Abstract
Just as the blood-brain barrier (BBB) is not a static barrier, the adipocytes are not inert storage depots. Adipokines are peptides or polypeptides produced by white adipose tissue; they play important roles in normal physiology as well as in the metabolic syndrome. Adipokines secreted into the circulation can interact with the BBB and exert potent CNS effects. The specific transport systems for two important adipokines, leptin and tumor necrosis factor alpha, have been characterized during the past decade. By contrast, transforming growth factor beta-1 and adiponectin do not show specific permeation across the BBB, but modulate endothelial functions. Still others, like interleukin-6, may reach the brain but are rapidly degraded. This review summarizes current knowledge and recent findings of the rapidly growing family of adipokines and their interactions with the BBB.
Keywords: adipocytes; adipokines; cytokines; peptides, polypeptides, blood-brain barrier; leptin; TNFα; TGF-β; IL-6, adiponectin; angiotensinogen; PAI-1; relaxin; resistin; ASP; RBP
1. Introduction
1.1. Blood-brain barrier (BBB)
The BBB constitutes a large interface between the circulation and the central nervous system (CNS), consisting of brain and spinal cord. Its primary component is a monolayer of endothelial cells forming the outer wall of capillaries and venules. These microvascular endothelial cells have few fenestrations, pinocytic vesicles, or transendothelial channels, and are joined by tight junctions. A continuous basement membrane, astrocytic endfeet, and pericytes reinforce barrier function from the basolateral side facing the extracellular matrix. The surface area of the BBB is 100 to 150 cm2/g, by contrast to the circumventricular organs (CVOs) which have a total surface area of only 0.02 cm2/g tissue (15;114). This immense neurovascular interface controls the penetration of amino acids, peptides, polypeptides, and proteins as well as many other molecules (5;39;49;52;53;55;95;137). In obesity and metabolic disorders, the BBB mediates the interactions of a specific subset of serum factors with the CNS. Adipokines are prominent among these players.
1.2. Adipokines
Adipokine is a term applied to biologically active substances found in the adipocytes of white fat (adipose) tissue. Adipokines may be synthesized at other sites and participate in functions unrelated to those within adipose tissue. Many exert proinflammatory effects and may be causally involved in obesity and diabetes. These adipokines include leptin, tumor necrosis factor alpha (TNFα), interleukin (IL)-6, plasminogen activator inhibitor-1 (PAI-1), angiotensinogen, and resistin. A few others, particularly adiponectin and transforming growth factor beta-1 (TGF-β1), are anti-inflammatory and may exert protective functions against metabolic disturbance.
1.3. Adipokines interact with the BBB
Certain adipokines act on receptors located in CNS regions participating in the regulation of nutritional disorders. The hypothalamus is one of the main targets. Contrary to a long-standing dogma, the hypothalamus has an intact BBB (50;99). The interactions of adipokines with the BBB can fall into three categories: changing endothelial function and signaling; modulating signals from other adipokine and cytokines; and permeation across the BBB by themselves. The rich information from leptin and TNFα provides insights into transcellular transport across the BBB and provides guidance and contrasts with other less well-studied adipokines. The recognition of signal amplification and secretory functions of the BBB by adipokines as well as other cytokines is also beginning to form a new subspecialty in neuroendocrinology.
2. Leptin
2.1. Leptin resistance
Leptin production correlates with the fat mass. An exception is the ob/ob mouse, which has a targeted deletion of leptin and develops adiposity, diabetes, and insulin resistance. The ob/ob mouse responds to peripheral leptin treatment with rapid weight loss, further supporting an anorexigenic role for leptin. The phenomenon of leptin resistance is seen in diet-induced obesity, in which peripheral administration or even elevated CSF levels of leptin fail to counteract the weight gain (22;35;104;107;125;131). As leptin concentrations in subjects with diet-induced obesity are high and the transport system for leptin to permeate the BBB has limited capacity, the BBB is a potential regulatory site for leptin resistance. In hypothalamic neurons, defective signal transduction downstream to the leptin receptors can also contribute to leptin resistance (18;27;31).
2.2. Physiological regulation of leptin transport
Transport of leptin into brain is reduced by fasting (45) and by genetic mutation and dysfunction of the transporting receptor ObRa (10;54). It is increased by pretreatment with glucose (46). The saturable transport system for leptin has been demonstrated both in vivo (9) and in vitro (67), and shows a diurnal rhythm (93). It is partially saturated in mice with normal weight (6), being even more so in obese mice (7).
2.3. Leptin and urocortin transport
Leptin activates the BBB transport of urocortin, in contrast to the lack of effect of many other ingestive peptides that we have tested so far (48;51). This facilitatory effect of leptin occurs in cultured endothelial cells as well as in intact animals (121). The interactions appear to involve membrane events at the level of their respective receptors. Urocortin, which can enter the cells by CRHR1 or CRHR2-mediated endocytosis (123), does not cause reciprocal enhancement of leptin endocytosis, but potentiates cellular signaling including activation of Signal transducer and activator for transcription (Stat) 1 and 3 (84). Thus, leptin, with anorexic effects limited by saturation at the BBB, can activate the blood-to-brain transport of another anorexic agent, urocortin.
2.4. Leptin receptors ObRa-ObRd
The short isoform of the leptin receptor, ObRa, plays a major role in mediating leptin transport across the BBB (9;10;19;54). ObRa has a high level of expression in microvessels and high efficacy in mediating endocytosis in cell systems. The long isoform, ObRb, contains cytoplasmic domains that interact with the Stats and plays a major role in JAK/Stat signaling. Nevertheless, ObRa, ObRb, ObRc, and ObRd receptor subtypes can all mediate the binding and endocytosis of leptin in HEK293 cells overexpressing these receptors. Internalized leptin can remain intact for at least an hour, and shows substantial exocytosis. The stability of endocytosed leptin inside the cells or in the exocytosis medium can be further enhanced by inhibition of lysosomal activity. Thus, ObRb also can function as a transporting receptor, blurring the distinction of transport and signaling functions by these receptors (124). Moreover, once internalized, the intracellular degradation pattern and exocytosis of leptin are independent of the receptor subtype.
2.5. ObRe
ObRe is the soluble leptin receptor circulating in blood. It binds leptin and interferes with ObRb-mediated signaling. Recent evidence in vitro and in vivo shows that this soluble receptor for leptin serves as an antagonist not only in the signaling but also in the transport of leptin across endothelial cells (122).
3. TNFα
3.1. Background
TNFα is an adipokine as well as a proinflammatory cytokine from other cellular sources. TNFα deficiency (knockout) and antibodies against TNFα improve insulin resistance, consistent with a role of TNFα in modulating insulin signaling (59). Originally identified as an endotoxin-induced serum factor that causes tumor necrosis, this cachectin induces weight loss in cancer (23). TNFα and leptin are transported across the BBB by their respective transport systems, in contrast to the other adipokines studied so far (82). Interactions of TNFα and leptin may take place at the BBB level. In immortalized cerebral microvessel endothelial cells, cross-talk of TNFα and leptin occurs rapidly and involves alteration of phosphoproteomes, as described below.
3.2 Cross-talk of TNFα and leptin at the BBB
The profound alterations in cellular signaling and the intriguing interactions between TNFα and leptin represent some of the most fascinating changes occurring at the BBB. Although investigations are ongoing, our preliminary study has identified several classes of phosphoproteins that participate in a variety of biological functions. This study used b.End3 cells which are derived from mouse brain endothelia and express receptors for both leptin and TNFα. After stimulation with leptin or TNFα for 15 min, a series of proteins was phophorylated. These phosphoproteins and their co-precipitating partners were isolated by use of a phosphoprotein purification kit, separated by two-dimensional gel electrophoresis, and subjected to MALDI-TOF mass spectrometric analyses. Among 1839 ± 66 spots (pI 3-10) and 2216 ± 116 (pI 4-7) on the gel, 75 showed significant changes in density (level of expression) after leptin or TNFα. Combined treatment with these adipokines caused further changes, indicating their interaction on cellular signaling. Interestingly, co-treatment caused a dramatic decrease in the phosphorylation of proteasome subunits, an effect not seen after single treatment. Table 1 lists 31 proteins identified by peptide mass fingerprinting, with their normalized intensities in each gel. There are five major groups: cytoskeleton components (1 - 4), proteasome subunits (5 - 10), translation elongation factors (11 - 13), ribonucleoproteins (14 - 16), and others (17 - 31).
Table 1.
Induction of phosphoproteome by control (C), leptin (L), TNFα (T), or co-treatment (TL). In this list of identified phophoproteins and their in-gel densities, the relative spot intensity, which reflects the level of expression, is normalized to volume, and expressed as PPM of the total spot volume within the gel. NCBI, %, pI, and MW denote NCBI accession number, sequence coverage, isoelectric point (theoretical), and molecular weight in kD (theoretical) for each protein. In the gel, proteins were usually found within ±0.5 pI units and ± 5 kD range from their theoretical values. Sp. denotes taxon: b - bovine, h - human, m - mouse, p - pig, r - rat.
