Summary
Active adult neurogenesis occurs in discrete brain regions of all mammals and is widely regarded as a neuronal replacement mechanism. Whether adult-born neurons make unique contributions to brain functions are largely unknown. Here we systematically characterized synaptic plasticity of retrovirally-labeled adult-born dentate granule cells at different stages during their neuronal maturation. We identified a critical period between 1 and 1.5 months of the cell age when adult-born neurons exhibit enhanced long-term potentiation with increased potentiation amplitude and decreased induction threshold. Furthermore, such enhanced plasticity in adult-born neurons depends on developmentally regulated synaptic expression of NR2B-containing NMDA receptors. Our study demonstrates that adult-born neurons exhibit classic critical period plasticity as neurons in the developing nervous system. The transient nature of such enhanced plasticity may provide a fundamental mechanism allowing adult-born neurons within the critical period to serve as major mediators of experience-induced plasticity while maintaining stability of the mature circuitry.
Introduction
New neurons are continuously generated from adult neural stem/progenitor cells throughout life in discrete regions of the mammalian central nervous system (Gage, 2000; Ming and Song, 2005). In the hippocampus, a portion of newly generated dentate granule cells (DGCs) become synaptically integrated into the existing neural network (van Praag et al., 2002) and are then maintained in the adult brain (Kempermann et al., 2003; Zhao et al., 2006). A central question in the field of adult neurogenesis relates to the physiological significance of the continuous addition of a small population of new neurons in the mammalian brain (Aimone et al., 2006; Doetsch and Hen, 2005; Kempermann et al., 2004; Kitabatake et al., 2007). Are these new neurons merely replacing dying mature neurons, or are they making unique contributions to specific brain functions that could not be achieved by existing mature neurons? Accumulating evidence from behavioral analysis suggests that adult neurogenesis is essential for some forms of learning, memory and mood regulation (Abrous et al., 2005; Doetsch and Hen, 2005; Kempermann et al., 2004; Kitabatake et al., 2007; Lledo et al., 2006). Recent studies using c-Fos and Arc expression as indicators for neuronal activation suggest an interesting possibility that new DGCs may be preferentially recruited into circuits in the adult brain that mediate spatial information processing and memory formation (Kee et al., 2007; Ramirez-Amaya et al., 2006; Tashiro et al., 2007). How such preferential incorporation of adult-born neurons is achieved remains largely unknown.
Synaptic plasticity is widely regarded as a substrate for many brain functions, including learning and memory (Malenka and Bear, 2004). Comparing properties of synaptic plasticity between adult-born neurons and existing mature neurons may provide important insights into the potential function of adult neurogenesis. Previous studies using field recordings from a heterogeneous neuronal population, consisting of mature DGCs and newborn DGCs of mixed ages, provided indirect evidence suggesting that new neurons exhibit an enhanced synaptic plasticity (Snyder et al., 2001). More recently, immature newborn DGCs in adult rats, identified by their expression of TOAD-64 (Wang et al., 2000) or PSA-NCAM (Schmidt-Hieber et al., 2004), were shown to exhibit a lower threshold for the induction of glutamatergic long-term potentiation (LTP) when compared with mature DGCs. In the adult rodent brain, new neurons express these immature neuronal makers transiently only during 2–3 weeks after they are born (Seki, 2002), a period when glutamatergic synaptic inputs are just beginning to form (Ge et al., 2006). A key question remains: Are special physiological properties maintained in adult-born neurons after fully integration into the existing circuitry, or are they transient in nature? Recent development of the retroviral approach permits permanent labeling and single cell analysis of new neurons with a defined birth-date (van Praag et al., 2002). Aided by electrophysiological and morphological analysis, studies using this approach have delineated the sequential synaptic integration process of new neurons in the adult brain (Carleton et al., 2003; Esposito et al., 2005; Ge et al., 2006; Zhao et al., 2006). The properties and underlying mechanisms of synaptic plasticity in fully integrated adult-born neurons, however, remain largely uncharacterized.
While the adult brain exhibits significant plasticity for life-long learning (Chklovskii et al., 2004), the magnitude of synaptic plasticity and anatomical changes in response to experience distinguish the adult form of plasticity from developmental plasticity (Katz and Shatz, 1996). Since the classic experiments by Hubel and Wiesel in the visual cortex (Hubel and Wiesel, 1962; Wiesel and Hubel, 1963), it has been well-established that there exists a critical period when neuronal properties are particularly susceptible to modification by experience, which is concurrent with large-scale anatomical changes that become irreversible after the closure of the critical period. The classic critical period plasticity occurs mostly in juvenile animals and has been considered as a central mechanism for establishing fine tuned neuronal circuits in the developing brain (Hensch, 2004). Whether the adult mammalian hippocampus retains such developmental plasticity through continuous neurogenesis is unknown.
One of the key mediators of plasticity is the N-methyl-D-asparate (NMDA) type of glutamate receptors, which are composed of subunit families of NMDA receptors (NMDARs) NR1 and NR2 (Cull-Candy and Leszkiewicz, 2004). Of four NR2 subtypes (NR2A, 2B, 2C and 2D), the NR2B subunit is expressed early during the postnatal development and appears to be associated with enhanced synaptic plasticity during the critical period (Barria and Malinow, 2005; Barth and Malenka, 2001; Cull-Candy and Leszkiewicz, 2004). It remains largely unknown, however, whether NR2B subtypes play a permissive role, being the major NR2 subtypes expressed during the critical period, or alternatively, play an instructive role that could not be substituted by other subtypes. During adult neurogenesis, NMDARs are expressed early starting from immature neuronal stages (Carleton et al., 2003; Nacher et al., 2007) and activation of NMDARs is known to regulate several initial steps of adult neurogenesis, especially neuronal survival (Cameron et al., 1995; Tashiro et al., 2006). The role of specific NMDAR subtypes in synaptically connected adult-born neurons is unknown.
