Abstract
AMP-activated protein kinase (AMPK) is widely recognized as an important regulator of glucose transport in skeletal muscle. The p38 mitogen-activated protein kinase (MAPK) has been proposed to be a component of AMPK-mediated signaling. Here we used several different models of altered AMPK activity to determine whether p38 MAPK is a downstream intermediate of AMPK-mediated signaling in skeletal muscle. First, L6 myoblasts and myotubes were treated with AICAR, an AMPK stimulator. AMPK phosphorylation was significantly increased, but there was no change in p38 MAPK phosphorylation. Similarly, AICAR incubation of isolated rat extensor digitorum longus (EDL) muscles did not increase p38 phosphorylation. Next, we used transgenic mice expressing an inactive form of the AMPKα2 catalytic subunit in skeletal muscle (AMPKα2i TG mice). AMPKα2i TG mice did not exhibit any defect in basal or contraction-induced p38 MAPK phosphorylation. We also used transgenic mice expressing an activating mutation in the AMPKγ1 subunit (γ1R70Q TG mice). Despite activated AMPK, basal p38 MAPK phosphorylation was not different between wild type and γ1R70Q TG mice. In addition, muscle contraction-induced p38 MAPK phosphorylation was significantly blunted in the γ1R70Q TG mice. In conclusion, increasing AMPK activity by AICAR and AMPKγ1 mutation does not increase p38 MAPK phosphorylation in skeletal muscle. Furthermore, AMPKα2i TG mice lacking contraction-stimulated AMPK activity have normal p38 MAPK phosphorylation. These results suggest that p38 MAPK is not a downstream component of AMPK-mediated signaling in skeletal muscle.
INTRODUCTION
AMP-activated protein kinase (AMPK), a member of a metabolite-sensing protein kinase family, has multiple functions in various tissues (1-3). When intracellular ATP concentrations decrease, AMPK acts to switch off ATP-consuming pathways and switch on alternative pathways for ATP regeneration. Thus, AMPK is thought to act as a cellular fuel sensor (4). In skeletal muscle, regulation of glucose transport is one of the best known functions of AMPK (5). Activation of AMPK by pharmacological stimulation (6-8) or transient expression of an AMPK active mutant (9) increases glucose transport. Activation of AMPK stimulates glucose transport through translocation of glucose transporter (GLUT) 4 from the intracellular pool to surface membranes in skeletal muscle (10;11). AMPK may also play a role in enhancing muscle insulin sensitivity and responsiveness for glucose transport (12-15). Thus, understanding the function of AMPK has potential therapeutic significance for the treatment of defects in glucose metabolism such as diabetes and the metabolic syndrome.
Although there is considerable evidence demonstrating an important role for AMPK in the regulation of glucose transport in skeletal muscle, the signaling mechanisms downstream of AMPK leading to GLUT4 translocation are not fully understood. One hypothesis is that the p38 mitogen-activated protein kinase (MAPK) is a mediator of glucose transport, and downstream of AMPK signaling. Treatment of Clone 9 cells, a rat liver epithelial cell line, with the AMPK activator 5-aminoimidazole-4-carboxamide riboside (AICAR) stimulated glucose transport (16). AICAR-stimulated glucose transport was inhibited by a p38 MAPK inhibitor, SB203580, and also by overexpression of a dominant-negative p38 MAPK mutant in these cells (16). Furthermore, overexpression of a constitutively-active AMPK mutant resulted in activation of p38 MAPK (16). Since Clone 9 cells express GLUT1 but not GLUT4, the authors concluded that AMPK stimulates GLUT1-mediated glucose transport through activation of the p38 MAPK cascade. More recently, Lemieux et al. (11) reported that AICAR activates p38 MAPK concomitant with activation of AMPK and glucose transport in skeletal muscle, and that AICAR-stimulated glucose transport was abrogated by SB203580. These data suggest that p38 MAPK is a key molecule that mediates AMPK signaling to increase glucose transport in skeletal muscle.
Since SB203580, a commonly used tool to study p38 MAPK function has been found to have non-specific inhibitory effects on glucose transport (17) and can block AICAR uptake into cells (18), we have performed experiments aimed at investigating the putative relationship between AMPK and p38 MAPK signaling in skeletal muscle using methods that do not involve the use of this inhibitor. In using several different models of altered AMPK activity, our studies find no evidence for a regulatory role for AMPK in p38 MAPK cell signaling.
