Abstract
Background:
The purpose of this study was to determine the material properties of the normal carpal tunnel subsynovial connective tissue in response to shear stress.
Methods:
The shear modulus and maximum shear strength were measured with a custom-made micro-tester in 10 specimens of subsynovial connective tissue from 10 wrists in 8 patients with idiopathic carpal tunnel syndrome and in 10 specimens from 5 fresh frozen cadavers without a history of carpal tunnel syndrome.
Findings:
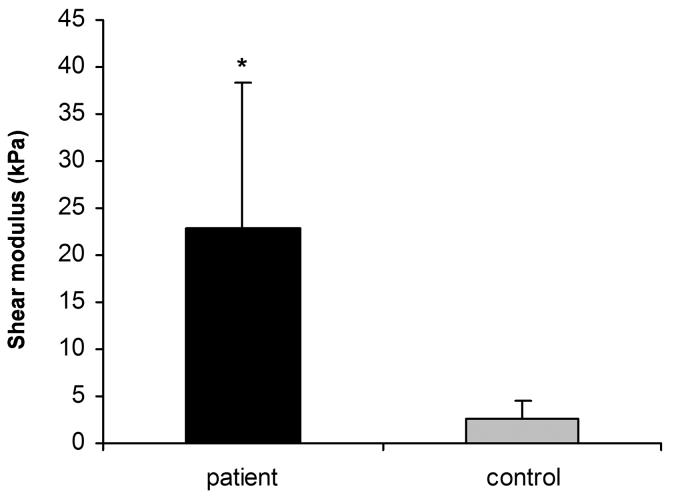
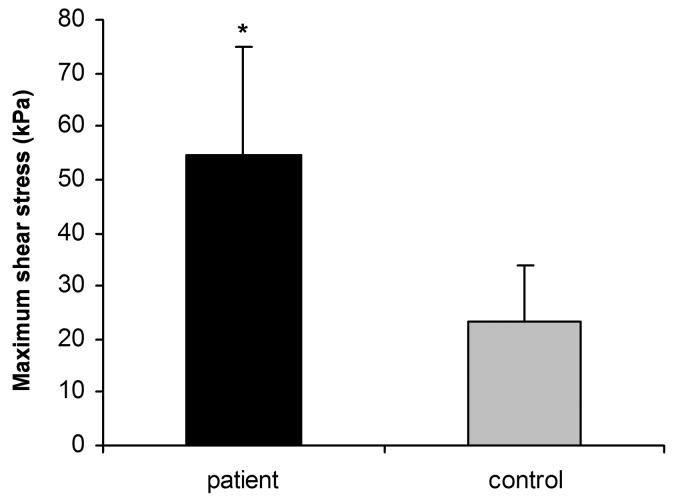
The mean shear modulus was 22.8 (SD 15.4) kPa for the patient group and 2.7 (SD 1.8) kPa for the control group. The mean maximum shear strength was 54.6 (SD 20.3) kPa for the patient group and 23.3 (SD 10.7) kPa for the control group. The values for the patient group were significantly higher than the control group (p < 0.05).
Interpretation:
The material properties of subsynovial connective tissue are altered in patients with idiopathic carpal tunnel syndrome. The impact, if any, of these altered properties on carpal tunnel syndrome remains to be elucidated.
Keywords: carpal tunnel syndrome, biomechanics, subsynovial connective tissue, shear stress
Introduction
The subsynovial connective tissue (SSCT) is the most characteristic structure in the carpal tunnel. It consists of multiple thin layers of collagenous fibers, in which blood and lymphatic vessels are richly represented (Guimberteau, 2001, Ettema et al., 2004). The SSCT plays an important role as a gliding unit to diminish friction and protect the vascular system within the SSCT. Noninflammatory fibrosis of the tenosynovium is the most common finding in idiopathic carpal tunnel syndrome (CTS), but its relationship to the neuropathy is unknown. Some have suggested that the changes in the SSCT might cause the neuropathy, through altered mechanics and vascularity in the carpal tunnel (Lluch, 1992; Sud et al., 2002).
Recent studies have demonstrated alterations in the gliding mechanism of the SSCT in patients with CTS (Ettema et al., 2006), as well as increased collagen type III, TGF-beta and abnormal angiogenesis (Ettema et al., 2004, Oh et al., 2004, Oh et al., 2005, Oh et al., 2006). These changes support the hypothesis that idiopathic CTS may be caused primarily by an injury to the SSCT, with secondary nerve involvement, rather than by direct damage of the nerve itself.