Using retrovirus mediated birth-dating and labeling, we examined the temporal regulation and underlying mechanism of synaptic plasticity of adult-born DGCs along their maturation. We identified a critical period when adult-born DGCs exhibit significantly enhanced synaptic plasticity. Importantly, such critical period plasticity depends on developmentally regulated synaptic expression of NR2B subtypes in adult-born neurons. Our study demonstrates that adult neurogenesis continuously produces cohorts of new neurons transiently exhibiting enhanced plasticity, thus potentially expand the capacity of the adult circuitry to be modified by experience throughout life.
Results
Enhanced synaptic plasticity by young adult-born neurons
To permanently label new neurons in the adult brain, we stereotaxically injected engineered retroviruses expressing green fluorescent protein (GFP) into the hilus region of adult mice (See Experimental Procedures). GFP+ newborn DGCs in slices acutely prepared from retrovirus-infected animals (See Figure S1 in the Supplementary Data) were recorded by whole-cell patch-clamp (Ge et al., 2006). Excitatory postsynaptic potentials (EPSPs) were monitored under the current-clamp in response to a low frequency stimulation (every 30s) of the medial perforant pathway (Figure 1). To examine the synaptic plasticity of newborn DGCs, we used a paradigm of theta burst stimulation (TBS; Figure S2) that corresponds to the physiological firing pattern of a DGC at the border of its place field in vivo (Schmidt-Hieber et al., 2004; Skaggs et al., 1996). Significant LTP of EPSPs was reliably induced with TBS in GFP+ DGCs examined at 1-month post infection (mpi; n = 7; Figure 1A), when most of new DGCs already fully integrated into the existing circuitry (Esposito et al., 2005; Ge et al., 2006). When the same stimulation paradigm was used to induce LTP in GFP− mature DGCs (Figure 1B), however, only 64% of neurons recorded exhibited significant LTP (Figure 1D). Thus, adult-born DGCs at 1 mpi in mice also exhibit a lower threshold for LTP induction, similar to those new DGCs at about 2–3 weeks of the cell age as reported in adult rats (Schmidt-Hieber et al., 2004). We then examined whether adult-born DGCs maintain this characteristic during its lifespan (Figure 1C). We recorded from GFP+ DGCs at 4 mpi when new neurons already reach both morphological (Zhao et al., 2006) and physiological (Laplagne et al., 2006) maturation. Interestingly, only 67% of these DGCs exhibited significant LTP (Figure 1D), similar to that of existing mature DGCs. Thus, the property of a lower threshold for LTP induction in adult-born neurons is not maintained once new neurons reach maturation.
Figure 1.
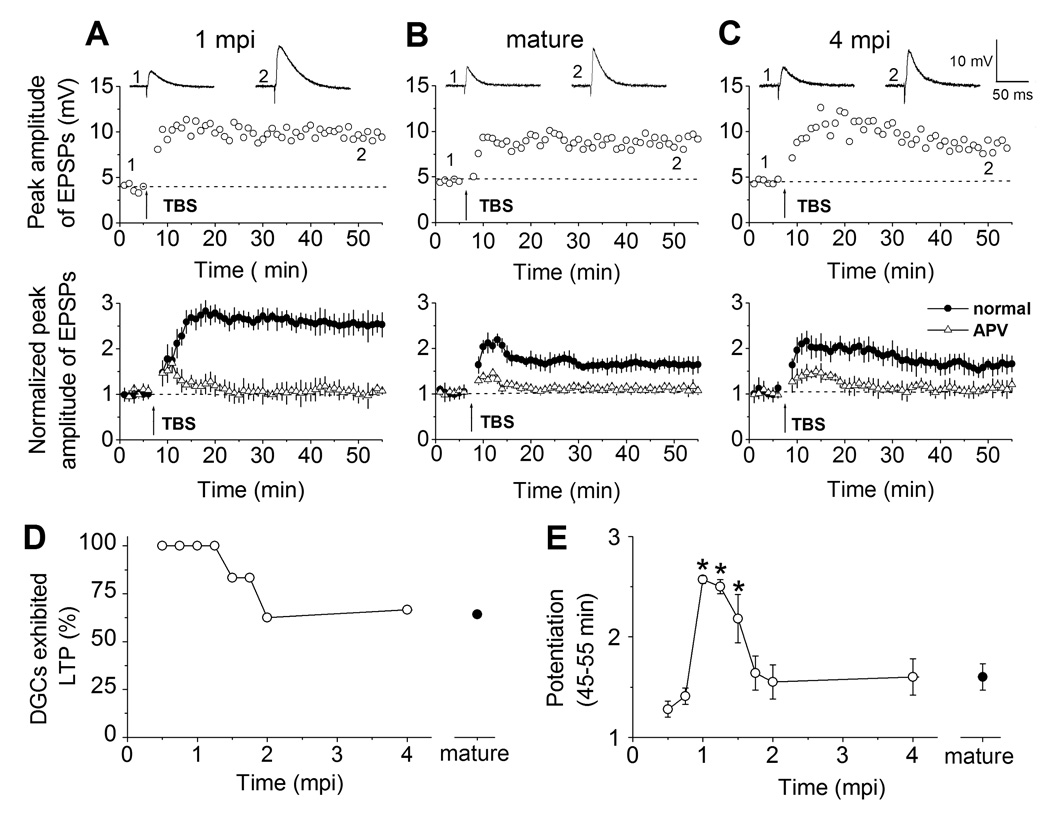
Adult-born neurons exhibit enhanced synaptic plasticity within a critical period. (A) LTP recorded from newborn DGCs at 1 mpi in the adult brain. Shown in the top row is an example of LTP of EPSPs recorded under the whole-cell current-clamp. Representative EPSPs averaged from 5 consecutive stimuli were taken before and after LTP induction by a physiological relevant TBS (arrow) at the time points (1 and 2) indicated in the graph. Shown at the bottom is the summary of LTP recorded from newborn DGCs at 1 mpi in the presence or absence of APV (50 µM). Normalized EPSP amplitudes are shown. Values represent mean ± SEM. (B, C) LTP recorded from mature DGCs and from newborn DGCs at 4 mpi. Same as in (A), except that GFP− mature DGCs (B) or newborn GFP+ DGCs at 4 mpi (C) were recorded. (D, E) A critical period with enhanced synaptic plasticity for adult-born neurons. Shown in (D) are percentages of DGCs at different stages of neuronal maturation that exhibited significant LTP. Shown in (E) is the summary of the mean enhancement of EPSPs (at 45–55 min during recording) from newborn GFP+ DGCs at different stages and from GFP− mature DGCs. Values represent mean ± SEM (*: p < 0.01; t-test). The value of LTP for individual cell examined under each condition is shown in Figure S3 in the Supplementary Data.