MATERIALS AND METHOD
Antibodies
Antibodies to p38 MAPK, phospho-p38 MAPK (Thr180/Tyr182), phospho-MKK3/6 (Ser189/207), phospho-ATF2 (Thr69/71) were from Cell Signaling Technology and phospho-AMPK (Thr172) and phosphor-acetyl CoA carboxylase (ACC, Ser79/218) were from Upstate Biotechnology. AMPK pan-α antibody was generated against a peptide N'-AEKQKHDGRVKIGHY-C'. AMPK α isoform- specific antibodies were made against the amino acid sequences 339-358 of rat α1 and 352-366 of rat α2 (19). Anti-mouse and anti-sheep IgG-horseradish peroxidase were from Upstate Biotechnology. The anti-rabbit IgG-HRP and ECL-Plus Western blotting detection kit were purchased from Amersham Pharmacia Biotechnology.
L6 myoblasts and myotubes
L6 cells were seeded into 12-well plates and maintained in growth medium containing αMEM supplemented with 10% fetal bovine serum in a humidified atmosphere containing 5% CO2-95% air at 37C (20). Myoblasts were grown in monolayers and allowed to reach confluence. Myoblasts were differentiated to myotubes by exposure to 2% fetal bovine serum for 8 days. L6 myoblasts and myotubes were serum-starved for 5 hr in αMEM before any treatment. Cells were washed twice in PBS and incubated in 2 mM of AICAR or 500 μM of 2,4-dinitrophenol (DNP) for 10 min. Cell culture dishes were then placed on ice and lysed for immunoblotting.
Transgenic Mice
Muscle specific transgenic mice carrying an inactive-form of the α2 catalytic subunit of AMPK (α2 D157A mutant; α2i TG mice) and muscle specific transgenic mice carrying an active mutant of the γ subunit of AMPK (γ1 R70Q mutant; γ1R70Q TG mice) were generated as reported by Fujii et al. (21) and Barre et al. (22), respectively. Wild type and heterozygous (+/−) transgenic mice were obtained from intercrosses between wild type mice and heterozygous (+/−) mice, and treatment groups were derived from littermates. All procedures were approved by the Institution Animal Care and Use Committee of the Joslin Diabetes Center.
In vitro muscle incubation with AICAR
Male Sprague-Dawley rats weighting ∼40 g (Taconic) were sacrificed, and the extensor digitorum longus (EDL) muscles were rapidly removed and treated as previously described (21). Both ends of each EDL muscle were tied with suture and mounted on an incubation apparatus. Muscles were preincubated in 6 ml of Krebs-Ringer bicarbonate (KRB) buffer containing 2 mM pyruvate for 20 min. The muscles were then incubated in KRB buffer in the absence or presence of 2 mM AICAR for 40 min.
In vivo AICAR treatment
Fed male Sprague-Dawley rats or ICR mice (Taconic) were given an intraperitioneal injection of either AICAR (1 mg/g body wt) or a comparable volume of saline and were allowed to remain in their cages. Animals were sacrificed via cervical dislocation 60 min after injections. Hindlimbs were removed, and gastrocnemius muscle was isolated, snap-frozen in liquid N2, and stored in liquid N2 until processing.
In situ muscle contraction
In situ muscle contraction was performed as described previously (23). Briefly, mice were anesthetized with an intraperitoneal injection of pentobarbital sodium (100 mg/kg). The sciatic nerves of both hindlimbs were exposed, and subminiature electrodes were attached to the nerves. Hindlimb muscles on one side were electrically stimulated to contract for 10 min with train rate 1/s, train duration 500 ms, rate 100 pulses/s, duration 0.1 ms, and 1-3 V, whereas the contra lateral side remained unstimulated and served as a sham-treated control.
Immunoblotting and AMPK activity assay
After the experimental protocols, L6 cells or skeletal muscles were homogenized using a Polytron (Brinkmann Instrument) in ice-cold lysis buffer containing 20mM Tris-HCl (pH 7.4), 1 % Triton X-100, 50 mM NaCl, 250 mM sucrose, 50 mM NaF, 5 mM sodium pyrophosphate, 2 mM DTT, 4 mg/L leupeptin, 50 mg/L trypsin inhibitor, 0.1 mM benzamidine and 0.5 mM PMSF. The homogenates were centrifuged at 14,000 g for 20 min at 4° C. Total protein concentrations were determined in the supernatant using the Bradford method (Bio-Rad). Immunoblotting was done using standard procedures as previously described (23). AMPK activity was measured as previously described (19). Briefly, muscle or cell lysates (100 μg) were immunoprecipitated with specific antibodies against the α1, α2, or both catalytic subunits and protein A beads. The kinase reaction was carried out in 40 mM HEPES, pH 7.0, 0.1 mM synthetic SAMS peptide, 0.2 mM AMP, 80 mM NaCl, 0.8 mM dithiothreitol, 5 mM MgCl2, and 0.2 mM ATP (2 μCi of [γ-32P]ATP ) for 20 min at 30C. Reaction products were spotted on Whatman P81 filter paper, the papers were extensively washed in 1% phosphoric acid, and radioactivity was assessed with a scintillation counter. AMPK kinase activity was expressed as the incorporation of [γ-32P]ATP (pmol) per immunoprecipitated protein (mg) per min incubation as previously described (24).
