Abstract
Objective
To identify clinical indicators for success of misoprostol treatment after early pregnancy failure.
Methods
A total of 473 women with early pregnancy failure received 800 μg of vaginal misoprostol on treatment day 1. At the follow-up visit on day 3, a second dose was given if expulsion was incomplete. On day 8, vacuum aspiration was offered if expulsion had not occurred. Ultrasonography was used as gold standard for success. A Classification and Regression Tree analysis was undertaken to derive two decision trees for the success of misoprostol treatment on study days 3 and 8.
Results
Heavy bleeding after the first dose and an open cervical os were identified as clinical indicators of treatment success on day 3. Treatment success occurred in 84% of women with either or both indicators. Reporting passage of tissue after a second misoprostol dose and old blood in the vagina were potential indicators of treatment success or failure on day 8. A woman with either of these indicators has a 65% chance of treatment success after the second dose. Conversely, a woman with neither indicator on day 8 has a 94% chance of treatment failure.
Conclusion
Standard clinical findings may be useful as indicators for success or failure of medical management of early pregnancy failure in settings with limited or no access to ultrasonography. More research to identify even better indicators is warranted.
Keywords: bleeding, cervical os, early pregnancy failure, indicator, misoprostol, treatment success
Introduction
One in four women will experience an early pregnancy failure during her reproductive life [1]. The current standard of care for early pregnancy failure is surgical management through vacuum aspiration [2]. Recently, medical management of early pregnancy failure with misoprostol, a synthetic prostaglandin E1 analogue, has been shown to be effective, safe, and convenient [3]. It has the same or an even higher acceptability rate as surgical treatment for early pregnancy loss [4,5].
In most previous research studies, transvaginal ultrasonography was used to verify the expulsion of the products of conception after medical management with misoprostol. During follow-up visits if ultrasonography showed a substantial endometrial lining, or if a gestational sac could still be seen, subjects received a second dose of misoprostol or vacuum aspiration [3,6]. In this instance ultrasonography enabled clinicians to validate the completion of the treatment in order to accurately diagnose and treat subjects. However, access to this type of technology in resource poor settings could limit the use of misoprostol treatment. The purpose of this study is to identify clinical signs and symptoms that correlate with ultrasound evidence of completion of expulsion. Quantitative assessment is used to ascertain whether clinical indicators can be used to guide further treatment when ultrasound is not readily accessible.
Materials and Methods
Data from a multi-center randomized clinical trial on the management of early pregnancy failure was used. Detailed description of the trial is provided elsewhere [3]. Briefly, 652 women who had early pregnancy failure in the first trimester (embryo/fetal demise, anembryonic gestation, incomplete or inevitable spontaneous abortion) were recruited from four university hospitals. Participants were randomly assigned in a 3:1 ratio to receive medical treatment or vacuum aspiration. Of the subjects, 94% had anembryonic gestation or embryonic/fetal demise, and 6% had incomplete or inevitable spontaneous abortion.
The current analysis focused on the 487 subjects who were assigned to receive misoprostol treatment only. On the first day of the study these women had four 200 μg tablets of misoprostol (Cytotec; Pfizer, New York, USA) inserted into the posterior fornix through a speculum. On day 3 the women returned for a follow-up visit with vaginal ultrasonography. If this examination indicated expulsion of the gestational sac, the subjects were asked to come back on day 15. If the examination indicated persistence of the gestational sac or an endometrial lining greater than 30 mm, the women received a second dose of 800 μg of misoprostol vaginally. On day 8 vaginal ultrasonography was repeated and vacuum aspiration offered if the gestational sac was visualized. All subjects returned for a follow-up visit on day 15. The trial was approved by the Institutional Review Boards of participating institutions.
Baseline demographic characteristics of each woman (e.g. age, race) were collected at enrollment. The women were asked to keep diaries of symptoms such as bleeding, fever, and temperature for two weeks. The women were given complete physical examinations at follow-up visits on treatment days 3 and 8. For the current analysis, 28 variables were included as potential indicators. In addition to those listed in Table 1, baseline characteristics such as gestational age, parity, type of pregnancy failure, Rh type, vaginal bleeding within 24 hours prior to treatment, and side effects related to misoprostol treatment (e.g. vomiting, diarrhea, chills, fever, and headache) were also examined.