We next quantified the amplitude of LTP, an important physiological characteristic of synaptic plasticity. Interestingly, the mean LTP amplitude for GFP+ DGCs at 1 mpi was significantly larger than that of GFP− mature DGCs (Figure 1E). GFP+ DGCs at 4 mpi, however, exhibited very similar LTP amplitude to that of mature DGCs (Figure 1E). Taken together, these results demonstrate that adult-born DGCs transiently exhibit enhanced synaptic plasticity, as reflected by both an increase in the LTP amplitude and a decrease in the LTP induction threshold.
A critical period for enhanced synaptic plasticity of adult-born neurons
To determine the precise window when adult-born DGCs exhibit enhanced synaptic plasticity, we systematically examined LTP from GFP+ DGCs of different cell ages using the same TBS paradigm. At 0.5 mpi and 0.75 mpi, all GFP+ DGCs recorded that had glutamatergic synaptic inputs exhibited significant LTP (Figure 1D), consistent with previous finding of a lower threshold for LTP induction in these immature neurons (Schmidt-Hieber et al., 2004; Wang et al., 2000). The LTP amplitude for GFP+ DGCs at 0.5 mpi and 0.75 mpi, however, was smaller in comparison with a selected population of GFP− mature DGCs that exhibited significant LTP (See Figure S3). When all cells were included, regardless of whether significant LTP was induced, the mean LTP amplitude for GFP+ DGCs during this period was comparable to that of mature DGCs (Figure 1E). Similarly, the LTP amplitude of GFP+ DGCs older than 1.75 mpi was also comparable to those of mature DGCs (Figure 1E). In contrast, almost all GFP+ DGCs at 1 mpi and 1.5 mpi exhibited significant LTP (Figure S3) with much larger amplitudes than those of mature DGCs and than those of adult-born neurons at all other stages examined (Figure 1E). Taken together, our systematic analysis identified a critical window between 1 and 1.5 months of the cell age when adult-born neurons exhibit enhanced synaptic plasticity. Interestingly, a high level of anatomical plasticity has also been observed for adult-born neurons during the same period (Zhao et al., 2006). This is reminiscent of the classic early postnatal critical period in which enhanced synaptic plasticity is associated with a large magnitude of structural modification and consolidation (Hensch, 2004).
Developmental expression of NR2B-containing NMDARs
We next examined the molecular mechanism underlying the critical period plasticity of adult-born neurons. Application of APV (50 µM), a specific antagonist of NMDARs, abolished LTP from both GFP− mature DGCs and GFP+ newborn DGCs at all stages examined (Figure 1A to Figure 1C; and data not shown). Thus, activation of NMDARs is required for LTP of DGCs regardless of the cell age. The expression of NR2B subtypes has been shown to be associated with an enhanced synaptic plasticity during early postnatal development (Barria and Malinow, 2005; Barth and Malenka, 2001), we therefore examined the contribution of NR2B-containing NMDARs to evoked postsynaptic currents (EPSCs) in adult-born DGCs during their maturation (Figure 2). We recorded pharmacologically isolated NMDAR-mediated EPSCs from GFP+ DGCs under the whole-cell voltage-clamp (Vm = −65 mV; See Experimental Procedures). Application of ifenprodil (3 µM), a NR2B-subtype specific antagonist (Barria and Malinow, 2005; Barth and Malenka, 2001), reduced the NMDAR-mediated EPSCs in GFP+ DGCs at 1 mpi by 72.1 ± 3.6% (n = 6; Figure 2A). In contrast, the same treatment led to only 25.1 ± 4.2% (n = 5) and 26.2 ± 4.2% (n = 4) reduction in GFP+ DGCs at 2 mpi and in mature DGCs, respectively (Figure 2B). Thus, there exists a developmental shift in the contribution of NR2B subtypes to the total NMDAR-mediated EPSCs during the maturation of adult-born neurons.
Figure 2.
