Abstract
Purpose
To characterize the clinical features and therapeutic outcome of Candida keratitis.
Design
Retrospective, observational case series.
Methods
We reviewed 26 patients treated for Candida keratitis, including 2 with recurrent keratitis and one with bilateral infection.
Results
Of 29 keratitis episodes due to C. albicans (20) or C. parapsilosis (9), 16 (55%) complicated chronic ocular surface disease, and 9 (31%) followed previous keratoplasty. Only 2 were clinically suspected to have keratomycosis at initial presentation, and 21 (72%) used antibacterial therapy before corneal scrapings. Reconstructive keratoplasty occurred more often in previously grafted eyes (P = .03). Visual outcome was 20/60 or better in 6 (100%) medically treated eyes with good presenting visual acuity but in only 5 (24%) with worse initial vision (P = .002).
Conclusions
Candida keratitis is an opportunistic infection of a compromised cornea that is often initially misdiagnosed, and despite antifungal therapy, occasionally requires corneal grafting.
Candida keratitis was first characterized in the mid-20th century,1 and clinical reports extended knowledge of its risk factors and management.2 Because filamentous fungal keratitis is more prevalent in the tropics, Candida accounts for proportionately more fungal corneal isolates at temperate latitudes.3 However, Candida keratitis is a worldwide problem, and we estimate its annual incidence as approximately one person per million.
After approval from our institutional review board, we reviewed the records of 54 corneal isolates of C. albicans (39) or related species (15) submitted to our ocular microbiology laboratory from 1975 through 2005 and identified 29 episodes affecting 15 females and 11 males averaging 50 years of age treated at our institution (Table). Nine had a hypopyon, and 2 a retrocorneal plaque. Four presented as infectious crystalline keratopathy. Initial diagnoses were bacterial keratitis (22), fungal keratitis (2), herpes simplex keratitis (2), allograft rejection (2), and toxicity (1). One patient had recurrent C. albicans keratitis 3 months after the first episode and contralateral infection 6 months later, and another had recurrent C. parapsilosis keratitis 4 months apart.
TABLE.
Clinical and Microbiological Characteristics of 29 Candida Keratitis Episodes
| Characteristics | No |
|---|---|
| Predisposing condition | |
| Ocular surface disorder, chronic keratopathy, or toxicity | 17 (59%) |
| Corneal graft | 9 (31%)* |
| Recent trauma | 2 (7%) |
| Contact lens | 5 (17%)** |
| Recent treatment | |
| Corticosteroid and antibacterial agent | 18 (62%) |
| Corticosteroid only | 2 (7%) |
| Antibacterial agent only | 3 (10%) |
| Initial assessment | |
| Bacterial keratitis | 22 (76%) |
| Fungal keratitis | 2 (7%) |
| Herpetic keratitis | 1 (3%) |
| Noninfectious keratopathy | 4 (14%) |
| Corneal isolate | |
| C. albicans | 20 (69%) |
| C. parapsilosis | 9 (31%) |
Four grafted eyes also had chronic ocular surface disease.
One had radial keratotomy, and another had a recent corneal foreign body. Three additional eyes were wearing a contact lens: two with ocular surface disease and one with a corneal graft.
Ocular surface disorders preceded infection in 17 (59%) eyes (Figure 1), including medicamentosa (2), radiation keratopathy (2), pemphigoid (1), dysthyroid exposure keratopathy (1), Sjögren syndrome (1), and Salzmann nodular degeneration (1). One occurred during herpetic stromal keratitis. Nine occurred 2 to 24 months after keratoplasty, and another 2 months after radial keratotomy. Two followed a fingernail scratch. A contact lens preceded 8 episodes (1 rigid gas-permeable, 3 cosmetic soft, and 4 therapeutic), including 3 without other predisposing conditions. Three patients had human immunodeficiency virus infection, and 3 were diabetic.
FIGURE 1.