Table 1.
Univariate analysis of clinical indicators for success of misoprostol treatment on day 3
| Variables | n | Success (%) | P value |
|---|---|---|---|
| Variables from Symptom Diary: After Dose 1, day 1 to day 3 | |||
| Heavy Bleeding Ever | |||
| Yes | 287 | 83 | |
| No | 183 | 58 | <0.0001 |
| Passed Tissue Ever | |||
| Yes | 382 | 77 | |
| No | 88 | 58 | < 0.001 |
| Tired Ever | |||
| Yes | 340 | 74 | |
| No | 130 | 73 | 0.921 |
| Light Headed Ever | |||
| Yes | 217 | 78 | |
| No | 253 | 69 | 0.025 |
| Faint Ever | |||
| Yes | 29 | 83 | |
| No | 441 | 73 | 0.239 |
| Called Doctors Ever | |||
| Yes | 87 | 75 | |
| No | 383 | 73 | 0.760 |
| Visit Doctors Ever | |||
| Yes | 22 | 73 | |
| No | 448 | 73 | 0.941 |
| Used Ibuprofen | |||
| Yes | 422 | 74 | |
| No | 48 | 71 | 0.671 |
| Used Codeine Ever | |||
| Yes | 291 | 78 | |
| No | 179 | 66 | 0.008 |
| Physical Findings at day 3 Visit | |||
| Uterine Mass | |||
| Yes | 12 | 92 | |
| No | 451 | 74 | 0.199a |
| Uterine Mobility | |||
| Yes | 456 | 74 | |
| No | 8 | 38 | 0.033a |
| Uterine Tenderness | |||
| None | 414 | 73 | |
| Mild/Moderate | 53 | 77 | 0.516 |
| Pool of Blood Present in the vagina | |||
| Yes | 164 | 87 | |
| No | 303 | 66 | <.0001 |
| Old Blood Only in the vagina | |||
| Yes | 186 | 73 | |
| No | 280 | 74 | 0.914 |
| Cervical os | |||
| Open | 105 | 89 | |
| Closed | 362 | 69 | <0.001 |
| Cervical Active Bleeding | |||
| None | 233 | 66 | |
| Mild or Heavy | 167 | 80 | 0.002 |
| Tissue at the cervical os | |||
| Yes | 77 | 91 | |
| No | 391 | 70 | < 0.001 |
Reported Fisher’s Exact two sided P value because cell counts were less than 5 therefore Chi square test may not be valid.
Statistical analysis
Univariate analyses were performed using SAS 9.1 (Cary, NC, USA) to identify any significant relationships between indicator variables and misoprostol treatment success on days 3 and 8. Chi square test and Fisher’s Exact Test were performed.
To obtain an optimal clinical decision rule Classification and Regression Tree (CART) analysis was used [7]. CART analysis is a nonparametric statistical method that uses an algorithm to classify observations based on the value of predictor variables. The program first selects a single predictor variable that best classifies the women in the study population into misoprostol treatment success or failure subgroups. Subgroups are then split based on the next best predictors until a decision tree has been derived with several levels [7,8]. Two separate CART analyses were performed to ascertain the best indicators of misoprostol treatment success on days 3 and 8.
The optimal clinical decision depends on the ratio of the “cost” of under-treating a woman who is misclassified as a success versus over-treating a woman misclassified as a failure. Here the “cost” is a general term for both monetary and non-monetary cost. On day 3 if a woman is classified as a success she would not undergo an ultrasound or a physical examination on day 8. The error of under-treating on day 3 would mean that the expulsion of a non-viable pregnancy is not completed. The consequences of under-treatment would include continued bleeding, pain, and potential emergent care for an incomplete abortion. Consequences of over-treating on day 3 would include an unnecessary dose of misoprostol and its consequences such as nausea, pain, or other side effects. In this situation, under-treatment was considered the greater error. Thus, the ratio of the cost of under-treating versus over-treating a woman was set between 2:1 and 4:1.