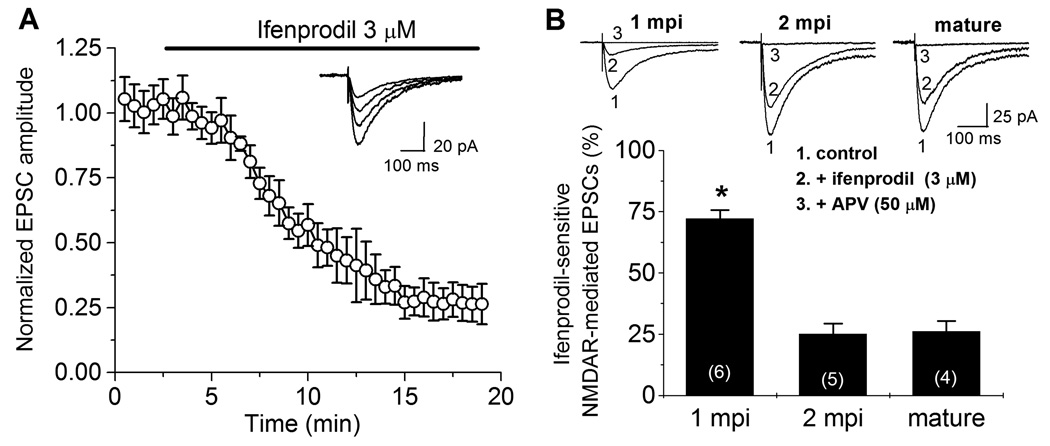
Developmental regulation of synaptic expression of NR2B-containing NMDARs in adult-born neurons during their maturation. (A) Blockade of NR2B-containing NMDAR-mediated EPSCs by ifenprodil. Shown are normalized NMDAR-mediated EPSCs from newborn DGCs at 1 mpi recorded under the whole-cell voltage-clamp (Vm = −65 mV) before and after bath application of ifenprodil (3 µM) as indicated. Glycine (10 µM), bicuculline (10 µM) and NBQX (100 µM) were present throughout the recording. Values represent mean ± SEM (n = 6). Insert shows sample EPSC traces recorded at different time points. Scale bars: 100 ms and 20 pA. (B) Contribution of NR2B-containing NMDARs to the total NMDAR-mediated EPSCs in newborn and mature DGCs in the adult brain. Top panel shows pharmacologically isolated NMDAR-mediated EPSCs from a newborn GFP+ DGC at 1 mpi, 2 mpi, and from a mature DGC recorded (1) before, (2) 15–20 min after bath application of ifenprodil (3 µM), and (3) followed by APV (50 µM). ottom panel shows the summary of inhibition of NMDAR-mediated EPSC by ifenprodil in newly generated DGCs at different maturation stages and in mature DGCs. Values represent mean ± SEM. The total number of cells examined under each condition is indicated in parentheses (*: p < 0.01; t-test).
Role of NR2B-containing NMDARs in the critical period plasticity
To determine the physiological role of NR2B subtypes in regulating synaptic plasticity of DGCs in the adult brain, we examined LTP from adult-born DGCs at 1 mpi, 2 mpi and from mature DGCs in the presence of pharmacological inhibition of NR2B-containing NMDARs. Interestingly, treatment of ifenprodil (3 µM) almost completely abolished LTP of GFP+ DGCs at 1 mpi (Figure 3A). In contrast, the same treatment only slightly affected LTP of GFP− mature DGCs or GFP+ DGCs at 2 mpi (Figure 3A). Similar results were also obtained in the presence of Ro25-6981 (0.5 µM; Figure 3B), another NR2B-subtype specific antagonist (Barria and Malinow, 2005; Barth and Malenka, 2001). Thus, LTP of adult-born DGCs exhibit differential dependence on NR2B subtypes during the maturation of these new neurons in the adult brain.
Figure 3.

Differential requirement of NR2B-signaling for LTP of mature and newborn DGCs in the adult brain. (A) Summary of LTP of EPSPs in GFP+ newborn DGCs at 1 mpi, 2 mpi and in mature DGCs, with or without the addition of ifenprodil (3 µM). EPSPs averaged from 5 consecutive stimuli were taken before and after LTP induction by TBS (arrow) at the time points (1 and 2) indicated in the graphs. Same groups of cells for 1 mpi were used as in Fig. 1A. (B) Summary of the modulation of the mean enhancement of EPSPs (at 45–55 min during recording) by ifenprodil (3 µM) or Ro25-6981 (0.5 µM). Values represent mean ± SEM. The total number of cells examined at each condition is indicated in parentheses (*: p < 0.01; t-test).
The role of NR2B subtypes for enhanced synaptic plasticity of adult-born neurons within the critical period could be simply permissive, as they constitute the majority of NMDARs; alternatively, NR2B subtypes could play an instructive role that cannot be substituted by other NMDARs. To differentiate these two possibilities, we first characterized dose-responses of APV and ifenprodil in inhibiting EPSCs recorded from GFP+ DGCs at 1 mpi (Figure S4). We next examined LTP of these new neurons in the presence of partial inhibition of NMDAR-mediated EPSCs to the same extent by either APV or ifenprodil (Figure 4). Interestingly, at 25% inhibition, ifenprodil (0.03 µM), but not APV (0.5 µM), significantly reduced the mean amplitude of LTP (Figure 4A and Figure 4D). Thus, LTP of adult-born neurons within the critical period exhibit differential requirement of NR2B and pan-NMDAR signaling. In further support of such notion, we found that at 50% inhibition, LTP is reduced to a much greater extent by ifenprodil (0.25 µM) than by APV (3 µM; Figure 4B and Figure 4D). At 75% inhibition, both ifenprodil (0.5 µM) and APV (7 µM) largely abolished LTP (Figure 4C and Figure 4D). Taken together, these results strongly suggest that NR2B-containing NMDARs play an instructive role in enhanced synaptic plasticity of adult-born neurons within the critical period that cannot be substituted by other NMDAR subtypes.
Figure 4.