If CTS is caused, or can be caused, by an injury to the SSCT, then the mechanism of that injury must be plausible. One such mechanism could be a shearing injury of the SSCT, due to excessive differential tendon motion. There are many reports describing highly repetitive work as one of the risk factors for idiopathic CTS (Finkel, 1985, Atcheson et al., 1998, Chin and Jones, 2002). One biomechanical study has reported that differential finger motion with the wrist flexed elevated the tendon gliding resistance in the carpal tunnel (Zhao et al., 2007). The thickening of the fibers, noted by Ettema et al. (2004) and Oh et al. (2006) could be the result of healing of such an injury. To date, there have been no reports describing the mechanical properties of the SSCT in the carpal tunnel. The purpose of this study was to compare the mechanical properties of the SSCT of normal human cadavers in response to shear stress with those of patients with CTS.
Materials and Methods
Specimen Preparation
This study was approved by our Institutional Review Board, and all patients gave informed consent for the use of their tissue in the study. Ten SSCT samples were obtained from 10 wrists of 8 adult patients (2 males and 6 females) with idiopathic CTS undergoing open carpal tunnel release surgery. The patients' ages ranged from 24 to 71 years (mean, 45 years). A full thickness SSCT biopsy, 5 mm wide by 10 mm long, was taken from the visceral synovium on the anterior middle finger flexor digitorum superficialis (FDS) tendon surface. The control group consisted of 10 similar SSCT samples, harvested from the palmar surface of the middle and ring finger FDS tendons of 5 fresh frozen cadavers (3 males, 2 females) aged 78 to 83 years (mean, 82 years). Medical records were available for the cadaver donors, and were reviewed for evidence of carpal tunnel syndrome and associated conditions. Exclusion criteria included any notation in the medical record of carpal tunnel syndrome, diabetes, inflammatory arthritis (rheumatoid arthritis, lupus, etc), or injury to the wrist. All tissues were stored at −70 °C until testing.
Measurement of shear force of SSCT
After thawing the sample, specimen thickness was measured with a charge-coupled device (CCD) laser displacement sensor (LK-081, Keyence Corporation, Osaka, Japan) as previously described (Osamura et al., 2007). The thickness was measured at 3 different points on the sample and the averaged data were considered as the thickness of each sample.
The palmar and dorsal surfaces of the SSCT were each attached with cyanoacrylate (Henkel Corp, Rocky Hill, CT) to a 3 mm wide by 20 mm long rectangular plastic plate (Fig. 1). Redundant SSCT extending beyond the edge of the plastic plate was trimmed. A pair of plastic plates with the specimen was mounted in neutral position, without any stretching, on a custom-made micro-tester which was composed of a linear servo motor (MX 80 Daedal, Irwin, PA) and a load cell accurate to 0.025 g (1.5 N, GSO-150, Transducer Techniques, Temecula, CA). The proximal side of the sample was connected to the linear servo motor and the distal side was connected to the load cell. Throughout testing, the specimen was kept moist in a chamber filled with phosphate-buffered saline. The SSCT was stretched until failure under displacement control at a rate of 1.0 mm/s using the linear servo motor. Shear force and displacement were recorded continuously. Shear stress was calculated by dividing the shear force by the surface area of the specimen (15 mm2). Shear strain was obtained by dividing the tissue stretch (mm) by the SSCT thickness (mm). A shear stress-shear strain curve was plotted and the shear modulus was determined from the slope of the linear portion of the post-toe region of this curve. Maximum shear stress was recorded from the peak of the curve.
Figure 1. Specimen preparation.
(a) A 5 mm wide by 10 mm long specimen is taken from the middle finger flexor digitorum superficialis tendon surface, and associated full thickness of SSCT.
(b) The deep (tendon) and superficial (SSCT) surfaces of the specimen are attached to a 3×20 mm plastic plate on each of SSCT with cyanoacrylate, and then the specimen is trimmed to 3 × 5 mm.
(c) Side view of the specimen with attached plastic plates, which connect to the load cell and linear motor for the shear testing.
Statistics
All data were expressed as the mean ± standard deviation (SD). The mechanical properties of the carpal tunnel patients and normal cadavers were compared using Student's t-test. P < 0.05 was considered to be statistically significant.
Results
There were no surgical complications as a result of the synovial biopsy. The mean SSCT thickness for the carpal tunnel patients; 1.46 mm (SD 0.56 mm) was significantly greater than the normal control, 0.48 mm (SD 0.12 mm) (p < 0.05).