| No. | Sp. | Protein | NCBI | % | pI | MW | C | T | L | TL |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | m | brain α-tropomyosin (TMBr-3) | 13270471 | 37 | 4.7 | 32.72 | 1671 | 2292 | 412 | 1182 |
| 2 | p | chain b, tubulin α-β dimer | 3745822 | 22 | 5.2 | 48.33 | 614 | 672 | 2449 | 1226 |
| 3 | m | syntaxin 7 | 7949144 | 19 | 5.6 | 29.97 | 679 | 1038 | 2027 | 2036 |
| 4 | m | villin 2 | 2832989 | 23 | 5.9 | 69.53 | 341 | 1928 | 2147 | 531 |
| 5 | b | proteasome subunit, α type 1 | 21465647 | 44 | 6.1 | 29.82 | 2447 | 2884 | 2173 | 10 |
| 6 | m | proteasome subunit, β type 2 | 6755200 | 55 | 6.5 | 23.06 | 4402 | 4664 | 3037 | 1020 |
| 7 | m | proteasome subunit Lmp7 | 673450 | 56 | 6.9 | 23.26 | 1109 | 1242 | 1193 | 10 |
| 8 | m | proteasome subunit, α type 2 | 6679497 | 59 | 8.6 | 26.02 | 4216 | 7358 | 4443 | 1262 |
| 9 | r | proteasome subunit, α type 3 | 8394066 | 48 | 5.3 | 28.63 | 4677 | 100 | 1737 | 906 |
| 10 | h | proteasome 26S subunit, type 2 | 4506209 | 40 | 5.7 | 49.02 | 351 | 1991 | 1242 | 335 |
| 11 | m | translation elongation factor 1δ-b | 12963597 | 42 | 4.9 | 31.39 | 767 | 2364 | 783 | 1086 |
| 12 | m | elongation factor Tu, mitochondrial | 20843609 | 34 | 7.3 | 49.89 | 739 | 1234 | 2373 | 2081 |
| 13 | m | translation elongation factor 1β | 12856949 | 30 | 4.9 | 28.82 | 244 | 1173 | 2544 | 1596 |
| 14 | m | hnRP C | 13435678 | 37 | 5.0 | 32.26 | 453 | 100 | 100 | 567 |
| 15 | m | hnRP A2/B1 | 21071091 | 47 | 8.7 | 36.02 | 10 | 446 | 588 | 10 |
| 16 | m | poly(rC) binding protein 1 | 6754994 | 42 | 6.7 | 37.99 | 849 | 100 | 560 | 785 |
| 17 | h | septin 7 | 4502695 | 25 | 9.0 | 49.05 | 10 | 10 | 725 | 10 |
| 18 | h | JM2 protein | 14759872 | 25 | 9.2 | 44.24 | 10 | 10 | 498 | 10 |
| 19 | m | aldehyde dehyrdogenase 8, A1 | 23512292 | 22 | 7.8 | 54.39 | 10 | 10 | 100 | 10 |
| 20 | r | serum-inducible kinase | 13929172 | 16 | 9.0 | 78.99 | 10 | 638 | 676 | 1117 |
| 21 | r | Cytochrome P450, subfamily IIC | 9506529 | 25 | 7.8 | 57.59 | 1264 | 3946 | 10 | 2970 |
| 22 | h | ketohexokinase, isoform a | 4557693 | 26 | 5.6 | 33.11 | 630 | 420 | 10 | 442 |
| 23 | r | collapsin response mediator 2 | 1351260 | 40 | 6.0 | 62.66 | 1806 | 3974 | 2226 | 813 |
| 24 | h | follistatin precursor | 120547 | 18 | 5.9 | 39.52 | 521 | 2586 | 2418 | 576 |
| 25 | m | similar to septin 6 | 20836152 | 21 | 5.9 | 52.86 | 554 | 2507 | 1903 | 827 |
| m | TNFA-IP3 interacting protein 2 | 20532344 | 21 | 6.0 | 49.82 | |||||
| m | pyruvate dehydrogenase kinase | 19526816 | 21 | 6.1 | 46.28 | |||||
| m | PI3-kinase p85-gamma subunit | 9910789 | 16 | 5.7 | 54.74 | |||||
| 26 | m | transaldolase 1 | 20846995 | 46 | 6.6 | 37.54 | 150 | 1121 | 1050 | 150 |
| 27 | m | transaldolase 1 | 20846995 | 33 | 6.6 | 37.54 | 3872 | 6217 | 6231 | 7766 |
| 28 | r | transaldolase 1 | 13929152 | 38 | 6.6 | 37.63 | 791 | 3634 | 2192 | 4711 |
| 29 | m | transaldolase 1 | 20846995 | 38 | 6.6 | 37.54 | 749 | 3777 | 532 | 5595 |
| 30 | m | kinesin family member C1 (NS) | 16195832 | 21 | 6.5 | 47.06 | 150 | 1795 | 740 | 1073 |
| 31 | h | helicase-like protein 2 | 13514813 | 22 | 6.7 | 73.65 | 261 | 1819 | 1129 | 394 |
The gel images in figure 1 include a range of isoelectric point from 5.8 to 6.6, and molecular weights from 35 to 90 kD. The distribution of spots in the pI - MW plot shows no significant clustering. This suggests that the pool of identified proteins is random and representative of the whole population of differentially regulated proteins. Compared with the PBS control, there was upregulation (greater than 50% changes in the spot intensity) of phosphoproteins by both TNFα (25 ng/ml) and leptin (1 μg/ml). The increase of overall abundance of phosphoproteins was 61.3 % in the TNFα-treated and 58.1 % in the leptin-treated cells. Combined treatment, however, counteracted some of the effects of single treatment, with an overall increase of phosphoprotein of only 38.7 % (Fig.1).
Fig.1.

Gel distribution in pI-MW plot.
Apart from upregulation of the phosphoproteome, there was also downregulation. TNFα treatment caused 9.7% decrease of selective phosphoproteins, measured by a greater than 50% decrease in spot intensity. Leptin caused a 16.1% decrease, whereas co-treatment with leptin and TNFα induced a further down-regulation of 41.9% and 48.4% of the identified proteins, respectively.
Among the cytoskeleton proteins in b.End3 cells that are upregulated by adipokine treatment, villin 2 links the cell membrane to actin filaments in the apical region of polarized cells (3), and is required for phagocytosis involving binding with phosphatidylinositol 4,5-biphosphate, a substrate for PI3 kinase (25). PI3 kinase participates in leptin signaling (36) and can be activated by TNFα in endothelial cells (12;66). Thus, upregulation of villin 2 might be implicated in the regulation of receptor-mediated endocytosis (21;127). Similarly, syntaxin 7 is a part of SNARE (soluble N-ethyl maleimide - sensitive factor attachment protein receptor) trafficking machinery and also has a prominent role in endocytosis. It is a plasma membrane protein that co-precipitates with late endosome compartments, and is required for the fusion of late endosome and lysosomes (44;75). α-Tropomyosin affects membrane blebbing (37) whereas tubulin is a major microtubule constituent and participates in translocation of the endocytotic recycling compartment (63). Thus, the altered phosphorylation patterns of these proteins suggest their involvement in the transport of TNFα and leptin across the endothelial cells.
The proteasome is a 700 kD multi-unit protease for ubiquitin-conjugated proteins and participates in a variety of cellular processes from oncogenesis to antigen presentation (26). Proteasome activity is essential for NFκB activation (78;119) and is implicated in TNFR1-mediated apoptosis or cell survival (101). We have shown recently that TNFα induced upregulation of the glycoprotein gp130 involves NFκB activity (135), whereas lysosomal degradation of the specific receptor gp190 for leukemia inhibitory factor can be partially counteracted by proteasomal and NFκB activities (136). Reduced phosphorylation of proteasome subunits by combined TNFα and leptin treatment suggests that these adipokines are involved in protein turnover.
Translation elongation factors (tEFs) are non-ribosomal proteins that perform the most energy-consuming phase of protein synthesis. Their activity is tightly regulated, phosphorylation being the major inhibitory signal (20). TNFα modulates tEF2 phosphorylation in endothelial and epithelial cells of non-BBB origin (56;68). But in the b.End3 cells, it was other subtypes (1β, 1δ-b, and Tu) that were upregulated by either TNFα or leptin treatment. De-phosphorylation of tEFs as a result of the combined treatment suggests that these two adipokines counteract each other in protein synthesis.
Heterogeneous nuclear ribonucleoproteins (hNRPs) bind to pre-mRNA during an early step of the pre-mRNA splicing pathway (70) and are subject to cytokine regulation (105). There was differential regulation among subtypes. hNRP-C was decreased by either TNFα or leptin, whereas hNRP-A2/B1 was upregulated. Co-treatment abolished the effects for both. By contrast, poly(rc) binding protein 1 was downregulated mainly by TNFα. The results indicate rapid modulation of transcriptional events by these adipokines.
Among other phophoproteins regulated by TNFα and leptin, septin 7 belongs to a group of GTPases that bind GTP and phosphatidylinositol 4,5-biphosphate in a mutually exclusive manner and interact with syntaxins and other components of SNARE machinery (43). Thus, it probably plays a role in intracellular trafficking. Collapsin response mediator protein 2 is a cytosolic substrate for Rho-kinase (4) and is involved in microtubule function (34). The implications of a changed phosphorylation status of follistatin precursor, ketohexokinase, serum-inducible kinase, and several others are not yet clear. Nevertheless, the cross-talk of phosphoproteomes by leptin and TNFα clearly illustrates the interactions of signaling pathways at multiple levels, and suggests linkage of signal transduction with adipokine transport.
3.3. Upregulation of TNFα transport
While regulation of leptin transport across the BBB has been mainly investigated in the context of altered feeding status and energy balance, the regulatory changes of TNFα transport have been mainly studied in inflammation, ischemia, and trauma (92;94;96). Concurrent with the dynamic changes of the BBB and blood-spinal cord barrier in these pathological processes, permeation of TNFα across the BBB follows temporal and spatial patterns unique to the insults. Not only can partial disruption of the BBB increase the availability of TNFα to the CNS, but the transport system also shows specific upregulation. This is reflected not only by saturability (self-inhibition) of the increased permeation of TNFα tracer, but also by a higher level of mRNA and protein expression of the transporting receptors, TNFR1 and TNFR2.
Upregulation of TNFα transport can be seen in mice after stroke (79), acute experimental autoimmune encephalomyelitis (90), minimal brain trauma (83), and different types of spinal cord injury (80;87;88;97). Downregulation of TNFα transport, on the other hand, is mainly seen in mice deficient in TNFR1 or TNFR2. Double receptor knockout mice have completely abolished transport of TNFα (81). The circadian rhythm of TNFα transport is present in spinal cord but not in the brain, but the functional implications of such an observation are yet to be explored (91).
3.5. TNFα trafficking
The trafficking route of TNFα is best shown by the application of small tags to exogenous TNFα in transport assays on cerebral microvessels in mice and cultured cells. In intact mice after intravenous (iv) delivery of 125I-TNFα, electron microscopic autoradiography of ultrathin sections of the brain showed passage of radioactivity representing TNFα from blood vessels to brain parenchyma. In the cultured RBE4 cerebral microvessel endothelial cell line, endocytosed biotinylated TNFα was detected by Quantum dots conjugated with streptavidin, and its time-dependent colocalization with intracellular organelles was shown by confocal microscopy. These new imaging approaches, combined with in vitro transport assays and degradation analyses, showed rapid kinetics for the vesicular trafficking of TNFα with a major role of TNFR2 leading to substantial exocytosis of the intact adipokine (98).