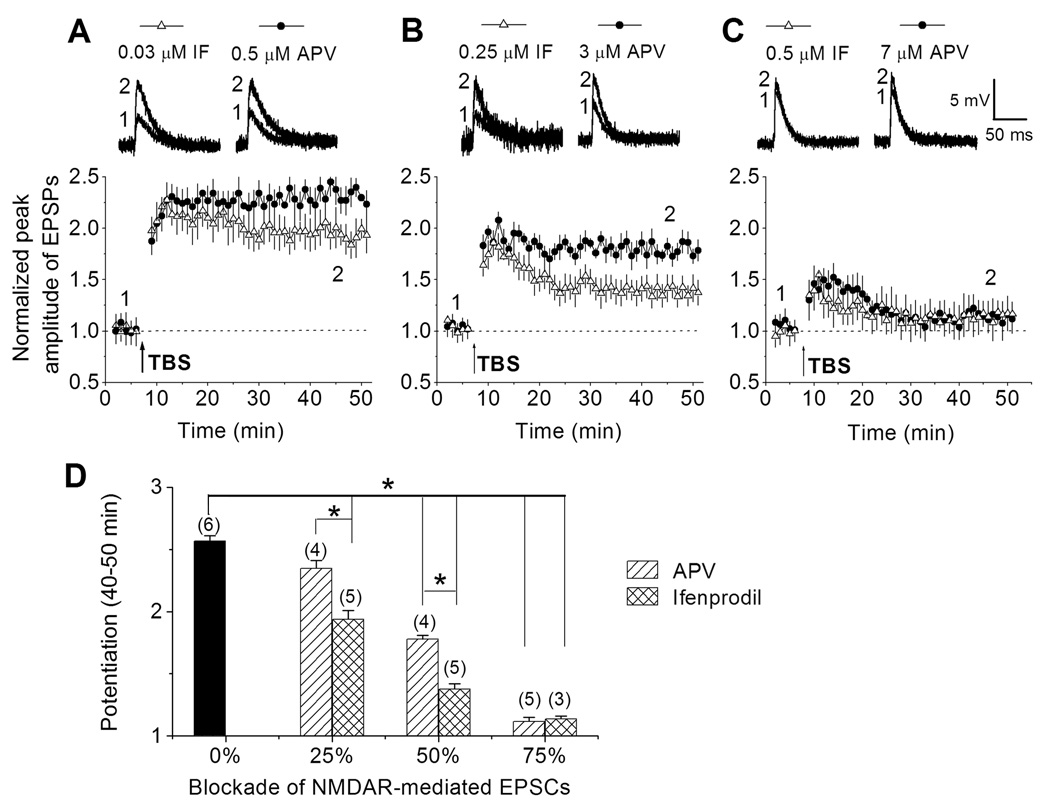
Differential modulation of enhanced synaptic plasticity of adult-born neurons by ifenprodil and APV. (A to C) LTP of EPSPs in GFP+ newborn DGCs at 1 mpi in the presence of different amount inhibition of NMDAR-mediated EPSCs by ifenprodil or APV. EPSPs averaged from 5 consecutive stimuli were taken before and after LTP induction by TBS (arrow) at the time points (1 and 2) indicated in the graphs. (D) Summary of modulation of the mean enhancement of EPSPs (at 45–55 min during recording) by ifenprodil or APV. Values represent mean ± SEM. The total number of cells examined at each condition is indicated in parentheses (*: p < 0.01; t-test).
Discussion
In summary, our systematic analysis of synaptic plasticity of newborn DGCs along their maturation in the adult brain demonstrates the existence of a critical period when adult-born neurons exhibit enhanced synaptic plasticity. Such critical period plasticity is associated with developmentally regulated synaptic expression of NR2B-subtypes in adult-born neurons, which, in turn, plays an instructive role in the enhanced plasticity. This adult form of critical period plasticity resembles features of the classic early postnatal critical period plasticity in juvenile animals, which is normally coincident with a high volume of information processing to establish fine tuned neuronal circuits (Hensch, 2004). Thus, adult-born neurons within the critical period may serve as major mediators for experience-driven plasticity, and therefore function as special units in the adult circuitry to contribute to specific brain functions throughout life. The transient nature of such critical period plasticity, on the other hand, may also allow maintenance of stability of the mature circuitry that is essential for proper functions of the adult brain (Rakic, 1985).
Our electrophysiological study identified a fairly sharp and narrow time window between 1– 1.5 months of the cell age when adult-born neurons exhibit enhanced synaptic plasticity with both increased LTP amplitude and decreased LTP induction threshold. Several studies have demonstrated that the retroviral approach provides an adequate time resolution for birth-dating of newborn cells and does not appear to affect the development of labeled newborn neurons and physiology of surrounding mature neurons despite its invasive nature (Ge et al., 2006; Zhao et al., 2006). Previous studies on synaptic plasticity of adult-born neurons focused mainly on immature neurons younger than 4 weeks of the cell age and without defined birth-dating (Schmidt-Hieber et al., 2004; Snyder et al., 2001; Wang et al., 2000). Interestingly, our results show that adult-born neurons at these immature stages, while indeed have a lower threshold for LTP induction (Figure 1D), exhibit smaller LTP amplitude (Figure S3). Consistent with our findings, a recent study using c-Fos and Arc immmunocytochemistry suggest that adult-born neurons with the cell age between 1 and 2 months, but not those younger than 1 month, are preferentially activated by specific behaviors (Kee et al., 2007). In addition, a high level of anatomical plasticity of adult-born DGCs was observed during the critical period we identified (Zhao et al., 2006). For example, the motility of dendritic spines of newborn DGCs is maximal between 1 and 2 months of the cell age and decreases thereafter. Our identification of the exact timing of such critical period for adult-born neurons will aid future efforts to understand the physiological significance of adult neurogenesis, given that previous behavior studies on the function of adult neurogenesis largely focused on immature neurons younger than one month old (Madsen et al., 2003; Snyder et al., 2005).