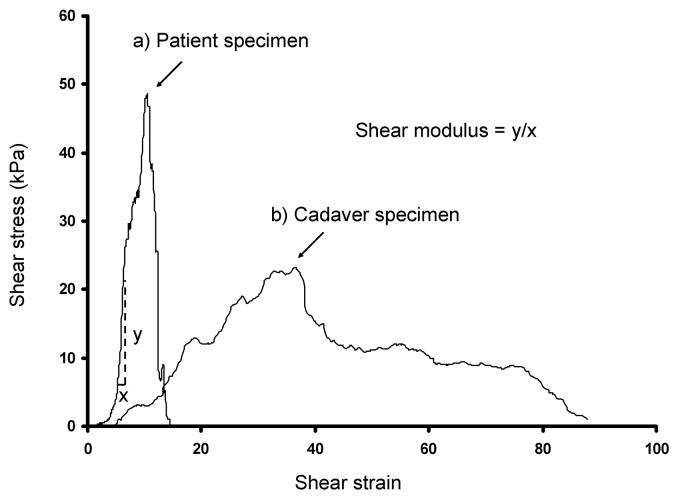
Figure 2 shows representative shear stress-shear strain curves. Typically, no load (i.e., a “toe” region) was observed during the initial strain phase. The failure mode during testing included mid-substance rupture (n = 5 patients, n = 9 controls) or avulsion from the plastic plates (n = 5 patients, n = 1 control). Four specimens did not completely rupture within the maximum displacement (29 mm) of the linear servo motor, although their peak shear stress was exceeded. The peak shear stress occurred at a mean shear strain of 10.9% (SD 7.7%) [mean actuator excursion of 12.7 mm (SD 5.0 mm)] in the patient specimens and 30.1% (SD 19.7%) shear strain [mean actuator excursion of 13.5 mm (SD 7.2 mm)] in the cadaver controls. The mean shear modulus was 22.8 kPa (SD 15.4 kPa) in the patient group and 2.7 kPa (SD 1.8 kPa) in the control group (Fig. 3). The mean maximum shear stress was 54.6 kPa (SD 20.3 kPa) (range; 31.3 – 97.3 kPa) for the patient group and 23.3 kPa (SD 10.7 kPa) (range; 8.1 – 43.2 kPa) for the control group (Fig. 4). The peak shear stress and shear modulus were significantly higher in the patient group. The shear strain at the point of peak shear stress was significantly lower in the patient group.
Figure 2.
Representative shear stress against shear strain curves (a; patient specimen, b; cadaver specimen). The shear modulus is determined as the slope of the linear portion of the curve. The shear strain is obtained by dividing the actuator displacement (mm) by the specimen thickness (mm). There is a period of initial laxity where the tissue stretches without any loading.
Figure 3.
Shear modulus of subsynovial connective tissue. The shear modulus for the patient group is significantly higher than the control group. (* p < 0.05)
Figure 4.
Maximum shear stress of subsynovial connective tissue. The maximum shear stress for the patient group is significantly higher than the control group. (* p < 0.05)
Discussion
Recently, several immunohistological (Ettema et al., 2004, Oh et al., 2005) and biochemical studies (Freeland et al., 2002, Hirata et al., 2004, Hirata et al., 2005, Tsujii et al., 2006) have been reported describing the SSCT in carpal tunnel syndrome patients. The SSCT of CTS patients is fibrotic, with increased amounts of type III collagen, tenascin-C, versican, VEGF, PGE-2, MMP-2, IL-6, and TGF-beta, and decreased elastin. Sud et al. (2002) have shown that the in vitro tenosynovial absorption rate is increased in CTS patients. We have added to this data an assessment of the material properties of the SSCT in the carpal tunnel, in individuals with and without CTS.
In the normal human carpal tunnel, the tendons are surrounded by a multilayered SSCT (Guimberteau, 2001, Ettema et al., 2004). Normally this SSCT allows differential gliding of the tendons, as when one finger flexes and an adjacent finger extends or remains still, as may occur in many tasks. Such activity imposes a shear strain on the SSCT. Here, we have measured the normal shear properties of the SSCT, and compared them with those in patients with CTS. While the cadavers were somewhat older than our patients, we were able to exclude cadavers with any evidence of abnormality within the carpal tunnel, as well as any with a documented history of carpal tunnel syndrome in the medical records we were able to access. In addition, none of the cadaver specimens showed any of the fibrosis that is characteristic of the SSCT in carpal tunnel syndrome. The cadaver specimens were also quite uniform, with narrower standard deviations than the patient specimens, again suggesting that there was no pathology associated with aging. Finally, while we have not been able to identify any data on the effect of aging on the material properties of synovial tissues, the available data on tendons and ligaments (Becker et al., 1994, Narici and Maganaris, 2006) suggests that with aging tendons and ligaments become roughly 10% weaker compared to younger adults. Our differences far exceeded this range, again suggesting that the fact that our controls were older did not have a major effect on the results we noted.