RESULTS
Effects of AICAR on AMPK and p38 MAPK phosphorylation in L6 cells and rat muscle
Since previous reports have suggested that p38 MAPK is downstream of AMPK, we reasoned that stimulation of AMPK should result in increases in p38 MAPK phosphorylation in skeletal muscle cells and tissue. We first used L6 myoblasts and myotubes and the AMPK activator AICAR. Cells were treated with AICAR, and AMPK phosphorylation on Thr172 and p38 MAPK phosphorylation on Thr180/Tyr182 were determined by immunoblotting. As shown in Figure 1A and B, AICAR significantly increased AMPK phosphorylation in myoblasts and myotubes. However, there was not a corresponding increase in p38 MAPK phosphorylation in both cell types (Fig. 1A and B). DNP, an uncoupler of mitochondrial electron transport and ATP synthase was used as a positive control (25;26) and was shown to phosphorylate p38 MAPK in the muscle cells (data not shown).
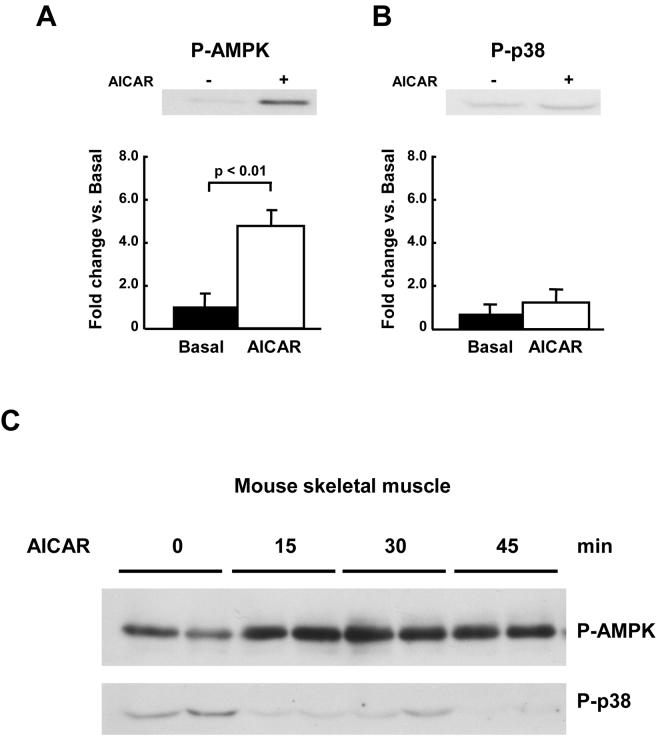
Figure 1. Activation of AMPK by AICAR is not associated with an increase in p38 MAPK phosphorylation in L6 cells.
L6 myoblasts (A) or myotubes (B) were treated with 2 mM AICAR for 10 min. Phosphorylation of AMPK and p38 MAPK were determined by immunoblotting using phospho-specific antibodies. Data are the means ± SEM of three independent experiments and each data point was assayed in duplicate.
We next determined the effects of AICAR in isolated rat EDL muscles in vitro. Consistent with the results in L6 cells, p38 MAPK phosphorylation did not change with AICAR treatment, despite a significant increase in AMPK phosphorylation (Fig. 2A and B). We also determined if in vivo AICAR treatment increases p38 MAPK phosphorylation concomitant with AMPK phosphorylation in vivo in mice. Although an intraperitoneal injection of AICAR (1 mg/g body wt) significantly increased AMPK phosphorylation in gastrocnemius muscles at 15, 30, and 45 min, p38 MAPK phosphorylation was not observed at any time point in the mice (Fig. 2C). Total protein expression AMPK and p38 MAPK was determined by immunoblotting and did not change with any treatment (data not shown). The dissociation of AMPK and p38 MAPK phosphorylation in response to AICAR stimulation in these three models suggest that p38 MAPK is not downstream of AMPK in skeletal muscle.
Figure 2. Activation of AMPK by AICAR is not associated with an increase in p38 MAPK phosphorylation in skeletal muscle.