Our findings of developmentally regulated expression of NR2B subtypes may provide a temporal mechanism to limit the enhanced plasticity of adult-born neurons within the critical period. NMDAR subtypes are known to be differentially expressed during early postnatal neuronal development and have recently been proposed to differentially regulate the form and degree of synaptic plasticity (Barria and Malinow, 2005; Barth and Malenka, 2001; Kim et al., 2005; Liu et al., 2004; Tang et al., 1999; Zhao et al., 2005). In particular, over-expression of NR2B in the forebrain of transgenic mice leads to enhanced LTP (Tang et al., 1999), while switching synaptic NR2B-containing NMDARs with those containing NR2A dramatically decreases LTP (Barria and Malinow, 2005). Our study not only shows a temporal correlation between synaptic expression of NR2B subtypes and critical period plasticity, but also provides strong evidence for an instructive role of NR2B signaling in the enhanced synaptic plasticity that could not be substituted by other NMDAR subtypes.
While the exact function of adult neurogenesis in mammals remains elusive, adult neurogenesis and specific behaviors appear to be reciprocally affected by many experimental manipulations (Doetsch and Hen, 2005; Kempermann et al., 2004; Kitabatake et al., 2007). One emerging view is that adult-born neurons may make unique contributions to specific brain functions. For example, recent studies suggest that adult-born neurons may be preferentially recruited into circuits that mediate spatial information processing and memory formation (Kee et al., 2007; Ramirez-Amaya et al., 2006). One potential mechanism for such biased recruitment is selective survival of over-produced immature neurons at the cell age of around 2 weeks in response to experience (Kee et al., 2007; Tashiro et al., 2007; Tashiro et al., 2006). Because immature neurons at these early stages are yet to fully integrate into the circuitry, survival mechanism is unlikely to be fully responsible for the selective integration of adult-born neurons into specific circuits. In contrast, NR2B-dependent critical period plasticity we described here could provide an important cellular mechanism for the fine-tuning of synaptic incorporation of adult-born neurons in response to experience.
The magnitude of synaptic and structural modification induced by experience differentiates developmental plasticity from the adult form of plasticity after the closure of the early postnatal critical period in most brain regions (Hensch, 2004; Katz and Shatz, 1996). Adult hippocampus may retain such enhanced form of plasticity through the continuous generation of cohorts of new neurons. Therefore, adult neurogenesis may represent, not merely a replacement mechanism for lost neurons, but instead an ongoing developmental process that continuously rejuvenates the mature nervous system by offering expanded capacity of plasticity in response to experience throughout life.
Experimental Procedures
Retrovirus production and stereotaxic injection
Engineered self-inactivating murine retroviruses expressing GFP were used to label proliferating cells and their progeny in the dentate gyrus of adult mice and high titers of engineered retroviruses (1 × 109 unit/ml) were produced as previously described (Ge et al., 2006). Adult (7–8 weeks old) female C57BL/6 mice (Charles River) housed under standard conditions were stereotaxically injected at 4 sites in the dentate gyrus (0.5 µl per site at 0.25 µl/min) as previously described (Ge et al., 2006). The following coordinates were used: anterioposterior = −2 mm from bregma; lateral = ±1.6 mm; ventral = 2.5 mm; anterioposterior = −3 mm from bregma; lateral = ±2.6 mm; ventral = 3.2 mm. All animal procedures were in accordance with institutional guidelines.
Electrophysiology
Mice were sacrificed at different time points after retroviral injection and processed for slice preparation as previously described (Ge et al., 2006). Brains were quickly removed into ice-cold solution containing (in mM): 110 choline chloride, 2.5 KCl, 1.3 KH2PO4, 25.0 NaHCO3, 0.5 CaCl2, 7 MgCl2, 10 dextrose, 1.3 sodium ascorbate, 0.6 sodium pyruvate, 5.0 kynurenic acid. Slices (300 µm thick) were cut using a vibrotome (Leica VT1000S) and transferred to a chamber containing the external solution (in mM: 125.0 NaCl, 2.5 KCl, 1.3 KH2PO4, 25.0 NaHCO3, 2 CaCl2, 1.3 MgCl2, 1.3 sodium ascorbate, 0.6 sodium pyruvate, 10 dextrose, pH 7.4), bubbled with 95% O2/5% CO2. Slices from adult animals were kept at 35°C for 40 min, followed by incubation at room temperature for at least 60 min before recording. Electrophysiological recordings were obtained at 32°C – 34°C as previously described (Ge et al., 2006). In all the studies, 10 µM bicuculline was added to the external solution to block GABAergic synaptic transmission. GFP+ DGCs in the granule cell layer were visualized by DIC and fluorescence microscopy and identified by their green fluorescence, neuronal morphology and ability to fire action potentials. Mature DGCs (GFP−) located in the outer DGC layer in the same slice were recorded as controls (Esposito et al., 2005; Schmidt-Hieber et al., 2004). The whole-cell patch-clamp configuration was used in the voltage-clamp or current-clamp modes as indicated. Microelectrodes (4–6 MΩ) were pulled from borosilicate glass capillaries and filled with the following (in mM): 120.0 potassium gluconate, 15 KCl, 4 MgCl2, 0.1 EGTA, 10.0 HEPES, 4 ATP (magnesium salt), 0.3 GTP (sodium salt), 7 phosphocreatine (pH 7.4).
A bipolar electrode (adapted from WPI, Sarasota, FL) was used to stimulate (100 µs stimulus every 30 s) the medial perforant pathway as previously described (Ge et al., 2006). The stimulus intensity (~10–30 µA) was adjusted to evoke EPSPs in the range of 3–6 mV. LTP was induced with a theta burst stimulation (TBS) paradigm consisting of four repeated episodes (at 0.1 Hz) of ten stimuli at 100 Hz, repeated ten times at 5Hz and paired with a 100 pA postsynaptic current injection (See Figure S2 in the Supplemental Data) as previously described (Schmidt-Hieber et al., 2004). Data were collected using an Axon 200B amplifier and acquired via a Digidata 1322A (Axon Instruments) at 10 kHz. The series and input resistances were monitored and only those that changed less than 20% during experiments were used for data analysis. The series resistance ranged between 10–30 MΩ and was uncompensated. The EPSPs peak amplitudes were measured for at least 7 min before the onset of the induction paradigm and for at least 45 min after the induction. We used only one GFP+ neuron with functional glutamatergic synaptic inputs in each animal for the plasticity study. t-test was used for statistical analysis.