More detailed analysis of the differential role of the two receptors for TNFα on cellular transcytosis has been determined in HEK293 endothelial cells overexpressing TNFR1 and TNFR2. The uptake of TNFα mediated by TNFR1 is faster than that mediated by TNFR2. TNFR2, however, has greater capacity, leading to a higher percentage exocytosis of TNFα. Moreover, TNFα inside the cell remains intact for 1 h. Thus, passage of an adipokine in intact form across an endothelial cell can involve the differential and cooperative functions of different receptors (85).
3.6. TNFα and MDR
Multi-drug resistance proteins (MDRs) are efflux pumps that serve important biological functions but hinder drug delivery to the CNS. Many chemotherapeutic agents and anti-epileptic drugs are substrates for MDRs. Recently, TNFα was found to specifically induce the expression and enhance the function of MDR1 in RBE4 cerebral endothelial cells (134). The increase in MDR1 mRNA was shown by cDNA microarray at 6, 12, and 24 h after TNF treatment, and this was confirmed by RT-PCR at 2 to 24 h. Protein expression of MDR1was increased 6 to 24 h after TNFα treatment and resulted in a significant reduction in the cellular uptake of 3H-vinblastine. Thus, the drug efflux transporter in cerebral endothelial cells can be upregulated by an adipokine, but it is uncertain whether this suggests that obese patients with higher TNFα concentrations in their blood would show higher drug resistance. Nonetheless, it raises the possibility that adjunctive treatment with anti-TNFα, or even with antibodies against other cytokines, has novel therapeutic potential in brain cancer and epilepsy.
4. IL-6
4.1. Background
IL-6 is produced by adipocytes, and its blood concentration correlates better than leptin and TNFα with insulin resistance, although all three are elevated in obese women (13). After 3 weeks of a very low-calorie diet, IL-6 levels decrease in adipose tissue as well as in serum. IL-6 knockout mice develop obesity at the age of about a half-year (130). Unlike the greater concentration of leptin in serum than in CSF in obesity, IL-6 may have higher CSF concentrations than serum in some obese but otherwise healthy men (112). Furthermore, intracerebroventricular (icv) administration of IL-6 can reduce obesity and leptin levels in rats (128). IL-6 receptors are also present in the plasma membrane of human adipocytes (14).
4.2. Transport
Because of excessive degradation of IL-6 in the brain, the relative contribution of peripheral IL-6 on actions in the CNS is not clear. Although radioactively labeled IL-6 can cross the BBB by a saturable transport system to enter both CSF and brain parenchyma in intact form, only about 16% of the radioactivity reaching the parenchyma of the brain and about 50% in the CSF represents intact cytokine (8). Yet, this small fraction of intact IL-6 or its fragment may be sufficient to produce biological effects. There also is evidence that 48 hours of exposure to IL-6 regulates Na-K-Cl co-transport in brain microvessel endothelial cells, helping maintain cerebral ionic homeostasis (115). Thus, IL-6 of both peripheral and CNS production can affect the functions of the BBB and the brain.
5. TGF-β1
5.1. Background
TGF-β1, a potent anti-inflammatory agent, can be produced by adipocytes as well as other tissues. It stimulates the secretion of plasminogen activator inhibitor-1 (PAI-1), another adipokine discussed later in this review (17). In visceral as well as subcutaneous fat tissue, TGF-β1 levels correlate with those of PAI-1 and body mass index (BMI), but not with TNFα (2). Release of TGF-β1 in explants of human subcutaneous adipose tissue is increased by an inhibitor of TNFα (soluble human TNFα receptor) and by insulin (30). These results indicate a role of TGF-β1 in the autocrine loop in adipose tissue.
5.2. Effect on the BBB
At the BBB level, TGF-β1 can be produced by pericytes at the basolaberal surface of the endothelial cells, and the inhibition of its production by cyclosporin A probably contributes to BBB dysfunction (117). Consistent with this finding, TGF-β1 appears to tighten the BBB (decreases endothelial permeability) and enhance P-glycoprotein efflux transporter activity in mouse brain capillary endothelia cells (29). Thus, TGF-β1 can act at the BBB.
5.3. Transport
Despite its effect on cerebral endothelial cells and stability in blood, TGF-β1 does not enter the brain any faster than the vascular marker albumin after iv delivery (47). The lack of blood-to-brain transport of TGF-β1 is probably caused by the complexing of TGF-β1 with serum binding proteins, because when perfused in blood-free buffer, the rate of entry of TGF-β1 is significantly faster than that of albumin.
This lack of BBB permeation of iv TGF-β1 contrasts with the saturable transport of TGF-β2 (73). Unlike TGF-β1, TGF-β2 can cross the BBB after iv administration. Moreover, its entry is inhibited by excess TGF-β2, indicating a saturable blood-to-brain transport system. After transcytosis, it is distributed throughout the brain, with the highest concentrations in the hypothalamus. Nonetheless, TGF-β2 is not considered an adipokine. Although TGF-β1 is the predominant form found in most cells, including endothelial cells, TGF-β2 is most abundant in neurons. Both decrease the expression of adipose genes (118).
6. PAI-1
6.1. Background
Adipocytes are the main tissue source of the elevated levels of PAI-1 seen in obesity. The biological role of this plasminogen activator inhibitor involves more than inhibition of fibrinolysis. Elevated plasma PAI-1 might dysregulate fibrinolysis and increase the cardiovascular risk already existing in obese subjects. In endothelial cells, activation of protein kinase C promotes transcription of PAI-1, as seen in the insulin resistance syndrome (71).
Opposite results on the effects of PAI-1 on body composition are seen in genetic models. On the one hand, transgenic mice overexpressing PAI-1 in adipocytes show adipocyte hypotrophy, and lower amplitude of increase in fat and body weight while on a high fat diet (62). By contrast, mice deficient in PAI-1 have faster weight gain and greater response to diet-induced obesity (74). These results indicate a protective role of PAI-1 against obesity.
On the other hand, disruption of the PAI-1 gene in ob/ob mice reduces obesity, effects perhaps explained by the actions of PAI-1 on TNFα gene expression (106). The decreased adiposity seen in PAI-1-deficient ob/ob mice is associated with a large decrease in the expression of TNFα by adipose tissue. The concentrations of PAI-1 in plasma and adipose tissue of obese animals and humans are increased by TNFα. In addition to TNFα, IL-1β also increases mRNA and protein of PAI-1 (17).
6.2. BBB
Although the permeation across the BBB of PAI-1 itself has not been reported, tissue-type plasminogen activator (tPA), used clinically to treat stroke, crosses the BBB without dramatic alteration of paracellular permeability (16). The lack of effect of PAI-1 on BBB permeability, as suggested by a study of a small sample of humans with acquired immunodeficiency syndrome (AIDS) dementia complex (ADC) (110), does not rule out the role of dysfunction of the CNS uPAR/uPA/PAI-1 system in inflammation.
PAI-1 appears to exhibit neurotrophic functions. This is shown in PAI-1 transgenic mice subjected to stroke, and by PAI-1 treatment by icv injection in normal mice after stroke. PAI-1 reduces infarct volume when stroke is induced by permanent ligation of the middle cerebral artery, but worsens the infarct area in a thrombosis model (77). These paradoxical findings raise the possibility that the effects of PAI-1 are modulated by reperfusion and its related secondary injury by oxidative stress. Stroke causes a unique pattern of BBB disruption. However, it is not yet clear whether elevated PAI-1 in the metabolic syndrome affects BBB function.
7. Adiponectin
7.1. Background
Adiponectin is decreased in obese subjects and increased by weight loss. It is proposed that CNS-mediated actions of adiponectin contribute to weight loss by increasing energy expenditure, but adiponectin does not seem to cross the BBB directly.
Adiponectin receptors are immunopositive in the postmortum human hypothalamus. In obese women, the CSF/serum ratios of adiponectin and leptin are inversely correlated with the BMI, a relationship not seen in men (57). In human microvascular endothelial cells, adiponectin reduces the passage of fluorescein-conjugated dextran, indicating a stabilizing effect on barrier integrity. Adiponectin can promote differentiation of peripheral blood CD14+ monocytes into endothelial cells, which might contribute to vascular healing and angiogenesis (133).
7.2. BBB
7.2.1. Studies with iv administration of radioactive tracer
The controversy about whether or not adiponection crosses the BBB started after the observation that CSF levels of immunoreactive adiponectin increase after its administration by the iv route (102). Apart from the possibility that there is blood-to-brain transport, endogenous adiponectin produced within the CNS might have been detected, or a fragment of the injected adiponectin could have been immunoactive.
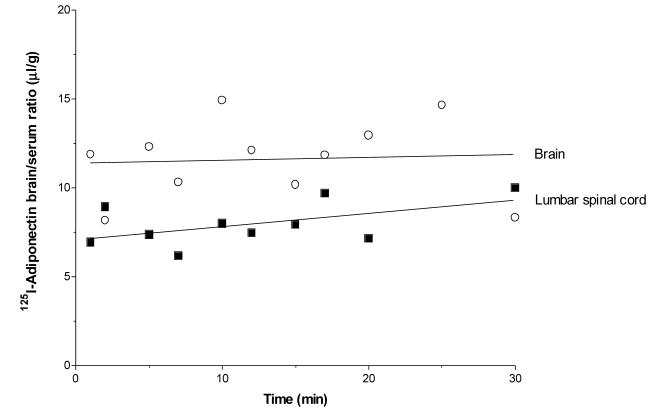
Further studies by two independent laboratories using sensitive radiotracer methods to quantify BBB influx after iv injection failed to detect significant permeation of adiponectin (86;111). The lumbar spinal cord showed a low rate of influx (Fig. 2), but the volume of distribution of 125I-adiponectin in both brain and spinal cord is close to that seen with the paracellular permeability marker albumin. Moreover, excess adiponectin does not modulate the negligible influx, despite the demonstration that adiponectin is extremely stable in the circulation (86).
Fig. 2.