Rat EDL muscles were isolated and preincubated in KRB buffer for 20 min. The muscles were then incubated with 2 mM AICAR for 40 min. Phosphorylation of AMPK (A) and p38 MAPK (B) were determined by immunoblotting using phospho-specific antibodies. Data are the means ± SEM. n = 4/group. (C); Mice were injected intraperitoneal with AICAR (1mg/g). Gastrocnemius muscles were harvested at each time point indicated. Phosphorylation of AMPK and p38 MAPK were determined by immunoblotting using phosphospecific antibodies. Representative images are shown.
Dissociation of AMPK activity and p38 MAPK phosphorylation in skeletal muscle expressing an activating AMPK mutant
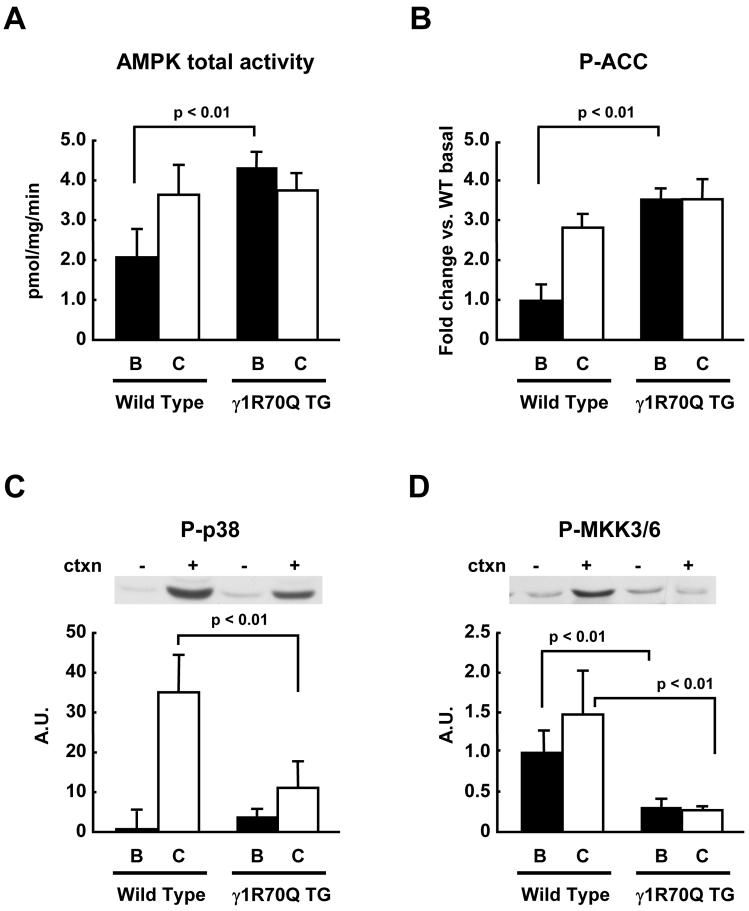
The R70Q mutation in one of the cystathionine β-synthase domains of the γ1 subunit of AMPK causes a marked increase in AMPK activity (27). More recently, we have shown that mice expressing the AMPKγ1 R70Q mutation in muscle have significantly higher AMPK activity in multiple skeletal muscles (22). We therefore tested the hypothesis that this model of increased AMPK activity would result in increased p38 MAPK phosphorylation. Here we show that γ1R70Q TG mice had an ∼ 2 fold increase in AMPK activity in the basal state (Fig. 3A), and that the phosphorylation of ACC, an established AMPK downstream substrate, was also increased by > 3 fold (Fig. 3B). Despite elevated AMPK activity and ACC phosphorylation, basal phosphorylation levels of p38 MAPK were not significantly different between wild type and γ1R70Q TG mice (Fig. 3C). As we have described previously, in the γ1R70Q mice, contraction did not further increase AMPK activity (22). Interestingly, contraction increased p38 MAPK phosphorylation in wild type mice, and this effect was significantly blunted in the γ1R70Q TG mice. Phosphorylation of MKK3/6, an upstream kinase of p38 MAPK, was also unaffected in the basal state and diminished in γ1R70Q TG mice in response to contraction (Fig. 3D). Thus, despite elevated AMPK activity, there was a significant decrease in contraction-stimulated p38 MAPK phosphorylation.
Figure 3. p38 MAPK phosphorylation in γ1R70Q TG mice.
Wild type and γ1R70Q TG mice were anesthetized, and the sciatic nerves were attached to electrodes. One leg was electrically stimulated for 10 min to induce muscle contractions, and the other leg served as sham control. Gastrocnemius muscles were dissected. In vitro kinase assay was done to determine total AMPK activity after immunoprecipitation with an antibody that recognizes both α1 and α2 AMPK isoforms (A). The same protein samples used for the kinase assays were used for immunoblotting of phospho-ACC (B), phospho-p38 MAPK (C), and phospho-MKK3/6 (D). Data are the means ± SEM. n = 6/group.
