
- Gouchie C, Kimura D. The relationship between testosterone levels and cognitive ability patterns. Psychoneuroendocrinology. 1991;16:323–334. doi: 10.1016/0306-4530(91)90018-o. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Four Factor Index of Social Status. Yale University Department of Sociology; New Haven: 1975. [Google Scholar]
- Johnson ES, Meade AC. Developmental patterns of spatial ability: an early sex difference. Child Dev. 1987;58:725–740. doi: 10.1111/j.1467-8624.1987.tb01413.x. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kraemer HC, Yesavage JA, Taylor JL, Kupfer D. How can we learn about developmental processes from cross-sectional studies, or can we? Am J Psychiatry. 2000;157:163–171. doi: 10.1176/appi.ajp.157.2.163. [DOI] [PubMed] [Google Scholar]
- Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, Wei R, Curtin LR, Roche AF, Johnson CL. 2000 CDC Growth Charts for the United States: methods and development. Vital Health Stat. 2002;11:1–190. [PubMed] [Google Scholar]
- Luders E, Steinmetz H, Jancke L. Brain size and grey matter volume in the healthy human brain. Neuroreport. 2002;13:2371–2374. [PubMed] [Google Scholar]
- Meek LR, Romeo RD, Novak CM, Sisk CL. Actions of testosterone in prepubertal and postpubertal male hamsters: dissociation of effects on reproductive behavior and brain androgen receptor immunoreactivity. Horm Behav. 1997;31:75–88. doi: 10.1006/hbeh.1997.1371. [DOI] [PubMed] [Google Scholar]
- Prothero J. Cortical scaling in mammals: a repeating units model. J Hirnforsch. 1997;38:195–207. [PubMed] [Google Scholar]
- Schulz KM, Sisk CL. Pubertal hormones, the adolescent brain, and the maturation of social behaviors: Lessons from the Syrian hamster. Mol Cell Endocrinol. 2006;254-255:120–126. doi: 10.1016/j.mce.2006.04.025. [DOI] [PubMed] [Google Scholar]
- Sisk CL, Foster DL. The neural basis of puberty and adolescence. Nat Neurosci. 2004;7:1040–1047. doi: 10.1038/nn1326. [DOI] [PubMed] [Google Scholar]
- Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998;17:87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- Steinberg L, Dahl RE, Keating D, Kupfer DJ, Masten AS, Pine DS. The study of developmental psychopathology in adolescence: integrating affective neuroscience with the study of context. In: Cicchetti D, Cohen DJ, editors. Developmental psychopathology. John Wiley & Sons; Hoboken, N.J.: 2006. pp. 710–741. [Google Scholar]
- Zhang K, Sejnowski TJ. A universal scaling law between gray matter and white matter of cerebral cortex. Proc Natl Acad Sci U S A. 2000;97:5621–5626. doi: 10.1073/pnas.090504197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zijdenbos AP, Forghani R, Evans AC. Automatic “pipeline” analysis of 3-D MRI data for clinical trials: application to multiple sclerosis. IEEE Trans Med Imaging. 2002;21:1280–1291. doi: 10.1109/TMI.2002.806283. [DOI] [PubMed] [Google Scholar]