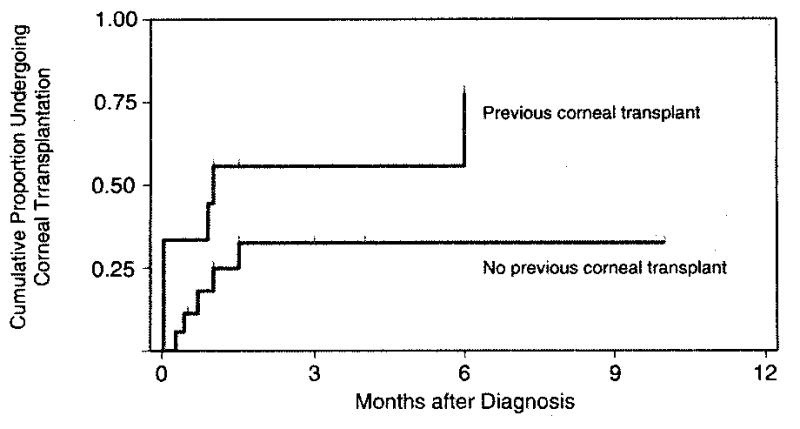
Kaplan-Meier curves for occurrence of reconstructive corneal transplantation following Candida keratitis, according to prior corneal surgery (P = .03, Wilcoxon-Breslow-Gehan test). Tick marks show when eyes completed follow up.
Fungal elements (7 showing yeasts, 6 yeasts and pseudohyphae, and 2 pseudohyphae only) were seen on 15 (52%) smears. Growth occurred on 77% of blood agar plates and 50% of Sabouraud agar, at an average of 2 days incubation. Treatment started an average of 12 days (range, 1 day to 3 months) after onset. Topical amphotericin B was used for 17 eyes, natamycin for 4, and miconazole for 2, while 3 had immediate keratoplasty. Oral ketoconazole or itraconazole was added in 10 episodes. Nine eyes underwent penetrating keratoplasty; infected grafts had earlier surgery (Figure 2). One eye was eviscerated, and another with perforation complicated by Streptococcus agalactiae endophthalmitis was enucleated. Visual outcome was at least 20/60 among 6 medically treated patients who had presenting visual acuity of 20/60 or better, and 5 of 21 with worse initial vision, of whom one was grafted (Supplemental Material at AJO.com).
FIGURE 2.
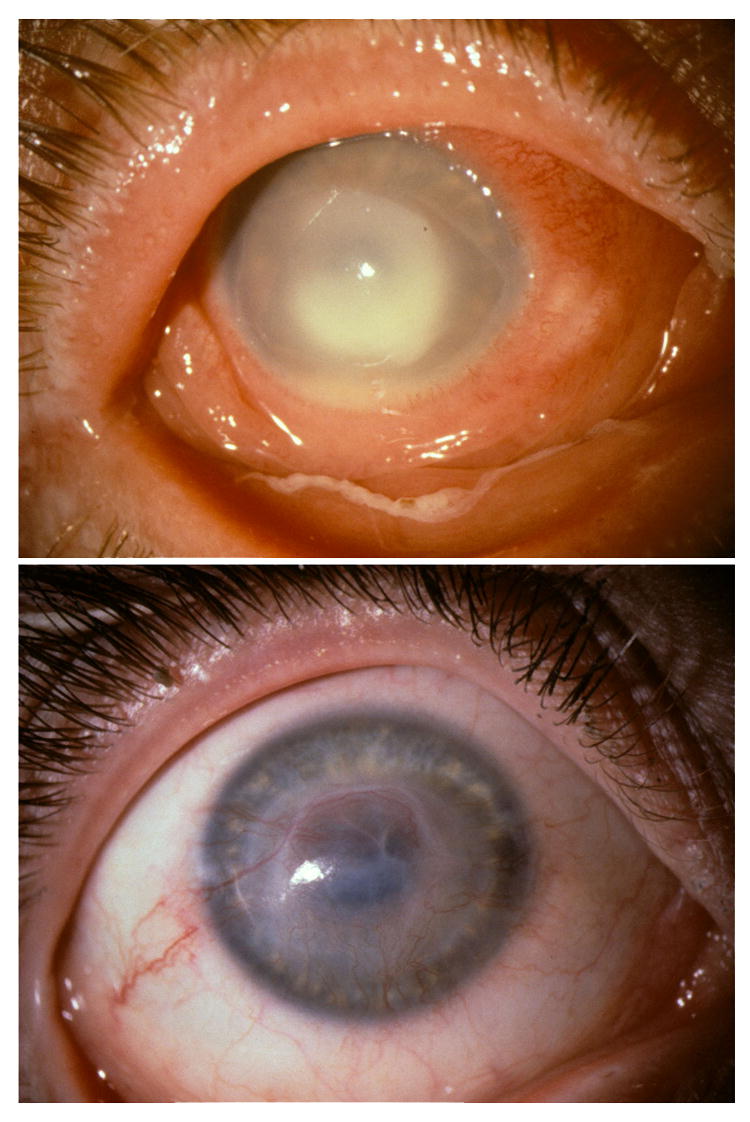
C. albicans keratitis during topical proparacaine overuse. TOP. Suppurative infiltrate with hypopyon at presentation. BOTTOM. Residual vascularized corneal opacity after 12 weeks of antifungal treatment
Candida species are opportunists that occasionally complicate chronic keratopathy and corneal grafts.4, 5 Immunosuppression and diabetes may predispose to candidiasis.5, 6 Our mainstay therapy is topical amphotericin B, with an oral azole for severe infection. New antifungal agents offer promising alternatives: voriconazole has good bioavailability and activity, and echinocandins such as caspofungin may be effective. Progressive keratomycosis may lead to keratoplasty in up to one third,5, 7 and an infected corneal transplant portends regrafting. Improved interventions are needed since visual outcome is often limited with advanced disease.