Ablation of AMPK α2 activity does not inhibit contraction-induced p38 MAPK phosphorylation in mouse skeletal muscle
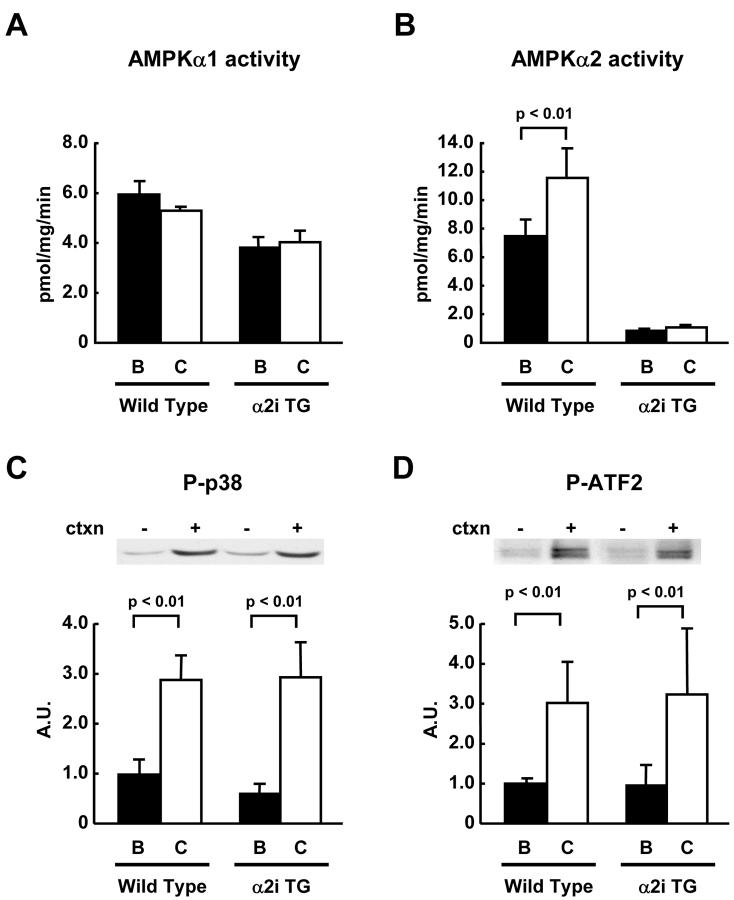
To directly determine if p38 MAPK is involved in downstream AMPK signaling in skeletal muscle, we used our AMPKα2i TG mice that have ablated AMPKα2 activity (21). These experiments were done in the basal state and in response muscle contraction, a physiological stimulus known to activate both AMPK and p38 MAPK. Muscle contraction in situ was produced by electrical stimulation of the sciatic nerve. Muscle contraction significantly increased AMPKα2 activity in the wild type mice, but both basal and contraction-stimulated muscles had near undetectable AMPK activity in AMPKα2i TG mice (Fig. 4A). AMPKα1 activity was not increased with in situ contraction even in wild type mice (Fig. 4B), consistent with work from our group and others using this type of contraction protocol (21;28;29). Despite ablated AMPKα2 activity, p38 MAPK phosphorylation was normally increased by contraction in gastrocnemius muscles of AMPKα2i TG mice compared to wild type mice (Fig. 4C and D). Furthermore, ATF2, a downstream substrate of p38 MAPK was also similarly increased in wild type and AMPKα2i TG mice. There was no change in protein levels of p38 MAPK in the transgenic mice (data not shown). These results strongly suggest that AMPKα2 is not an essential upstream kinase for the regulation of p38 MAPK activity in contracting muscles.
Figure 4. Muscle contraction-induced p38 MAPK phosphorylation in AMPK activity in α2i TG mice.
Wild type and α2i TG mice were anesthetized, and sciatic nerves were exposed. One leg was electrically stimulated for 10 min to induce muscle contractions, and the other leg served as sham control. Gastrocnemius muscles were dissected. In vitro kinase assay was done to determine AMPKα1 (A) and α2 (B) activities after immunoprecipitation with their specific antibodies as described in Material and Methods. The same protein samples used for the kinase assays were used for immunoblotting of phospho-p38 MAPK (C) and phospho-ATF2 (D). Data are the means ± SEM. n = 5-9/group.