Restricted CNS permeation of adiponectin
7.2.2. Studies with in-situ perfusion
When adiponectin is perfused in blood-free buffer, the non-glycosylated form shows a trend (p = 0.06) toward uptake in the brain whereas the glycosylated form does not. The large molecular weight complex seen in gel electrophoresis appears to be related to protein binding or aggregation of adiponectin. Nonetheless, the mRNAs for both adiponectin receptor-1 and receptor-2 are present in brain microvessels; receptor-1 mRNA can be upregulated by fasting. Further, adiponectin suppresses IL-6 release from brain endothelial cells, indicating that it can modulate BBB function (111).
A discrepancy between results of BBB transport obtained after iv administration and those after in-situ perfusion usually can be attributed to instability of the test substance injected into the circulation or its binding to blood proteins, as opposed to the blood-free medium used for perfusion. Moreover, the influx rate measured by in-situ brain perfusion is not directly comparable to that from iv bolus injection, and control experiments are necessary to rule out BBB disruption caused by the perfusion procedure (89;95).
Regardless, the trend toward influx by perfusion is not sufficient to support the postulation of an active transport mechanism for adiponectin. The CSF concentration of adiponectin is not high enough to hamper blood-to-brain transport by saturation or equilibrium among the compartments. The 1000-fold lower CSF concentration than serum concentration certainly implies the presence of an effective barrier (57;60).
7.2.3. CVOs
It has also been speculated that adiponectin enters the brain through the circumventricular organs (CVOs) (1). However, since the astrocytic barrier is joined by tight junctions between the cells that separate the CVOs from the rest of the parenchyma, diffusion from the small region of CVOs (less than 5 %) (100) to the parenchyma covered by the BBB (a surface area of 150 cm2) would be negligible for detection in the CSF.
In rats, neurons in the area postrema express receptors for adiponectin and show alterations of membrane potentials. Microinjection of adiponectin into this CVO increases arterial blood pressure, suggesting a role for adiponectin in cardiovascular homeostasis by actions in the brain (32).
7.2.4. Summary of interactions between adiponection and the BBB
A potential transport system for adiponectin across the BBB is not supported by any active transport, saturation by high CSF concentrations, or binding proteins in the periphery. Rather, there may be intrathecal production of adiponectin. The major effect of circulating adiponectin on the BBB seems to be the modulation of proinflammatory signals, shown by decreased IL-6 production.
8. Angiotensinogen
8.1. Background
White adipose tissue is a major extrahepatic source of angiotensinogen, the angiotensin precursor. Although the renin-angiotensin system is implicated in obesity-induced hypertension and insulin resistance, the plasma concentration of angiotensinogen is similar in obese and lean humans. However, β-adrenergic stimulation causes release of angiotensinogen from adipose tissue and muscle, which increases plasma angiotensin II level although its own concentration is unaltered (33). In cultured human aortic endothelial cells, insulin suppresses mRNA expression of angiotensinogen as well as its cellular levels measured by radioimmunoassay (42). Thus, dysregulation of angiotensinogen production may further worsen vasculopathy in the metabolic syndrome.
Angiotensinogen knockout mice are protected from diet-induced obesity, gaining less weight than the wildtype mice in response to a chow or high fat diet. There is hypotrophy of adipcytes and decrease in fatty acid synthase activity. Since locomotor activity is increased whereas metabolic rate and the mRNA of uncoupling proteins is not, it is thought that a CNS component mediates the increased locomotor activity along with reduced lipogenesis (69).
8.2. BBB
In the angiotensinogen knockout mice, the BBB is more susceptible to cold injury. This is related to reduced expression of glial fibrially acidic protein, a marker for astrocytes that normally produce antiogensiogen. Laminin, a constituent of the extracellular matrix related to the tight junctions, is also decreased in the knockout mice. Disruption of the BBB after focal injury by dry ice, determined by exudation of Evans blue, is seen at day 7 and day 16 in the knockout mice. This contrasts with a more rapid repair of the BBB permeability by day 5 in the wildtype mice (41). The study suggests a protective role of angiotensinogen in maintaining the integrity of the BBB. This contrasts with the lack of changes of BBB function in the renin knockout mice (132). Thus, angiotensinogen, but not renin, is probably involved in the functional maintenance of the BBB, but transport rates have not bee reported.
9. Relaxin
9.1. Background
Microarray analysis of neonatal pig adipose tissue shows the expression of relaxin, considered a member of the insulin superfamily (11). Injection of relaxin-3 into the paraventricular nucleus of the hypothalamus can cause hyperphagia (72).
9.2. CVOs
Circulating relaxin stimulates water drinking by exerting its main action on two CVOs, the subfornical organ (SFO) and perhaps the organum vasculosum of the lamina terminalis (OVLT) (116). This is seen by increased Fos expression in the SFO and OVLT after iv infusion of a dipsogenic dose of relaxin. Furthermore, ablation of the SFO, but not the OVLT, abolishes relaxin-induced water drinking in vivo, and in vitro relaxin increases electrical activity of neurons in isolated slices of the SFO.
There is confusion about the barrier function of the CVOs, and even the hypothalamus. As summarized recently (50), although CVOs don’t possess a classic BBB, penetration from blood into a CVO only occurs for a few cell layers because the ependyma/tanicytes, especially β1 tanicytes (99), form a continuous membrane of tight junctions, preventing direct penetration in the parenchyma of the brain. Once within the CVO, the peptide/polypeptide is trapped inside, unable to enter the CSF or brain regions adjacent to the CVO such as the hypothalamus (40).
9.3. Vascular endothelial cells
Relaxin treatment of human umbilical vein endothelial cells (HUVEC) results in increased expression of inducible nitric oxide synthase (iNOS) and hence increased NO generation mediated by NFκB (103). Although these findings suggest that relaxin in the umbilical vein might contributed to the NO-dependent regulation of vascular tone, this does not necessarily apply to the microvasculature of the BBB. The actions of relaxin on the brain probably are best explained by its effects on the CVOs mentioned above.
10. ASP
10.1. Background
Aacylation-stimulating protein (ASP), or complement C3a-desArg, arises from carboxypeptidase cleavage of the Arg from complement C3a. ASP is therefore deficient in C3 knockout mice, which have decreased body weight, adipose tissue, food efficiency (increased energy intake in calories/g body weight), and plasma concentrations of leptin (76). By contrast, serum ASP levels are increased in IL-6 knockout mice; this may increase triacylglycerol in adipocytes, thus contributing to the development of obesity in these knockouts (130). In obese humans, plasma ASP concentrations are increased (109). This increase begins in very young obese children and may predispose toward increased storage of fat (24).
10.2. BBB
Although there are no specific studies of the transport of the C3a protein ASP across the BBB, the CSF concentrations of C3 increase with age, a finding Loeffler et al. suggest may be explained by BBB leakage (64). Moreover, Kossmann et al. showed that C3 levels are increased in the CSF of patients with severe head injury, explaining the increase by leakage across the BBB (58). They suggest that activation of complement in the CNS of head-injured patients could lead to inflammation and alteration of the BBB through the generation of C3a and C5a, which increase vascular permeability.
11. RBP
11.1. Background
Adipocytes synthesize and secrete retinol-binding protein (RBP)(120). Animal studies indicate a vital role for RBP in glucose regulation, but this may not occur in humans (38).
11.2. BBB
In both rat and human brain, MacDonald et al. showed by immunohistochemistry that RBP is localized in the endothelial cells of the BBB and by autoradiography in the epithelial cells of the choroid plexus, but not in the vascular system of other tissues (65). They indicate that transport of retinol across the BBB may occur after RBP uptake from blood with subsequent transcytosis as a complex with cellular RBP.
12. Resistin
12.1. Background
Like RBP, resistin may have different actions in rodents and humans. It was named because it can cause resistance to insulin. Its concentrations are increased in plasma of genetic and diet-induced obese mice (113), but its mRNA is decreased in white adipose tissue of obese rodents (61;129).
12.2. Vascular endothelial cells
Resistin-treated cells express lower levels of TNFα receptor-associated factor 3 (TRAF3), which inhibits CD40 ligand-mediated endothelial cell activation (126) Although human saphenous vein endothelial cells were used, the results raise the possibility that the dysfunction of the endothelial cells of the BBB might also be caused by elevated resistin levels.
In other in vitro studies with human endothelial cells (HUVEC), resistin induced production of the angiogenic factor vascular endothelial cell growth factor (VEGF) and endothelial cell tube formation (28). Another study with these cells showed increased vascular cell adhesion molecule-1 (VACM-1) expression after exposure to resistin (108). It is possible that such increases could contribute to the risk of atherosclerosis in some patients.
12.3. CSF
Kos et al. measured concentrations of resistin in serum and CSF and found no correlation between the two (57). Resistin concentrations in human CSF were about 100-times lower than in serum, and were not influenced by gender, age, BMI, or diabetic status. This suggests that the passage of resistin across the BBB is restricted (57).
13. Conclusion
Adipokines interact with the BBB and the CNS, providing novel aspects of neuroendocrine regulation. In obesity and other metabolic syndromes, altered adipokine concentrations affect the BBB in multiple ways. The permeation of selective adipokines with saturable transport systems, such as leptin and TNFα, is responsive to physiological and pharmacological regulation. This affects feeding behavior and probably provides negative feedback. Obesity reduces adiponectin concentrations, thus reducing its tonic inhibition on selective TNFα signaling pathways. The effects of adipokines on the endothelial cells composing the BBB are largely related to the presence of receptors, some of which can serve a transport function as well. It is not known whether the atherosclerotic effects of some adipokines on larger vessels can apply to the microvasculature of the BBB. It is conceivable that adipokines could change membrane functions involving metabolism of lipids, carbohydrates, and proteins at the BBB level. Elucidation of the interactions of adipokines and the BBB has many implications, perhaps the most importing being help in the understanding of how obesity affects brain function.