Supplementary Material
Visual acuity of eyes with Candida keratitis upon presentation compared to visual acuity at follow up. Visual outcome of 20/60 or better occurred in all 6 eyes with initial visual acuity of at least 20/60, but in only 5 (24%) of eyes with worse initial visual acuity (P = .002). Reasons for poor visual outcome included corneal opacification or graft failure, enucleation or evisceration (2), and retinal detachment (1).
Acknowledgments
This study was supported by research grant EY013782 and core grant EY02520 from the National Eye Institute, Bethesda, Maryland; an unrestricted departmental grant from Research to Prevent Blindness, Inc. New York, New York; and the Sid W. Richardson Foundation, Fort Worth, Texas. The authors indicate no financial conflict of interest. Contributions of authors: design of the study (R.L.S., K.R.W., D.B.J.); conduct of the study and collection, management, analysis, and interpretation of data (R.L.S.); preparation of the manuscript (R.L.S., K.R.W.); and review and approval of the manuscript (K.R.W., D.B.J.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sykes E. Fungal infections of the cornea. Tex State J Med. 1946;42:330–332. [PubMed] [Google Scholar]
- 2.Behrens-Baumann W. Mycosis of the Eye and its Adnexa. Basel: S Karger; 1999. p. 80. [Google Scholar]
- 3.Leck AK, Thomas PA, Hagan M, et al. Aetiology of suppurative corneal ulcers in Ghana and south India, and epidemiology of fungal keratitis. Br J Ophthalmol. 2002;86:1211–1215. doi: 10.1136/bjo.86.11.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kelly L, Pavan-Langston D, Baker A. Keratomycosis in a New England referral center: spectrum of pathogenic organisms and predisposing factors. In: Bialasiewicz AA, Schaal KP, editors. Infectious Diseases of the Eye. Buren: Æolus Press; 1994. pp. 184–190. [Google Scholar]
- 5.Tanure MAG, Cohen EJ, Sudesh S, Rapuano CJ, Laibson PR. Spectrum of fungal keratitis at Wills Eye Hospital, Philadelphia, Pennsylvania. Cornea. 2000;19:307–312. doi: 10.1097/00003226-200005000-00010. [DOI] [PubMed] [Google Scholar]
- 6.Hemady RK. Microbial keratitis in patients infected with the human immunodeficiency virus. Ophthalmology. 1995;102:1026–1030. doi: 10.1016/s0161-6420(95)30917-7. [DOI] [PubMed] [Google Scholar]
- 7.Xie L, Dong X, Shi W. Treatment of fungal keratitis by penetrating keratoplasty. Br J Ophthalmol. 2001;85:1070–1074. doi: 10.1136/bjo.85.9.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Visual acuity of eyes with Candida keratitis upon presentation compared to visual acuity at follow up. Visual outcome of 20/60 or better occurred in all 6 eyes with initial visual acuity of at least 20/60, but in only 5 (24%) of eyes with worse initial visual acuity (P = .002). Reasons for poor visual outcome included corneal opacification or graft failure, enucleation or evisceration (2), and retinal detachment (1).