DISCUSSION
p38 MAPK has been proposed to be a downstream mediator of AMPK signaling in several different cell types and tissues (16;26;30-35). Much of this work has been in the heart (30;31) and primary cultured cardiomyocytes (26;32;33). In primary cultures of rat cardiomyocytes, DNP, an uncoupler of oxidative phosphorylation, increased AMPK and p38 MAPK phosphorylation coincident with an increase in glucose transport (26). Adenine 9-beta-D arabinofuranoside, a non-specific AMPK inhibitor, and adenoviral infection with a dominant-negative AMPK abolished DNP-mediated p38 MAPK phosphorylation and partially decreased DNP-stimulated glucose uptake (26). Studies in rat neonatal cardiomyocytes have shown that multiple stimuli that activate AMPK (simulated ischemia, AICAR, metformin) also increase p38 MAPK phosphorylation. Although it was concluded that p38 MAPK is downstream of AMPK, it was not determined if AMPK inhibition blocked p38 MAPK phosphorylation (32). Perhaps the most convincing evidence for AMPK mediation of p38 MAPK signaling in the heart comes from a study where AICAR increased p38 phosphorylation, and hearts deficient in AMPKα2 activity had significantly reduced p38 MAPK activation in response to low-flow ischemia (30). In contrast to AMPK mediating p38 phosphorylation, it was recently reported that adenosine-stimulated phosphorylation of AMPK was attenuated by the p38 MAPK inhibitors SB202190 and SB203580 in the perfused rat heart (30). These results suggest that AMPK can be downstream of p38 MAPK in mediating the effects of adenosine in the heart (31), as opposed to other reports were p38 MAPK was proposed to be downstream of AMPK.
In C2C12 myotubes, AICAR has been shown to increase p38 MAPK phosphorylation, with maximal responses observed with 6-24 hours of AICAR treatment (36). In adult skeletal muscle, AICAR treatment in vivo and in incubated muscles resulted in increased p38 MAPK α and β activities. These findings are in direct contrast with our study, where we used several different models of AMPK activation and inhibition and show no relationship between AMPK and p38 MAPK. Using the AMPKα2i TG mouse, a model of ablated AMPKα2 activity (21), we showed no decrease in p38 MAPK phosphorylation in the basal state or in response to muscle contraction. Although we can not completely rule out the possibility that the remaining AMPKα1 activity is responsible for p38 MAPK phosphorylation, it is unlikely since the contraction model we used does not increase AMPKα1 activity [Fig. 4A and (21;28;29)]. This result demonstrates that AMPKα2 is not essential for p38 MAPK activation in contracting skeletal muscle. Furthermore, we found that activation of AMPK using AICAR or a transgenic model of chronic AMPK activation did not increase p38 MAPK phosphorylation. In fact, contraction-stimulated p38 MAPK phosphorylation is actually decreased in the γ1R70Q transgenic mice, despite high levels of AMPK activity. Taken together, our findings clearly demonstrate a disconnect between AMPK and p38 MAPK signaling.
Dissociation of signaling between AMPK and p38 MAPK has also been reported by other groups. In rat epididymal fat cells, isoproterenol, a β-adrenergic agonist, similarly increased p38 MAPK and AMPK activities (37). However, since activation of AMPK with AICAR did not change p38 MAPK activity in the cells, it was concluded that the activation of p38 MAPK is not a direct result of AMPK activation. Studies in Chinese hamster ovary (CHO) cells found that hyperosmotic stress induced by sorbitol increased both AMPK and p38 MAPK activities (38). Nevertheless, this group concluded AMPK was not involved in p38 MAPK activation, since treatment of CHO cells with AICAR did not increase the effect on p38 MAPK activation, although a clear increase in AMPK phosphorylation was observed. Expression of a dominant-negative AMPK did not affect p38 MAPK activation in sorbitol-treated cells, further supporting the conclusion that AMPK is not involved in the activation of p38 MAPK by hyperosmotic stress. Thus, AMPK regulation of p38 MAPK signaling does not exist in all tissue and cell types.
In summary, activation of AMPK by AICAR in L6 myoblasts and myotubes and in rat EDL muscles did not increase p38 MAPK phosphorylation. There was also a clear dissociation of AMPK activity and p38 MAPK-related signaling in muscles from transgenic mice expressing an inactive-form of AMPKα2 catalytic subunit or an activating mutation in the AMPKγ1 subunit. We conclude that AMPK is not an essential upstream regulator of p38 MAPK signaling in skeletal muscle.