Acknowledgement
The authors receive support from NIH (DK54880, NS45751, and NS46528). We thank other members of the BBB Group for their preliminary observations and active discussions, and Ms. Loula Burton for editorial assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Ahima R, Qi Y, Singhal N, Jackson M, Scherer P. Brain adipocytokine action and metabolic regulation. Diabetes. 2006;55:S145–S154. doi: 10.2337/db06-s018. [DOI] [PubMed] [Google Scholar]
- 2.Alessi M, Bastelica D, Morange P, Berthet B, Leduc I, Verdier M, et al. Plasminogen activator inhibitor 1, transforming growth factor-beta 1, and BMI are closely associated in human adipose tissue during morbid obesity. Diabetes. 2000;49:1374–80. doi: 10.2337/diabetes.49.8.1374. [DOI] [PubMed] [Google Scholar]
- 3.Algrain M, Turunen O, Vaheri A, Louvard D, Arpin M. Ezrin contains cytoskeleton and membrane binding domains accounting for its proposed role as a membrane-cytoskeletal linker. J.Cell.Biol. 1993;120:129–39. doi: 10.1083/jcb.120.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arimura A, Inagaki N, Chihara K, Menager C, Nakamura N, Amano M, et al. Phosphorylation of collapsin response mediator protein-2 by Rho-kinase. Evidence for two separate signaling pathways for growth cone collapse. J.Biol.Chem. 2000;275:23973–80. doi: 10.1074/jbc.M001032200. [DOI] [PubMed] [Google Scholar]
- 5.Banks WA. Denial versus dualism: the blood-brain barrier as an interface of the gutbrain axis. Endocrinology. 2006;147:2609–10. doi: 10.1210/en.2006-0335. [DOI] [PubMed] [Google Scholar]
- 6.Banks WA, Clever CM, Farrell CL. Partial saturation and regional variation in the transport of leptin across the blood-brain barrier in normal weight mice. Am.J.Physiol. 2000;278:E1158–E1165. doi: 10.1152/ajpendo.2000.278.6.E1158. [DOI] [PubMed] [Google Scholar]
- 7.Banks WA, DiPalma CR, Farrell CL. Impaired transport of leptin across the blood-brain barrier in obesity. Peptides. 1999;20:1341–5. doi: 10.1016/s0196-9781(99)00139-4. [DOI] [PubMed] [Google Scholar]
- 8.Banks WA, Kastin AJ, Gutierrez EG. Penetration of interleukin-6 across the murine blood-brain barrier. Neurosci.Lett. 1994;179:53–6. doi: 10.1016/0304-3940(94)90933-4. [DOI] [PubMed] [Google Scholar]
- 9.Banks WA, Kastin AJ, Huang W, Jaspan JB, Maness LM. Leptin enters the brain by a saturable system independent of insulin. Peptides. 1996;17:305–11. doi: 10.1016/0196-9781(96)00025-3. [DOI] [PubMed] [Google Scholar]
- 10.Banks WA, Niehoff ML, Martin D, Farrell CL. Leptin transport across the blood-brain barrier of the Koletsky rat is not mediated by a product of the leptin receptor gene. Brain Res. 2002;950:130–6. doi: 10.1016/s0006-8993(02)03013-5. [DOI] [PubMed] [Google Scholar]
- 11.Barb C, Hausman G, Czaja K. Leptin: a metabolic signal affecting central regulation of reproduction in the pig. Domest Anim Endocrinol. 2005;29:186–92. doi: 10.1016/j.domaniend.2005.02.024. [DOI] [PubMed] [Google Scholar]
- 12.Barsacchi R, Perrotta C, Bulotta S, Moncada S, Borgese N, Clementi E. Activation of endothelial nitric-oxide synthase by tumor necrosis factor-alpha: a novel pathway involving sequential activation of neutral sphingomyelinase, phosphatidylinositol-3′ kinase, and Akt. Mol.Pharmacol. 2003;63:886–95. doi: 10.1124/mol.63.4.886. [DOI] [PubMed] [Google Scholar]
- 13.Bastard J, Jardel C, Bruckert E, Blondy P, Capeau J, Laville M, et al. Elevated levels of interleukin 6 are reduced in serum and subcutaneous adipose tissue of obese women after weight loss. J.Clin.Endocrinol.Metab. 2000;85:3338–42. doi: 10.1210/jcem.85.9.6839. [DOI] [PubMed] [Google Scholar]
- 14.Bastard J, Maachi M, Van Nhieu J, Jardel C, Bruckert E, Grimaldi A, et al. Adipose tissue IL-6 content correlates with resistance to insulin activation of glucose uptake both in vivo and in vitro. J.Clin.Endocrinol.Metab. 2002;87:2084–9. doi: 10.1210/jcem.87.5.8450. [DOI] [PubMed] [Google Scholar]
- 15.Begley DJ. Peptides and the blood-brain barrier: the status of our understanding. Ann.NY Acad.Sci. 1994;739:89–100. doi: 10.1111/j.1749-6632.1994.tb19810.x. [DOI] [PubMed] [Google Scholar]
- 16.Benchenane K, Berezowski V, Ali C, Fernandez-Monreal M, Lopez-Atalaya J, Brillaut J, et al. Tissue-type plasminogen activator crosses the intact blood-brain barrier by low-density lipoprotein receptor-related protein-mediated transcytosis. Circulation. 2005;111:2241–9. doi: 10.1161/01.CIR.0000163542.48611.A2. [DOI] [PubMed] [Google Scholar]
- 17.Birgel M, Gottschling-Zeller H, Hrohrig K, Hauner H. Role of cytokines in the regulation of plasminogen activator inhibitor-1 expression and secretion in newly differentiated subcutaneous human adipocytes. Arterioscler.Thromb.Vasc.Biol. 2000;20:1682–7. doi: 10.1161/01.atv.20.6.1682. [DOI] [PubMed] [Google Scholar]
- 18.Bjørbæk C, Kahn BB. Leptin signaling in the central nervous system and the periphery. Recent Prog.Horm.Res. 2004;59:305–31. doi: 10.1210/rp.59.1.305. [DOI] [PubMed] [Google Scholar]
- 19.Bjørbæk C, Elmquist JK, Michl P, Ahima RS, van Bueren A, McCall AL, et al. Experession of leptin receptor isoforms in rat brain microvessels. Endocrinology. 1998;139:3485–91. doi: 10.1210/endo.139.8.6154. [DOI] [PubMed] [Google Scholar]
- 20.Browne G, Proud C. Regulation of peptide-chain elongation in mammalian cells. Eur J Biochem. 2002;269:5360–8. doi: 10.1046/j.1432-1033.2002.03290.x. [DOI] [PubMed] [Google Scholar]
- 21.Cardone M, Mostov K. Wortmannin inhibits transcytosis of dimeric IgA by the polymeric immunoglobulin receptor. FEBS Lett. 1995;376:74–6. doi: 10.1016/0014-5793(95)01251-8. [DOI] [PubMed] [Google Scholar]
- 22.Caro JF, Kolaczynski JW, Nyce MR, Ohannesian JP, Opentanova I, Goldman WH, et al. Decreased cerebrospinal-fluid/serum leptin ratio in obesity: a possible mechanism for leptin resistance. Lancet. 1996;348:159–61. doi: 10.1016/s0140-6736(96)03173-x. [DOI] [PubMed] [Google Scholar]
- 23.Carswell EA, Old LJ, Dassel RL. An endotoxin-induced serum factor that causes necrosis of tumors. Proc.Natl.Acad.Sci.USA. 1975;72:3666–70. doi: 10.1073/pnas.72.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cianflone K, Lu H, Smith J, Yu W, Wang H. Adiponectin, acylation stimulating protein and complement C3 are altered in obesity in very young children. Clin.Endocrinol. 2005;62:567–72. doi: 10.1111/j.1365-2265.2005.02260.x. [DOI] [PubMed] [Google Scholar]
- 25.Defacque H, Boes E, Garvalov B, Barret C, Roy C, Mangeat P, et al. Phosphoinositides regulate membrane-dependent actin assembly by latex bead phagosomes. Mol.Biol.Cell. 2002;13:1190–202. doi: 10.1091/mbc.01-06-0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DeMartino G, Slaughter C. The proteasome, a novel protease regulated by multiple mechanisms. J.Biol.Chem. 1999;274:22123–6. doi: 10.1074/jbc.274.32.22123. [DOI] [PubMed] [Google Scholar]
- 27.Dhillon H, Zigman JM, Ye CP, Lee CE, McGovern RA, Tang VS, et al. Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron. 2006;49:191–203. doi: 10.1016/j.neuron.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 28.Di Simone N, Di Nicuolo F, Sanguinetti M, Castellani R, D’Asta M, Caforio L, et al. Resistin regulates human choriocarcinoma cell invasive behaviour and endothelial cell angiogenic processes. J.Endocrinol. 2006;189:691–9. doi: 10.1677/joe.1.06610. [DOI] [PubMed] [Google Scholar]
- 29.Dohgu S, Yamauchi A, Takata F, Naito M, Tsuruo T, Higuchi S, et al. Transforming growth factor-beta-1 upregulates the tight junction and P-glycoprotein of brain microvascular endothelial cells. Cell Mol Neurobiol. 2004;24:491–7. doi: 10.1023/B:CEMN.0000022776.47302.ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fain J, Tichansky D, Madan A. Transforming growth factor beta 1 release by human adipose tissue is enhanced in obesity. Metabolism. 2005;54:1546–51. doi: 10.1016/j.metabol.2005.05.024. [DOI] [PubMed] [Google Scholar]
- 31.Friedman JM. Obesity in the new millennium. Nature. 2000;404:632–4. doi: 10.1038/35007504. [DOI] [PubMed] [Google Scholar]
- 32.Fry M, Smith P, Hoyda T, Duncan M, Ahima R, Sharkey K, et al. Area postrema neurons are modulated by the adipocyte hormone adiponectin. J.Neurosci. 2006;26:9695–702. doi: 10.1523/JNEUROSCI.2014-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goossens G, Jocken J, Blaak E, Schiffers P, Saris W, van Baak M. Endocrine role of the renin-angiotensin system in human adipose tissue and muscle. Effect of beta-adrenergic stimulation. Hypertension. 2007;49:542–7. doi: 10.1161/01.HYP.0000256091.55393.92. [DOI] [PubMed] [Google Scholar]