Conversely, the weight of error was considered differently for day 8. On day 8, if the expulsion had not occurred, a dilation and curettage (D&C) would be recommended. Therefore, over-treatment would be referring a patient to surgical treatment. Because this recommendation would involve surgical management on all subjects, it was considered a greater error than that of under-treatment on day 8. The consequences of under-treatment on day 8 would potentially mean that the miscarriage was not fully treated, which could result in prolonged bleeding and pain, and potential need for emergency services. Therefore, the ratio of the cost of under-treating versus over-treating a woman was set between 1:2 and 1:4.
CART produced a regression tree for each cost ratio [7]. Final trees were selected after considering their interpretability and application in a clinical setting. To assess the sensitivity, specificity, and positive and negative predictive values, we used ten-fold cross validation. This method, described by Breiman et al. [7] partitions the data into 10 subsets, and uses all but one subset at a time to derive classifiers that are each assessed by the one-tenth of the data not used in its construction. The estimates resulting from the subsets were averaged, giving a single cross-validated estimate for each of the various measures.
Results
Of the 487 women who received misoprostol treatment, women who were lost to follow-up (n=2) or had an unknown outcome for day 3 (n=12) were excluded. Success of treatment was analyzed in the remaining 473 women. Table 1 shows that women had a significantly higher rate of success on day 3 if they reported to have had heavy bleeding, passed tissue, used codeine, or had one of the following physical signs: a pool of blood in the vagina, open cervical os, active bleeding, or tissue at cervical os.
The regression trees that resulted with the cost ratio of 2:1 and 3:1 were the same for day 3. This tree was chosen as the final predictor model (Fig. 1). It included two indicators of success of misoprostol treatment: heavy bleeding after the treatment, and cervical os open. The sensitivity, specificity, and positive and negative predictive values of this diagnostic algorithm are estimated by cross validation as 78%, 60%, 84% and 51%, respectively, i.e. 84% of women who are considered a treatment success according to clinical indicators after first dose can be confirmed by ultrasonography.
Figure 1.
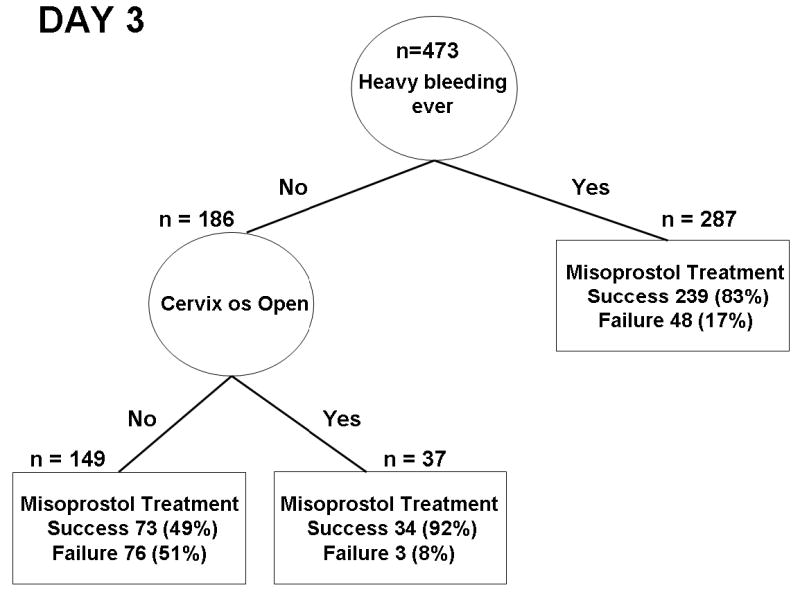
Decision tree obtained from Classification and Regression Tree analysis to predict success of misoprostol treatment on day 3.
To identify clinical indicators of success among 107 women who received a second dose of misoprostol, one subject who had an unknown outcome for day 8 was excluded. Table 2 indicates that women had a significantly higher rate of success if they reported to have had heavy bleeding, passed tissue, used analgesic, or had one of the following physical findings at the day 8 visit: a pool of blood in the vagina, old blood only in the vagina, or cervical active bleeding.
Table 2.