Our study suggests that the normal SSCT can withstand only relatively low loads before failure, and that shearing motion of 26 mm or less (mean 13.5 mm in our specimens) may be sufficient to produce these loads. This motion is within the physiological limits of tendon motion within the carpal tunnel; total tendon excursion of the FDS is more than 60 mm (Smith, 1987). Given the moment arms of the FDS in the fingers, any differential digit motion totaling more than 120 degrees at the proximal interphalangeal and metacarpophalangeal joints would be sufficient to create 25 mm of displacement, (Brand and Hollister, 1993) and thus, sufficient shear strain to exceed the threshold for SSCT rupture in any of our specimens. To create 13.5 mm of displacement, less than 90 degrees of differential digit composite motion would be needed. While our fixation of the SSCT surface to the slide, with glue, is not physiological, there is no way to test material properties without fixing both ends of the specimen to be tested. Thus, while it is possible that there may be more “give” in the system in situ, we believe that it is reasonable to suppose that, in everyday activities, strains approaching those associated with SSCT shear failure may occur. We find it interesting that for the CTS patients, the SSCT not only fails at higher loads, as would be expected with fibrosis, but also at lower strains. This could suggest the possibility of a vicious cycle: the injured SSCT is liable to injury at lower strains than normal SSCT; in addition the greater stiffness of the injured SSCT may increase the work needed to move the tendons surrounded by an injured SSCT, which may lead to increased tendon loading, so that higher forces are applied to the tendons, increasing the risk of further SSCT injury.
In order to apply a shear force to the SSCT specimen, we attached the superficial and deep surfaces of the SSCT to plastic plates with cyanoacrylate and then displaced the plates in opposite directions. The influence of cyanoacrylate on the SSCT specimen depends on how much cyanoacrylate penetrates into the specimen, so that a thinner specimen could be more susceptible to cyanoacrylate. However, even though the control group was thinner than the patient group (and thus theoretically more susceptible to cyanoacrylate), the control group SSCT stiffness was significantly lower than that of the patient group. Additionally, some specimens peeled off of the plastic plates during testing, suggesting that the strength of some SSCT specimens was larger than the adhesive force of the cyanoacrylate. We regarded the recorded peak of shear strength as the maximum strength even if the specimen peeled off the plates. In terms of avulsion failure, there were 7 specimens for the patient group but only 2 specimens for the control group. Thus, the true maximum shear strength of SSCT could be much higher than our results, especially for the patient group.
Another limitation of this study was the use of cadavers for the control group. We chose cadavers for controls, as we believed it was not ethical to open the carpal tunnels of normal living individuals to biopsy the synovium, and we were able to exclude cadavers with any evidence of abnormality within the carpal tunnel. Thus, we believe that our control sample is a reasonable one.
In summary, we have measured the material properties of the SSCT in response to shear stress in specimens from individuals with and without a history of carpal tunnel syndrome. The shear modulus of the SSCT in idiopathic CTS patients was significantly higher than that of the control SSCT, consistent with the fibrosis of the SSCT which is known to be present in CTS patients. We have further shown that the SSCT of cadaver carpal tunnels fails in shear load at displacements which are well within the physiological range, and that the SSCT of CTS patients fails not only at higher loads, but also at lower displacements. We believe that this evidence supports the hypothesis, proposed by Lluch (1992) and others (Lluch, 1992, Tucci et al., 1997, Guimberteau, 2001, Freeland et al., 2002, Sud et al., 2002, Ettema et al., 2004, Hirata et al., 2004, Hirata et al., 2005, Oh et al., 2006) that CTS is the result of the response of the SSCT to injury, possibly a shearing injury, with secondary pressure elevation within the carpal tunnel as a result of altered SSCT permeability. This hypothesis deserves further testing, preferably in an animal model where injury to the SSCT can be induced and its sequelae studied.