ACKNOWLEDGMENTS
This work was supported by National Institute of Health Grants RO1 AR42238 and AR45670 (L.J.G.) and DK35712 (L.A.W).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCE
- 1.Hardie DG, Carling D, Carlson M. Annu.Rev.Biochem. 1998;67:821–855. doi: 10.1146/annurev.biochem.67.1.821. [DOI] [PubMed] [Google Scholar]
- 2.Kemp BE, Mitchelhill KI, Stapleton D, Michell BJ, Chen ZP, Witters LA. Trends Biochem.Sci. 1999;24:22–25. doi: 10.1016/s0968-0004(98)01340-1. [DOI] [PubMed] [Google Scholar]
- 3.Carling D. Trends Biochem.Sci. 2004;29:18–24. doi: 10.1016/j.tibs.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 4.Kahn BB, Alquier T, Carling D, Hardie DG. Cell Metab. 2005;1:15–25. doi: 10.1016/j.cmet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 5.Fujii N, Jessen N, Goodyear LJ. Am J Physiol Endocrinol.Metab. 2006;291:E867–E877. doi: 10.1152/ajpendo.00207.2006. [DOI] [PubMed] [Google Scholar]
- 6.Merrill GF, Kurth EJ, Hardie DG, Winder WW. Am.J Physiol. 1997;273:E1107–E1112. doi: 10.1152/ajpendo.1997.273.6.E1107. [DOI] [PubMed] [Google Scholar]
- 7.Hayashi T, Hirshman MF, Kurth EJ, Winder WW, Goodyear LJ. Diabetes. 1998;47:1369–1373. doi: 10.2337/diab.47.8.1369. [DOI] [PubMed] [Google Scholar]
- 8.Bergeron R, Russell RR, III, Young LH, Ren JM, Marcucci M, Lee A, Shulman GI. Am.J Physiol. 1999;276:E938–E944. doi: 10.1152/ajpendo.1999.276.5.E938. [DOI] [PubMed] [Google Scholar]
- 9.Fryer LG, Foufelle F, Barnes K, Baldwin SA, Woods A, Carling D. Biochem.J. 2002;363:167–174. doi: 10.1042/0264-6021:3630167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kurth-Kraczek EJ, Hirshman MF, Goodyear LJ, Winder WW. Diabetes. 1999;48:1667–1671. doi: 10.2337/diabetes.48.8.1667. [DOI] [PubMed] [Google Scholar]
- 11.Lemieux K, Konrad D, Klip A, Marette A. FASEB J. 2003;17:1658–1665. doi: 10.1096/fj.02-1125com. [DOI] [PubMed] [Google Scholar]
- 12.Iglesias MA, Ye JM, Frangioudakis G, Saha AK, Tomas E, Ruderman NB, Cooney GJ, Kraegen EW. Diabetes. 2002;51:2886–2894. doi: 10.2337/diabetes.51.10.2886. [DOI] [PubMed] [Google Scholar]
- 13.Fisher JS, Gao J, Han DH, Holloszy JO, Nolte LA. Am.J Physiol Endocrinol.Metab. 2002;282:E18–E23. doi: 10.1152/ajpendo.2002.282.1.E18. [DOI] [PubMed] [Google Scholar]
- 14.Iglesias MA, Furler SM, Cooney GJ, Kraegen EW, Ye JM. Diabetes. 2004;53:1649–1654. doi: 10.2337/diabetes.53.7.1649. [DOI] [PubMed] [Google Scholar]
- 15.Ye JM, Dzamko N, Hoy AJ, Iglesias MA, Kemp B, Kraegen E. Diabetes. 2006;55:2797–2804. doi: 10.2337/db05-1315. [DOI] [PubMed] [Google Scholar]
- 16.Xi X, Han J, Zhang JZ. J.Biol.Chem. 2001;276:41029–41034. doi: 10.1074/jbc.M102824200. [DOI] [PubMed] [Google Scholar]
- 17.Antonescu CN, Huang C, Niu W, Liu Z, Eyers PA, Heidenreich KA, Bilan PJ, Klip A. Endocrinology. 2005;146:3773–3781. doi: 10.1210/en.2005-0404. [DOI] [PubMed] [Google Scholar]
- 18.Fryer LG, Parbu-Patel A, Carling D. FEBS.Lett. 2002;531:189–192. doi: 10.1016/s0014-5793(02)03501-9. [DOI] [PubMed] [Google Scholar]
- 19.Fujii N, Hayashi T, Hirshman MF, Smith JT, Habinowski SA, Kaijser L, Mu J, Ljungqvist O, Birnbaum MJ, Witters LA, Thorell A, Goodyear LJ. Biochem.Biophys.Res.Commun. 2000;273:1150–1155. doi: 10.1006/bbrc.2000.3073. [DOI] [PubMed] [Google Scholar]