For characterization of NMDAR mediated EPSCs, ATP-magnesium and MgCl2 were substituted with the sodium salt and KCl in the internal and external solution, respectively. Additional drugs were added to the external solution as indicated with the following final concentrations: glycine (10 µM, Sigma), bicuculline (10 µM, Sigma), NBQX (100 µM, Sigma), APV (0.5–50 µM, Sigma), ifenprodil (0.03–3 µM, Tocris), Ro 25-6981 (0.5 µM, Tocris). The stimulation intensity was maintained for all experiments. Under our conditions ifenprodil and Ro25-6981 reached the maximum inhibition within 20 min with continuous low frequency stimulations (Figure 2A and data not shown).
Supplementary Material
Acknowledgements
We thank R. Huganir, D. Linden, J. Bischofberger and D. Jordan for comments and suggestions. This work was supported by the Whitehall Foundation, Sloan Scholar, and Klingenstein Fellowship Award in the Neuroscience to G-1. M, by the National Institute of Health (NS047344 and AG024984), McKnight Scholar Award and the Rett Syndrome Research Foundation to H.S. and by by a postdoctoral fellowship from the American Heart Association to S.G.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrous DN, Koehl M, Le Moal M. Adult neurogenesis: from precursors to network and physiology. Physiol Rev. 2005;85:523–569. doi: 10.1152/physrev.00055.2003. [DOI] [PubMed] [Google Scholar]
- Aimone JB, Wiles J, Gage FH. Potential role for adult neurogenesis in the encoding of time in new memories. Nat Neurosci. 2006;9:723–727. doi: 10.1038/nn1707. [DOI] [PubMed] [Google Scholar]
- Barria A, Malinow R. NMDA Receptor Subunit Composition Controls Synaptic Plasticity by Regulating Binding to CaMKII. Neuron. 2005;48:289–301. doi: 10.1016/j.neuron.2005.08.034. [DOI] [PubMed] [Google Scholar]
- Barth AL, Malenka RC. NMDAR EPSC kinetics do not regulate the critical period for LTP at thalamocortical synapses. Nat Neurosci. 2001;4:235–236. doi: 10.1038/85070. [DOI] [PubMed] [Google Scholar]
- Cameron HA, McEwen BS, Gould E. Regulation of adult neurogenesis by excitatory input and NMDA receptor activation in the dentate gyrus. J Neurosci. 1995;15:4687–4692. doi: 10.1523/JNEUROSCI.15-06-04687.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carleton A, Petreanu LT, Lansford R, Alvarez-Buylla A, Lledo PM. Becoming a new neuron in the adult olfactory bulb. Nat Neurosci. 2003;6:507–518. doi: 10.1038/nn1048. [DOI] [PubMed] [Google Scholar]
- Chklovskii DB, Mel BW, Svoboda K. Cortical rewiring and information storage. Nature. 2004;431:782–788. doi: 10.1038/nature03012. [DOI] [PubMed] [Google Scholar]
- Cull-Candy SG, Leszkiewicz DN. Role of distinct NMDA receptor subtypes at central synapses. Sci STKE. 2004;2004:re16. doi: 10.1126/stke.2552004re16. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Hen R. Young and excitable: the function of new neurons in the adult mammalian brain. Curr Opin Neurobiol. 2005;15:121–128. doi: 10.1016/j.conb.2005.01.018. [DOI] [PubMed] [Google Scholar]
- Esposito MS, Piatti VC, Laplagne DA, Morgenstern NA, Ferrari CC, Pitossi FJ, Schinder AF. Neuronal differentiation in the adult hippocampus recapitulates embryonic development. J Neurosci. 2005;25:10074–10086. doi: 10.1523/JNEUROSCI.3114-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer J, Zhao X, van Praag H, Wodtke K, Gage FH, Christie BR. Effects of voluntary exercise on synaptic plasticity and gene expression in the dentate gyrus of adult male Sprague-Dawley rats in vivo. Neuroscience. 2004;124:71–79. doi: 10.1016/j.neuroscience.2003.09.029. [DOI] [PubMed] [Google Scholar]
- Gage FH. Mammalian neural stem cells. Science. 2000;287:1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- Ge S, Goh EL, Sailor KA, Kitabatake Y, Ming GL, Song H. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature. 2006;439:589–593. doi: 10.1038/nature04404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensch TK. Critical period regulation. Annu Rev Neurosci. 2004;27:549–579. doi: 10.1146/annurev.neuro.27.070203.144327. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J Physiol. 1962;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- Kee N, Teixeira CM, Wang AH, Frankland PW. Preferential incorporation of adult-generated granule cells into spatial memory networks in the dentate gyrus. Nat Neurosci. 2007;10:355–362. doi: 10.1038/nn1847. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Gast D, Kronenberg G, Yamaguchi M, Gage FH. Early determination and long-term persistence of adult-generated new neurons in the hippocampus of mice. Development. 2003;130:391–399. doi: 10.1242/dev.00203. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Wiskott L, Gage FH. Functional significance of adult neurogenesis. Curr Opin Neurobiol. 2004;14:186–191. doi: 10.1016/j.conb.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Dunah AW, Wang YT, Sheng M. Differential roles of NR2A- and NR2B-containing NMDA receptors in Ras-ERK signaling and AMPA receptor trafficking. Neuron. 2005;46:745–760. doi: 10.1016/j.neuron.2005.04.031. [DOI] [PubMed] [Google Scholar]