- 34.Gu Y, Ihara Y. Evidence that collapsin response mediator protein-2 is involved in the dynamics of microtubules. J.Biol.Chem. 2000;275:17917–20. doi: 10.1074/jbc.C000179200. [DOI] [PubMed] [Google Scholar]
- 35.Halaas J, Boozer C, Blair-West J, Fidahusein N, Denton DA, Friedman JM. Physiological response to long-term peripheral and central leptin infusion in lean and obese mice. Proc.Natl.Acad.Sci.USA. 1997;94:8878–83. doi: 10.1073/pnas.94.16.8878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harvey J, Ashford M. Leptin in the CNS: much more than a satiety signal. Neuropharmacology. 2003;44:845–54. doi: 10.1016/s0028-3908(03)00076-5. [DOI] [PubMed] [Google Scholar]
- 37.Houle F, Rousseau S, Morrice N, Luc M, Mongrain S, Turner C, et al. Extracellular signal-regulated kinase mediates phosphorylation of tropomyosin-1 to promote cytoskeleton remodeling in response to oxidative stress: impact on membrane blebbing. Mol.Biol.Cell. 2003;14:1418–32. doi: 10.1091/mbc.E02-04-0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Janke J, Engeli S, Boschmann M, Adams F, Bohnke J, Luft F, et al. Retinol-binding protein 4 in human obesity. Diabetes. 2006;55:2805–10. doi: 10.2337/db06-0616. [DOI] [PubMed] [Google Scholar]
- 39.Johanson CE. Ventricles and cerebrospinal fluid. In: Conn PM, editor. Neuroscience in Medicine. J.B.Lippincott; Philadelphia: 1995. pp. 171–96. [Google Scholar]
- 40.Johanson CE. The Choroid Plexus-CNF Nexus. In: Conn PM, editor. Neuroscience in Medicine. Humana Press Inc; Totowa, NJ: 2003. pp. 165–95. [Google Scholar]
- 41.Kakinuma Y, Hama H, Sugiyama F, Yagami K, Goto K, Murakami K, et al. Impaired blood-brain barrier function in angiotensinogen-deficient mice. Nat.Med. 1998;4:1078–80. doi: 10.1038/2070. [DOI] [PubMed] [Google Scholar]
- 42.Kaminde K, Rakugi H, Nagai M, Takiuchi S, Matsukawa N, Higaki J, et al. Insulin-mediated regulation of the endothelial renin-angiotensin system and vascular cell growth. J.Hypertens. 2004;22:121–7. doi: 10.1097/00004872-200401000-00021. [DOI] [PubMed] [Google Scholar]
- 43.Kartmann B, Roth D. Novel roles for mammalian septins: from vesicle trafficking to oncogenesis. J.Cell Sci. 2001;114:839–44. doi: 10.1242/jcs.114.5.839. [DOI] [PubMed] [Google Scholar]
- 44.Kasai K, Akagawa K. Roles of the cytoplasmic and transmembrane domains of syntaxins in intracellular localization and trafficking. J.Cell Sci. 2001;114:3115–24. doi: 10.1242/jcs.114.17.3115. [DOI] [PubMed] [Google Scholar]
- 45.Kastin AJ, Akerstrom V. Fasting, but not adrenalectomy, reduces transport of leptin into the brain. Peptides. 2000;21:679–82. doi: 10.1016/s0196-9781(00)00195-9. [DOI] [PubMed] [Google Scholar]
- 46.Kastin AJ, Akerstrom V. Glucose and insulin increase the transport of leptin through the blood-brain barrier in normal mice but not in streptozotocin-diabetic mice. Neuroendocrinology. 2001;73:237–42. doi: 10.1159/000054640. [DOI] [PubMed] [Google Scholar]
- 47.Kastin AJ, Akerstrom V, Pan W. Circulating TGF beta 1 does not cross the intact blood-brain barrier. J.Molec.Neurosci. 2003;21:43–8. doi: 10.1385/JMN:21:1:43. [DOI] [PubMed] [Google Scholar]
- 48.Kastin AJ, Akerstrom V, Pan W. Activation of urocortin transport into brain by leptin. Peptides. 2000;21:1811–8. doi: 10.1016/s0196-9781(00)00349-1. [DOI] [PubMed] [Google Scholar]
- 49.Kastin AJ, Banks WA, Ahmed B, Zadina JE. Principles and Practice of Endocrinology & Metabolism. J.B. Lippincott; Philadelphia: 1995. Influence of hormones and messenger peptides on normal brain function; pp. 1682–91. [Google Scholar]
- 50.Kastin AJ, Pan W. Editorial: Intranasal leptin: blood-brain barrier bypass (BBBB) for obesity? Endocrinology. 2006;147:2086–7. doi: 10.1210/en.2006-0208. [DOI] [PubMed] [Google Scholar]
- 51.Kastin AJ, Pan W, Akerstrom V, Hackler L, Wang C, Kotz CM. Novel peptide-peptide cooperation may transform feeding behavior. Peptides. 2002;23:2189–96. doi: 10.1016/s0196-9781(02)00247-4. [DOI] [PubMed] [Google Scholar]
- 52.Kastin AJ, Pan W, Zadina JE. Banks WAThe endocrine brain. In: Becker KL, editor. Principles and Practice of Endocrinology and Metabolism. Lippincott Willimas & Wilkins; Philadelphia: 2001. pp. 1611–5. [Google Scholar]
- 53.Kastin AJ, Pan W, Maness LM, Banks WA. Peptides crossing the blood-brain barrier: some unusual observations. Brain Res. 1999;848:96–100. doi: 10.1016/s0006-8993(99)01961-7. [DOI] [PubMed] [Google Scholar]
- 54.Kastin AJ, Pan W, Maness LM, Koletsky RJ, Ernsberger P. Decreased transport of leptin across the blood-brain barrier in rats lacking the short form of the leptin receptor. Peptides. 1999;20:1449–53. doi: 10.1016/s0196-9781(99)00156-4. [DOI] [PubMed] [Google Scholar]
- 55.Kastin AJ, Zadina JE, Olson RD, Banks WA. The history of neuropeptide research: version 5.a. In: Crawley JN, McLean S, editors. Neuropeptides: Basic and Clinical Advances. Ann.NY Acad.Sci.; New York: 1996. pp. 1–18. [DOI] [PubMed] [Google Scholar]
- 56.Knebel A, Haydon C, Morrice N, Cohen P. Stress-induced regulation of eukaryotic elongation factor 2 kinase by SB 203580-sensitive and -insensitive pathways. Biochem J. 2002;367:525–32. doi: 10.1042/BJ20020916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kos K, Harte A, da Silva N, Tontchev A, Chalakov G, James S, et al. Adiponectin and resistin in human cerebrospinal fluid and expression of adiponectin receptors in the human hypothalamus. J.Clin.Endocrinol.Metab. 2007;92:1129–36. doi: 10.1210/jc.2006-1841. [DOI] [PubMed] [Google Scholar]
- 58.Kossmann T, Stahel P, Morganti-Kossmann M, Jones J, Barnum S. Elevated levels of the complement components C3 and factor B in ventricular cerebrospinal fluid of patients with traumatic brain injury. J.Neuroimmunol. 1997;73:63–9. doi: 10.1016/s0165-5728(96)00164-6. [DOI] [PubMed] [Google Scholar]
- 59.Kroder G, Bossenmaier B, Kellerer M, Capp E, Stoyanov B, Muhlhofer A, et al. Tumor necrosis factor-alpha- and hyperglycemia-induced insulin resistance. Evidence for different mechanisms and different effects on insulin signaling. J.Clin.Invest. 1996;97:1471–7. doi: 10.1172/JCI118569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kusminski C, McTernan P, Schraw T, Kos K, O’hare J, Ahima R, et al. Adiponectin complexes in human cerebrospinal fluid: distinct complex distribution from serum. Diabetologia. 2007;50:634–42. doi: 10.1007/s00125-006-0577-9. [DOI] [PubMed] [Google Scholar]
- 61.Le Lay S, Boucher J, Rey A, Castan-Laurell I, Krief S, Ferre P, et al. Decreased resistin expression in mice with different sensitivities to a high-fat diet. Biochem Biophys Res Commun. 2001;289:564–7. doi: 10.1006/bbrc.2001.6015. [DOI] [PubMed] [Google Scholar]
- 62.Lijnen H, Maquoi E, Morange P, Voros G, Van Hoef B, Kopp F, et al. Nutritionally induced obesity is attenuated in transgenic mice overexpressing plasminogen activator inhibitor-1. Arterioscler.Thromb.Vasc.Biol. 2003;23:78–84. doi: 10.1161/01.atv.0000044457.60665.dd. [DOI] [PubMed] [Google Scholar]
- 63.Lin S, Gundersen G, Maxfield F. Export from pericentriolar endocytic recycling compartment to cell surface depends on stable, detyrosinated (glu) microtubules and kinesin. Mol.Biol.Cell. 2002;13:96–109. doi: 10.1091/mbc.01-05-0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Loeffler D, Brickman C, Juneau P, Perry M, Pomara N, Lewitt P. Cerebrospinal fluid C3a increases with age, but does not increase further in Alzheimer’s disease. Neurobiol.Aging. 1997;18:555–7. doi: 10.1016/s0197-4580(97)00110-3. [DOI] [PubMed] [Google Scholar]
- 65.MacDonald P, Bok D, Ong D. Localization of cellular retinol-binding protein and retinol-binding protein in cells comprising the blood-brain barrier of rat. Proc.Natl.Acad.Sci.USA. 1989;87:4265–9. doi: 10.1073/pnas.87.11.4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Madge LA, Pober JS. A phosphatidylinositol 3-kinase/Akt pathway, activated by tumor necrosis factor or interleukin-1, inhibits apatosis but does not activate NFkappaB in human endothelial cells. J.Biol.Chem. 2000;275:15458–65. doi: 10.1074/jbc.M001237200. [DOI] [PubMed] [Google Scholar]
- 67.Maresh GA, Maness LM, Zadina JE, Kastin AJ. In vitro demonstration of a saturable transport system for leptin across the blood-brain barrier. Life Sci. 2001;69:67–73. doi: 10.1016/s0024-3205(01)01093-1. [DOI] [PubMed] [Google Scholar]