Acknowledgements
This study was funded by grants from NIH (NIAMS AR49823) and Mayo Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Atcheson SG, Ward JR, Lowe W. Concurrent medical disease in work-related carpal tunnel syndrome. Archives of Internal Medicine. 1998;158:1506–1512. doi: 10.1001/archinte.158.14.1506. [see comment] [DOI] [PubMed] [Google Scholar]
- Becker CK, Savelberg HH, Barneveld A. In vitro mechanical properties of the accessory ligament of the deep digital flexor tendon in horses in relation to age. Equine Veterinary Journal. 1994;26:454–459. doi: 10.1111/j.2042-3306.1994.tb04049.x. [DOI] [PubMed] [Google Scholar]
- Brand PW, Hollister A. Mosby; St. Louis: 1993. Clinical Mechanics of the Hand. [Google Scholar]
- Chin DH, Jones NF. Repetitive motion hand disorders. Journal of the California Dental Association. 2002;30:149–160. [PubMed] [Google Scholar]
- Ettema AM, Amadio PC, Zhao C, Wold LE, An KN. A histological and immunohistochemical study of the subsynovial connective tissue in idiopathic carpal tunnel syndrome. J Bone Joint Surg Am. 2004;86-A:1458–1466. doi: 10.2106/00004623-200407000-00014. [DOI] [PubMed] [Google Scholar]
- Finkel ML. The effects of repeated mechanical trauma in the meat industry. American Journal of Industrial Medicine. 1985;8:375–379. doi: 10.1002/ajim.4700080418. [DOI] [PubMed] [Google Scholar]
- Freeland AE, Tucci MA, Barbieri RA, Angel MF, Nick TG. Biochemical evaluation of serum and flexor tenosynovium in carpal tunnel syndrome. Microsurgery. 2002;22:378–385. doi: 10.1002/micr.10065. [DOI] [PubMed] [Google Scholar]
- Guimberteau JC. New ideas in hand flexor tendon surgery. Institut Aquitain De La Main; 2001. The sliding system. Vascularized flexor tendon transfers. [Google Scholar]
- Hirata H, Nagakura T, Tsujii M, Morita A, Fujisawa K, Uchida A. The relationship of VEGF and PGE2 expression to extracellular matrix remodeling of the tenosynovium in the carpal tunnel syndrome. J Pathol. 2004;204:605–612. doi: 10.1002/path.1673. [DOI] [PubMed] [Google Scholar]
- Hirata H, Tsujii M, Yoshida T, Imanaka-Yoshida K, Morita A, Okuyama N, Nagakura T, Sugimoto T, Fujisawa K, Uchida A. MMP-2 expression is associated with rapidly proliferative arteriosclerosis in the flexor tenosynovium and pain severity in carpal tunnel syndrome. J Pathol. 2005;205:443–450. doi: 10.1002/path.1709. [DOI] [PubMed] [Google Scholar]
- Lluch AL. Thickening of the synovium of the digital flexor tendons: cause or consequence of the carpal tunnel syndrome? J Hand Surg [Br] 1992;17:209–212. doi: 10.1016/0266-7681(92)90091-f. [DOI] [PubMed] [Google Scholar]
- Narici MV, Maganaris CN. Adaptability of elderly human muscles and tendons to increased loading. Journal of Anatomy. 2006;208:433–443. doi: 10.1111/j.1469-7580.2006.00548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh J, Zhao C, Amadio PC, An KN, Zobitz ME, Wold LE. Vascular pathologic changes in the flexor tenosynovium (subsynovial connective tissue) in idiopathic carpal tunnel syndrome. J Orthop Res. 2004;22:1310–1315. doi: 10.1016/j.orthres.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Oh J, Zhao C, Amadio PC, An KN, Zobitz ME, Wold LE. Immunolocalization of collagen types in the subsynovial connective tissue within the carpal tunnel in humans. J Orthop Res. 2005;23:1226–1231. doi: 10.1016/j.orthres.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Oh J, Zhao C, Zobitz ME, Wold LE, An KN, Amadio PC. Morphological changes of collagen fibrils in the subsynovial connective tissue in carpal tunnel syndrome. J Bone Joint Surg Am. 2006;88:824–831. doi: 10.2106/JBJS.E.00377. [DOI] [PubMed] [Google Scholar]
- Smith RJ. Tendon Transfers of the Hand and Forearm. Little, Brown; Boston: 1987. [Google Scholar]
- Sud V, Tucci MA, Freeland AE, Smith WT, Grinspun K. Absorptive properties of synovium harvested from the carpal tunnel. Microsurgery. 2002;22:316–319. doi: 10.1002/micr.10051. [DOI] [PubMed] [Google Scholar]
- Tsujii M, Hirata H, Yoshida T, Imanaka-Yoshida K, Morita A, Uchida A. Involvement of tenascin-C and PG-M/versican in flexor tenosynovial pathology of idiopathic carpal tunnel syndrome. Histol Histopathol. 2006;21:511–518. doi: 10.14670/HH-21.511. [DOI] [PubMed] [Google Scholar]
- Tucci MA, Barbieri RA, Freeland AE. Biochemical and histological analysis of the flexor tenosynovium in patients with carpal tunnel syndrome. Biomed Sci Instrum. 1997;33:246–251. [PubMed] [Google Scholar]