- 20.Ho RC, Alcazar O, Fujii N, Hirshman MF, Goodyear LJ. Am.J Physiol Regul.Integr.Comp Physiol. 2004;286:R342–R349. doi: 10.1152/ajpregu.00563.2003. [DOI] [PubMed] [Google Scholar]
- 21.Fujii N, Hirshman MF, Kane EM, Ho RC, Peter LE, Seifert MM, Goodyear LJ. J Biol.Chem. 2005;280:39033–39041. doi: 10.1074/jbc.M504208200. [DOI] [PubMed] [Google Scholar]
- 22.Barre L, Richardson C, Hirshman MF, Brozinick J, Fiering S, Kemp BE, Goodyear LJ, Witters LA. Am J Physiol Endocrinol.Metab. 2007;292:E802–E811. doi: 10.1152/ajpendo.00369.2006. [DOI] [PubMed] [Google Scholar]
- 23.Fujii N, Boppart MD, Dufresne SD, Crowley PF, Jozsi AC, Sakamoto K, Yu H, Aschenbach WG, Kim S, Miyazaki H, Rui L, White MF, Hirshman MF, Goodyear LJ. Am J Physiol Cell Physiol. 2004;287:C200–C208. doi: 10.1152/ajpcell.00415.2003. [DOI] [PubMed] [Google Scholar]
- 24.Winder WW, Hardie DG. Am.J.Physiol. 1996;270:E299–304. doi: 10.1152/ajpendo.1996.270.2.E299. [DOI] [PubMed] [Google Scholar]
- 25.Taha C, Tsakiridis T, McCall A, Klip A. Am J Physiol. 1997;273:E68–E76. doi: 10.1152/ajpendo.1997.273.1.E68. [DOI] [PubMed] [Google Scholar]
- 26.Pelletier A, Joly E, Prentki M, Coderre L. Endocrinology. 2005;146:2285–2294. doi: 10.1210/en.2004-1565. [DOI] [PubMed] [Google Scholar]
- 27.Hamilton SR, Stapleton D, O'Donnell JB, Kung JT, Dalal SR, Kemp BE, Witters LA. FEBS Lett. 2001;500:163–168. doi: 10.1016/s0014-5793(01)02602-3. [DOI] [PubMed] [Google Scholar]
- 28.Vavvas D, Apazidis A, Saha AK, Gamble J, Patel A, Kemp BE, Witters LA, Ruderman NB. J.Biol.Chem. 1997;272:13255–13261. doi: 10.1074/jbc.272.20.13255. [DOI] [PubMed] [Google Scholar]
- 29.Musi N, Hayashi T, Fujii N, Hirshman MF, Witters LA, Goodyear LJ. Am.J Physiol. 2001;280:E677–E684. doi: 10.1152/ajpendo.2001.280.5.E677. [DOI] [PubMed] [Google Scholar]
- 30.Li J, Miller EJ, Ninomiya-Tsuji J, Russell RR, III, Young LH. Circ Res. 2005;97:872–879. doi: 10.1161/01.RES.0000187458.77026.10. [DOI] [PubMed] [Google Scholar]
- 31.Jaswal JS, Gandhi M, Finegan BA, Dyck JR, Clanachan AS. Am J Physiol Heart Circ Physiol. 2007;292:H1978–H1985. doi: 10.1152/ajpheart.01121.2006. [DOI] [PubMed] [Google Scholar]
- 32.Du JH, Xu N, Song Y, Xu M, Lu ZZ, Han C, Zhang YY. Biochem.Biophys.Res Commun. 2005;337:1139–1144. doi: 10.1016/j.bbrc.2005.09.174. [DOI] [PubMed] [Google Scholar]
- 33.Capano M, Crompton M. Biochem.J. 2006;395:57–64. doi: 10.1042/BJ20051654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamaguchi S, Katahira H, Ozawa S, Nakamichi Y, Tanaka T, Shimoyama T, Takahashi K, Yoshimoto K, Ohara-Imaizumi M, Nagamatsu S, Ishida H. Am J Physiol Endocrinol.Metab. 2005 doi: 10.1152/ajpendo.00456.2004. [DOI] [PubMed] [Google Scholar]
- 35.Walker J, Jijon HB, Diaz H, Salehi P, Churchill T, Madsen KL. Biochem.J. 2005;385:485–491. doi: 10.1042/BJ20040694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ouchi N, Shibata R, Walsh K. Circ Res. 2005;96:838–846. doi: 10.1161/01.RES.0000163633.10240.3b. [DOI] [PubMed] [Google Scholar]
- 37.Moule SK, Denton RM. FEBS Lett. 1998;439:287–290. doi: 10.1016/s0014-5793(98)01392-1. %20. [DOI] [PubMed] [Google Scholar]
- 38.Mao X, Bravo IG, Cheng H, Alonso A. Exp.Cell Res. 2004;292:304–311. doi: 10.1016/j.yexcr.2003.09.012. [DOI] [PubMed] [Google Scholar]