- 68.Mario M, Dunbar J, Wu L, Ngaiza J, Han H, Guo D, et al. Inhibition of tumor necrosis factor signal transduction in endothelial cells by dimethylaminopurine. J.Biol.Chem. 1996;271:28624–9. doi: 10.1074/jbc.271.45.28624. [DOI] [PubMed] [Google Scholar]
- 69.Massiera F, Seydoux J, Geloen A, Quignard-Boulange A, Turban S, Saint-Marc P, et al. Angiotensin-deficient mice exhibit impairment of diet-induced weight gain with alteration in adipose tissue development and increased locomotor activity. Endocrinology. 2001;142:5220–5. doi: 10.1210/endo.142.12.8556. [DOI] [PubMed] [Google Scholar]
- 70.Mayrand S, Dwen P, Pederson T. Serine/threonine phosphorylation regulates binding of C hnRNP proteins to pre-mRNA. Proc Natl Acad Sci. 1993;90:7764–8. doi: 10.1073/pnas.90.16.7764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McCarty M. De novo synthesis of diacylglycerol in enothelium may mediate the association between PAI-1 and the insulin resistance syndrome. Med.Hypotheses. 2005;64:388–93. doi: 10.1016/j.mehy.2004.03.038. [DOI] [PubMed] [Google Scholar]
- 72.McGowan B, Stanley S, Smith K, White N, Connolly M, Thompson E, et al. Central relaxin-3 administration causes hyperphagia in male Wistar rats. Endocrinology. 2005;146:3295–300. doi: 10.1210/en.2004-1532. [DOI] [PubMed] [Google Scholar]
- 73.McLennan I, Weible M, 2, Hendry I, Koishi K. Transport of transforming growth factor-beta 2 across the blood-brain barrier. Neuropharmacology. 2005;48:274–82. doi: 10.1016/j.neuropharm.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 74.Morange P, Lijnen H, Alessi M, Kopp F, Collen D, Juhan-Vague I. Influence of PAI-1 on adipose tissue growth and metabolic parameters in a murine model of diet-induced obesity. Arterioscler.Thromb.Vasc.Biol. 2000;20:1150–4. doi: 10.1161/01.atv.20.4.1150. [DOI] [PubMed] [Google Scholar]
- 75.Mullock B, SMith C, Ihrke G, Bright N, Lindsay M, Parkinson E, et al. Syntaxin 7 is localized to late endosome compartments, associates with Vamp 8, and Is required for late endosome-lysosome fusion. Mol.Biol.Cell. 2000;11:3137–53. doi: 10.1091/mbc.11.9.3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Murray I, Havel P, Sniderman A, Cianflone K. Reduced body weight, adipose tissue, and leptin levels despite increased energy intake in female mice lacking acylation-stimulating protein. Endocrinology. 2000;141:1041–9. doi: 10.1210/endo.141.3.7364. [DOI] [PubMed] [Google Scholar]
- 77.Nagai N, Suzuki Y, Van Hoef B, Lijnen H, Collen D. Effects of plasminogen activator inhibitor-1 on ischemic brain injury in permanent and thrombotic middle cerebral artery occlusion models in mice. J Thromb Haemost. 2005;3:1379–84. doi: 10.1111/j.1538-7836.2005.01466.x. [DOI] [PubMed] [Google Scholar]
- 78.Palombella V, Rando O, Goldberg A, Maniatis T. The ubiquitin-proteasome pathway is required for processing the NF-kappa B1 precursor protein and the activation of NF-kappa B. Cell. 1994;78:773–85. doi: 10.1016/s0092-8674(94)90482-0. [DOI] [PubMed] [Google Scholar]
- 79.Pan W, Ding Y, Yu Y, Ohtaki H, Nakamichi T, Kastin AJ. Stroke upregulates TNF alpha transport across the blood-brain barrier. Exp.Neurol. 2006;198:222–33. doi: 10.1016/j.expneurol.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 80.Pan W, Kastin AJ. Increase in TNFα transport after SCI is specific for time, region and type of lesion. Exp.Neurol. 2001;170:357–63. doi: 10.1006/exnr.2001.7702. [DOI] [PubMed] [Google Scholar]
- 81.Pan W, Kastin AJ. TNFα transport across the blood-brain barrier is abolished in receptor knockout mice. Exp.Neurol. 2002;174:193–200. doi: 10.1006/exnr.2002.7871. [DOI] [PubMed] [Google Scholar]
- 82.Pan W, Kastin AJ. Why study transport of peptides and proteins at the neurovascular interface. Brain Res.Rev. 2004;46:32–43. doi: 10.1016/j.brainresrev.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 83.Pan W, Kastin AJ, Rigai T, McLay RN, Pick CG. Increased hippocampal uptake of tumor necrosis factor α and behavioral changes in mice. Exp.Brain Res. 2003;149:195–9. doi: 10.1007/s00221-002-1355-7. [DOI] [PubMed] [Google Scholar]
- 84.Pan W, Tu H, Hsuchou H, Daniel J, Kastin AJ. Amplification of leptin-induced Stat3 signaling by urocortin. Mol.Endocrinol. 2007 doi: 10.1007/s12031-007-0071-y. in press.
- 85.Pan W, Tu H, Hsuchou H, Yang Y, Peng T, Kastin AJ. Differential role of TNF receptors in cellular trafficking of intact TNF. Cell Physiol Biochem. 2007 doi: 10.1159/000107539. in press.
- 86.Pan W, Tu H, Kastin AJ. Differential BBB interactions of three ingestive peptides: obestatin, ghrelin, and adiponectin. Peptides. 2006;27:911–6. doi: 10.1016/j.peptides.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 87.Pan W, Zhang L, Liao J, Csernus B, Kastin AJ. Selective increase in TNF alpha permeation across the blood-spinal cord barrier after SCI. J.Neuroimmunol. 2003;134:111–7. doi: 10.1016/s0165-5728(02)00426-5. [DOI] [PubMed] [Google Scholar]
- 88.Pan W, Banks WA, Kastin AJ. Blood-brain barrier permeability to ebiratide and TNF in acute spinal cord injury. Exp.Neurol. 1997;146:367–73. doi: 10.1006/exnr.1997.6533. [DOI] [PubMed] [Google Scholar]
- 89.Pan W, Banks WA, Kastin AJ. Permeability of the blood-brain barrier to neurotrophins. Brain Res. 1998;788:87–94. doi: 10.1016/s0006-8993(97)01525-4. [DOI] [PubMed] [Google Scholar]
- 90.Pan W, Banks WA, Kennedy MK, Gutierrez EG, Kastin AJ. Differential permeability of the BBB in acute EAE: enhanced transport of TNF-α. Am.J.Physiol. 1996;34:E636–E642. doi: 10.1152/ajpendo.1996.271.4.E636. [DOI] [PubMed] [Google Scholar]
- 91.Pan W, Cornelissen G, Halberg F, Kastin AJ. Selected contribution: circadian rhythm of tumor necrosis factor-alpha uptake into mouse spinal cord. J.Appl.Physiol. 2002;92:1357–62. doi: 10.1152/japplphysiol.00915.2001. [DOI] [PubMed] [Google Scholar]
- 92.Pan W, Csernus B, Kastin AJ. Upregulation of p55 and p75 receptors mediating TNF alpha transport across the injured blood-spinal cord barrier. J Mol.Neurosci. 2003;21:173–84. doi: 10.1385/JMN:21:2:173. [DOI] [PubMed] [Google Scholar]
- 93.Pan W, Kastin AJ. Diurnal variation of leptin entry from blood to brain involving partial saturation of the transport system. Life Sci. 2001;68:2705–14. doi: 10.1016/s0024-3205(01)01085-2. [DOI] [PubMed] [Google Scholar]
- 94.Pan W, Kastin AJ. Upregulation of the transport system for TNFα at the blood-brain barrier. Arch.Physiol.Biochem. 2001;109:350–3. doi: 10.1076/apab.109.4.350.4238. [DOI] [PubMed] [Google Scholar]
- 95.Pan W, Kastin AJ. Interactions of cytokines with the blood-brain barrier: implications for feeding. Curr.Pharm.Des. 2003;9:827–31. doi: 10.2174/1381612033455332. [DOI] [PubMed] [Google Scholar]
- 96.Pan W, Kastin AJ. Transport of cytokines and neurotrophins across the BBB and their regulation after spinal cord injury. In: Shanker H, Westman J, editors. Blood-Spinal Cord and Brain Barriers in Health and Disease. Elsevier, Academic Press; San Diego, CA: 2003. pp. 395–407. [Google Scholar]
- 97.Pan W, Kastin AJ, Bell RL, Olson RD. Upregulation of tumor necrosis factor alpha transport across the blood-brain barrier after acute compressive spinal cord injury. J.Neurosci. 1999;19:3649–55. doi: 10.1523/JNEUROSCI.19-09-03649.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pan W, Kastin AJ, Daniel J, Yu C, Baryshnikova LM, von Bartheld CS. TNF alpha trafficking in cerebral vascular endothelial cells. J Neuroimmunol. 2007;185:47–56. doi: 10.1016/j.jneuroim.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Peruzzo B, Pastor FE, Blazquez JL, Schobitz K, Pelaez B, Amat P, et al. A second look at the barriers of the medial basal hypothalamus. Exp.Brain Res. 2000;132:10–26. doi: 10.1007/s002219900289. [DOI] [PubMed] [Google Scholar]
- 100.Plotkin SR, Banks WA, Kastin AJ. Comparison of saturable transport and extracellular pathways in the passage of interleukin-1α across the blood-brain barrier. J.Neuroimmunol. 1996;67:41–7. doi: 10.1016/0165-5728(96)00036-7. [DOI] [PubMed] [Google Scholar]
- 101.Pradillo J, Romera C, Hurtado O, Cardenas A, Moro M, Leza J, et al. TNFR1 upregulation mediates tolerance after brain ischemic preconditioning. J.Cereb.Blood Flow Metab. 2005;25:193–203. doi: 10.1038/sj.jcbfm.9600019. [DOI] [PubMed] [Google Scholar]
- 102.Qi Y, Takahashi N, Hileman SM, Patel H, Berg AH, Pajvani UB, et al. Adiponectin acts in the brain to decrease body weight. Nat.Med. 2004;10:524–9. doi: 10.1038/nm1029. [DOI] [PubMed] [Google Scholar]