- Kitabatake Y, Sailor KA, Ming GL, Song H. Adult neurogenesis and hippocampal memory function: new cells, more plasticity, new memories? Neurosurg Clin N Am. 2007;18:105–113. doi: 10.1016/j.nec.2006.10.008. x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laplagne DA, Esposito MS, Piatti VC, Morgenstern NA, Zhao C, van Praag H, Gage FH, Schinder AF. Functional Convergence of Neurons Generated in the Developing and Adult Hippocampus. PLoS Biol. 2006;4:e409. doi: 10.1371/journal.pbio.0040409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Wong TP, Pozza MF, Lingenhoehl K, Wang Y, Sheng M, Auberson YP, Wang YT. Role of NMDA receptor subtypes in governing the direction of hippocampal synaptic plasticity. Science. 2004;304:1021–1024. doi: 10.1126/science.1096615. [DOI] [PubMed] [Google Scholar]
- Lledo PM, Alonso M, Grubb MS. Adult neurogenesis and functional plasticity in neuronal circuits. Nat Rev Neurosci. 2006;7:179–193. doi: 10.1038/nrn1867. [DOI] [PubMed] [Google Scholar]
- Madsen TM, Kristjansen PE, Bolwig TG, Wortwein G. Arrested neuronal proliferation and impaired hippocampal function following fractionated brain irradiation in the adult rat. Neuroscience. 2003;119:635–642. doi: 10.1016/s0306-4522(03)00199-4. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Ming GL, Song H. Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci. 2005;28:223–250. doi: 10.1146/annurev.neuro.28.051804.101459. [DOI] [PubMed] [Google Scholar]
- Nacher J, Varea E, Miguel Blasco-Ibanez J, Gomez-Climent MA, Castillo-Gomez E, Crespo C, Martinez-Guijarro FJ, McEwen BS. N-methyl-d-aspartate receptor expression during adult neurogenesis in the rat dentate gyrus. Neuroscience. 2007;144:855–864. doi: 10.1016/j.neuroscience.2006.10.021. [DOI] [PubMed] [Google Scholar]
- Rakic P. Limits of neurogenesis in primates. Science. 1985;227:1054–1056. doi: 10.1126/science.3975601. [DOI] [PubMed] [Google Scholar]
- Ramirez-Amaya V, Marrone DF, Gage FH, Worley PF, Barnes CA. Integration of new neurons into functional neural networks. J Neurosci. 2006;26:12237–12241. doi: 10.1523/JNEUROSCI.2195-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Hieber C, Jonas P, Bischofberger J. Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature. 2004;429:184–187. doi: 10.1038/nature02553. [DOI] [PubMed] [Google Scholar]
- Seki T. Expression patterns of immature neuronal markers PSA-NCAM, CRMP-4 and NeuroD in the hippocampus of young adult and aged rodents. J Neurosci Res. 2002;70:327–334. doi: 10.1002/jnr.10387. [DOI] [PubMed] [Google Scholar]
- Skaggs WE, McNaughton BL, Wilson MA, Barnes CA. Theta phase precession in hippocampal neuronal populations and the compression of temporal sequences. Hippocampus. 1996;6:149–172. doi: 10.1002/(SICI)1098-1063(1996)6:2<149::AID-HIPO6>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Snyder JS, Hong NS, McDonald RJ, Wojtowicz JM. A role for adult neurogenesis in spatial long-term memory. Neuroscience. 2005;130:843–852. doi: 10.1016/j.neuroscience.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Snyder JS, Kee N, Wojtowicz JM. Effects of adult neurogenesis on synaptic plasticity in the rat dentate gyrus. J Neurophysiol. 2001;85:2423–2431. doi: 10.1152/jn.2001.85.6.2423. [DOI] [PubMed] [Google Scholar]
- Tang YP, Shimizu E, Dube GR, Rampon C, Kerchner GA, Zhuo M, Liu G, Tsien JZ. Genetic enhancement of learning and memory in mice. Nature. 1999;401:63–69. doi: 10.1038/43432. [DOI] [PubMed] [Google Scholar]
- Tashiro A, Makion H, Gage FH. Experience-specific functional modification of the dentate gyrus through adult neurogenesis: a critical period during an immature stage. J Neurosci. 2007;27:3252–3259. doi: 10.1523/JNEUROSCI.4941-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashiro A, Sandler VM, Toni N, Zhao C, Gage FH. NMDA-receptor-mediated, cell-specific integration of new neurons in adult dentate gyrus. Nature. 2006;442:929–933. doi: 10.1038/nature05028. [DOI] [PubMed] [Google Scholar]
- van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci U S A. 1999;96:13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Scott BW, Wojtowicz JM. Heterogenous properties of dentate granule neurons in the adult rat. J Neurobiol. 2000;42:248–257. [PubMed] [Google Scholar]
- Wiesel TN, Hubel DH. Single-Cell Responses In Striate Cortex Of Kittens Deprived Of Vision In One Eye. J Neurophysiol. 1963;26:1003–1017. doi: 10.1152/jn.1963.26.6.1003. [DOI] [PubMed] [Google Scholar]
- Zhao C, Teng EM, Summers RG, Jr, Ming GL, Gage FH. Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J Neurosci. 2006;26:3–11. doi: 10.1523/JNEUROSCI.3648-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao MG, Toyoda H, Lee YS, Wu LJ, Ko SW, Zhang XH, Jia Y, Shum F, Xu H, Li BM, et al. Roles of NMDA NR2B Subtype Receptor in Prefrontal Long-Term Potentiation and Contextual Fear Memory. Neuron. 2005;47:859–872. doi: 10.1016/j.neuron.2005.08.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