- 103.Quattrone S, Chiappini L, Scapagnini G, Bigazzi B, Bani D. Relaxin potentiates the expression of inducible nitric oxide synthase by endothelial cells from human umbilical vein in in vitro culture. Mol.Hum.Reprod. 2004;10:325–30. doi: 10.1093/molehr/gah040. [DOI] [PubMed] [Google Scholar]
- 104.Ramsey JJ, Kemnitz JW, Colman RJ, Cunningham D, Swick AG. Different central and peripheral responses to leptin in rhesus monkeys: brain transport may be limited. J.Clin.Endocrinol.Metab. 1998;83:3230–5. doi: 10.1210/jcem.83.9.5073. [DOI] [PubMed] [Google Scholar]
- 105.Rousseau S, Morrice N, Peggie M, Campbell D, Gaestel M, Cohen P. Inhibition of SAPK2a/p38 prevents hnRNP A0 phosphorylation by MAPKAP-K2 and its interaction with cytokine mRNAs. EMBO J. 2002;21:6505–14. doi: 10.1093/emboj/cdf639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Schafer K, Fujisawa K, Konstantinides S, Loskutoff D. Disruption of the plasminogen activator inhibitor 1 gene reduces the adiposity and improves the metabolic profile of genetically obese and diabetic ob/ob mice. FASEB J. 2001;15:1840–2. doi: 10.1096/fj.00-0750fje. [DOI] [PubMed] [Google Scholar]
- 107.Schwartz MW, Peskind E, Raskind M, Boyko EJ, Porte D., Jr Cerebrospinal fluid leptin levels: relationship to plasma levels and to adiposity in humans. Nature Med. 1996;2:589–93. doi: 10.1038/nm0596-589. [DOI] [PubMed] [Google Scholar]
- 108.Skilton M, Nakhla S, Sieveking D, Caterson I, Celermajer D. Pathophysiological levels of the obesity related peptides resistin and ghrelin increase adhesion molecule expression on human vascular endothelial cells. Clin.Exp.Pharmacol.Physiol. 2005;32:839–44. doi: 10.1111/j.1440-1681.2005.04274.x. [DOI] [PubMed] [Google Scholar]
- 109.Sniderman A, Cianflone K, Eckel R. Levels of acylation stimulating protein in obese women before and after moderate weight loss. Int.J.Obes. 1991;15:333–6. [PubMed] [Google Scholar]
- 110.Sporer B, Koedel U, Popp B, Paul R, Pfister H. Evaluation of cerebrospinal fluid uPA, PAI-1, and soluble uPAR levels in HIV-infected patients. J Neuroimmunol. 2005;163:190–4. doi: 10.1016/j.jneuroim.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 111.Spranger J, Verma S, Gohring I, Bobbert T, Seifert J, Sindler A, et al. Adiponectin does not cross the blood-brain barrier but modifies cytokine expression of brain endothelial cells. Diabetes. 2006;55:141–7. [PubMed] [Google Scholar]
- 112.Stenlof K, Wernstedt I, Fjallman T, Wallenius V, Wallenius K, Jansson J. Interleukin-6 levels in the central nervous system are negatively correlated with fat mass in overweight/obese subjects. J.Clin.Endocrinol.Metab. 2003;88:4379–83. doi: 10.1210/jc.2002-021733. [DOI] [PubMed] [Google Scholar]
- 113.Steppan C, Bailey S, Bhat S, Brown E, Banerjee R, Wright C, et al. The hormone resistin links obesity to diabetes. Nature. 2001;409:307–12. doi: 10.1038/35053000. [DOI] [PubMed] [Google Scholar]
- 114.Strand FL. Neuropeptides: Regulators of Physiological Processes. MIT Press; Cambridge, MA: 1999. [Google Scholar]
- 115.Sun D, Lytle C, O’Donnell M. IL-6 secreted by astroglial cells regulates Na-K-Cl cotransport in brain microvessel endothelial cells. Am.J.Physiol. 1997;272:C1829–C1835. doi: 10.1152/ajpcell.1997.272.6.C1829. [DOI] [PubMed] [Google Scholar]
- 116.Sunn N, Egli M, Burazin T, Burns P, Colvill L, Davern P, et al. Circulating relaxin acts on subfornical organ neurons to stimulate water drinking in the rat. Proc.Natl.Acad.Sci.USA. 2002;99:1701–6. doi: 10.1073/pnas.022647699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Takata F, Dohgu S, Yamauchi A, Sumi N, Nakagawa S, Naito M, et al. Inhibition of transforming growth factor-beta production in brain pericytes contributes to cyclosporin A-induced dysfunction of the blood-brain barrier. Cell Mol Neurobiol. 2007 doi: 10.1007/s10571-006-9125-x. in press.
- 118.Torti F, Torti S, Larrick J, Ringold G. Modulation of adipocyte differentiation by tumor necrosis factor and transforming growth factor beta. J.Cell.Biol. 1989;108:1105–13. doi: 10.1083/jcb.108.3.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Traenckner E, Wilk S, Baeuerle P. A proteasome inhibitor prevents activation of NF-kappa B and stabilizes a newly phosphorylated form of I kappa B-alpha that is still bound to NF-kappa B. EMBO J. 1994;13:5433–41. doi: 10.1002/j.1460-2075.1994.tb06878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tsutsumi C, Okuno M, Tannous L, Piantedosi R, Allan M, Goodman D, et al. Retinoids and retinoid-binding protein expression in rat adipocytes. J Biol.Chem. 1992;267:1805–10. [PubMed] [Google Scholar]
- 121.Tu H, Kastin AJ, Bjorbaek C, Pan W. Urocortin trafficking in cerebral microvessel endothelial cells. J.Mol.Neurosci. 2007 doi: 10.1385/jmn/31:02:171. in press.
- 122.Tu H, Kastin AJ, Hsuchou H, Pan W. The soluble receptor ObRe inhibits leptin transport. Mol.Endocrinol. 2007 doi: 10.1002/jcp.21195. in press.
- 123.Tu H, Kastin AJ, Pan W. CRH-R1 and CRH-R2 are both trafficking and signaling receptors for urocortin. Mol.Endocrinol. 2007;21:700–11. doi: 10.1210/me.2005-0503. [DOI] [PubMed] [Google Scholar]
- 124.Tu H, Pan W, Feucht L, Kastin AJ. Convergent trafficking pattern of leptin after endocytosis mediated by ObRa - ObRd. J.Cell Physiol. 2007 doi: 10.1002/jcp.21020. in press.
- 125.Van Heek M, Compton DS, France CF, Tedesco RP, Fawzi AB, Graziano MP, et al. Diet-induced obese mice develop peripheral, but not central, resistance to leptin. J.Clin.Invest. 1997;99:385–90. doi: 10.1172/JCI119171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Verma S, Li S-H, Wang C-H, Fedak PWM, Li R-K, Weisel R, et al. Resistin promotes endothelial cell activation: Further evidence of adipokine-endothelial interaction. Circulation. 2003;108:736–40. doi: 10.1161/01.CIR.0000084503.91330.49. [DOI] [PubMed] [Google Scholar]
- 127.Walker E, Perisic O, Ried C, Stephens L, Williams R. Structural insights into phosphoinositide 3-kinase catalysis and signalling. Nature. 1999;402:313–20. doi: 10.1038/46319. [DOI] [PubMed] [Google Scholar]
- 128.Wallenius K, Wallenius V, Sunter D, Dickson S, Jansson J. Intracereboventricular interleukin-6 treatment decreases body fat in rats. Biochem Biophys Res Commun. 2002;293:560–5. doi: 10.1016/S0006-291X(02)00230-9. [DOI] [PubMed] [Google Scholar]
- 129.Way J, Gorgun C, Tong Q, Uysal K, Brown K, Harrington W, et al. Adipose tissue resistin expression is severely suppressed in obesity and stimulated by peroxisome proliferator-activated receptor gamma agonists. J.Biol.Chem. 2001;276:25651–3. doi: 10.1074/jbc.C100189200. [DOI] [PubMed] [Google Scholar]
- 130.Wernstedt I, Olsson B, Jernas M, Paglialunga S, Carlsson L, Smith U, et al. Increased levels of acylation-stimulating protein in interleukin-6-deficient (IL-6(-/-)) mice. Endocrinology. 2006;147:2690–5. doi: 10.1210/en.2005-1133. [DOI] [PubMed] [Google Scholar]
- 131.Wu-Peng XS, Chua SC, Jr., Okada N, Liu S-M, Nicolson M, Leibel RL. Phenotype of obese Koletsky (f) rat due to Tyr763 stop mutation in the extracellular domain of the leptin receptor (lepr) Diabetes. 1997;46:513–8. doi: 10.2337/diab.46.3.513. [DOI] [PubMed] [Google Scholar]
- 132.Yanai K, Saito T, Kakinuma Y, Kon Y, Hirota K, Taniguchi-Yanai K, et al. Renin-dependent cadiovascular functions and renin-independent blood-brain barrier functions revealed by renin-deficient mice. J.Biol.Chem. 2000;275:5–8. doi: 10.1074/jbc.275.1.5. [DOI] [PubMed] [Google Scholar]
- 133.Yang H, Zhang R, Mu H, Li M, Yao Q, Chen C. Adiponectin promotes endothelial cell differentiation form human peripheral CD14+ monocytes in vitro. J.Cell.Mol.Med. 2006;10:459–69. doi: 10.1111/j.1582-4934.2006.tb00411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yu C, Pan W, Tu H, Waters S, Kastin AJ. TNF activates MDR1 in cerebral microvascular endothelial cells. J.Neurochem. 2007 doi: 10.1159/000110445. in press.
- 135.Yu C, Kastin AJ, Pan W. TNF reduces LIF endocytosis despite increasing NFκB-mediated gp130 expression. J.Cell Physiol. 2007 doi: 10.1002/jcp.21105. in press.
- 136.Yu C, Kastin AJ, Tu H, Pan W. Opposing effects of proteasomes and lysosomes on LIFR: modulation by TNF. J.Mol.Neurosci. 2007 doi: 10.1007/s12031-007-0017-4. in press.
- 137.Zlokovic BV. Cerebrovascular permeability to peptides: manipulations of transport systems at the blood-brain barrier. Pharm.Res. 1995;12:1395–406. doi: 10.1023/a:1016254514167. [DOI] [PubMed] [Google Scholar]