Univariate analysis of clinical indicators for success of misoprostol treatment on day 8
| Variables | n | Success (%) | P value |
|---|---|---|---|
| Variables from Symptom Diary: After Dose 2, From day4 to day 8 | |||
| Heavy Bleeding Ever | |||
| Yes | 44 | 39 | |
| No | 60 | 73 | < 0.001 |
| Passed Tissue Ever | |||
| Yes | 27 | 26 | |
| No | 77 | 70 | < 0.0001 |
| Tired Ever | |||
| Yes | 43 | 51 | |
| No | 61 | 64 | 0.193 |
| Light Headed Ever | |||
| Yes | 64 | 53 | |
| No | 40 | 68 | 0.148 |
| Faint Ever | |||
| Yes | 94 | 56 | |
| No | 10 | 80 | 0.189a |
| Called Doctors Ever | |||
| Yes | 88 | 56 | |
| No | 16 | 75 | 0.177a |
| Visit Doctors Ever | |||
| Yes | 97 | 58 | |
| No | 7 | 71 | 0.694a |
| Used Ibuprofen | |||
| Yes | 21 | 33 | |
| No | 83 | 65 | 0.008 |
| Used Codeine Ever | |||
| Yes | 51 | 49 | |
| No | 53 | 68 | 0.050 |
| Physical Findings After Dose 2, at day 8 | |||
| Uterine Tenderness | |||
| None | 96 | 59 | |
| Mild/Moderate | 8 | 63 | 1.000a |
| Pool of Blood in the vagina | |||
| Yes | 22 | 81 | |
| No | 83 | 53 | 0.016a |
| Old Blood Only in the vagina | |||
| Yes | 46 | 74 | |
| No | 59 | 47 | 0.006 |
| Cervical os | |||
| Open (1) | 21 | 62 | |
| Closed (2) | 84 | 58 | 0.766 |
| Cervical Active Bleeding | |||
| None | 67 | 49 | |
| Mild or Heavy | 26 | 77 | 0.016 |
| Tissue at the Cervical os | |||
| Yes | 16 | 69 | |
| No | 89 | 57 | 0.391 |
Reported Fisher’s Exact two sided P value because cell counts were less than 5 therefore Chi square test may not be valid.
The regression trees for misoprostol treatment success on day 8 with cost ratios between 1:2 and 1:4 were identical (Fig. 2). Passing tissue after a second dose and old vaginal blood present were indicators of treatment success on day 8. The sensitivity, specificity, and positive and negative predictive values of this diagnostic algorithm are estimated by cross validation as 90%, 34%, 67% and 94%, respectively, i.e. two-thirds of women who report to have passed tissue or have old blood present in the vagina have completed expulsion. Conversely, 94% of women who have neither indicators positive are a treatment failure.
Figure 2.
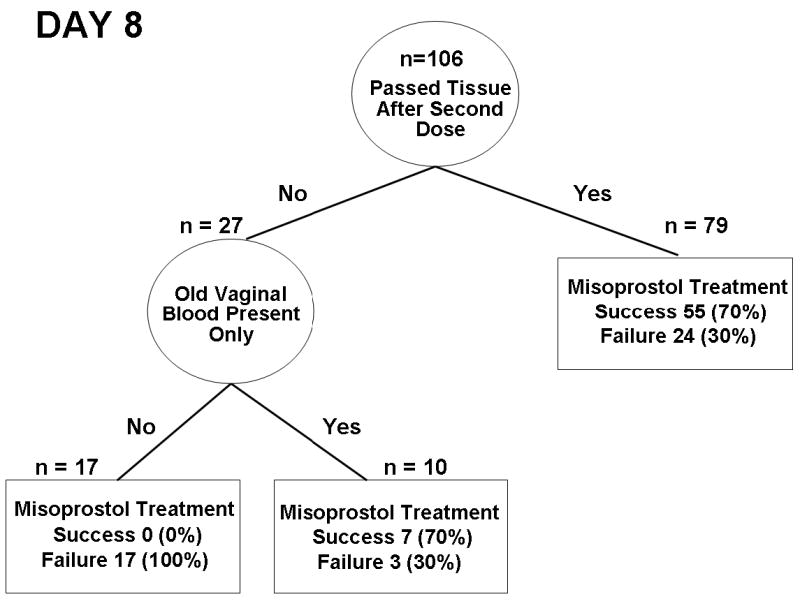
Decision tree obtained from Classification and Regression Tree analysis to predict success of misoprostol treatment on day 8.
The above analyses were repeated for women with only anembryonic gestation or fetal demise. The results essentially remained the same (not shown).
Discussion
In the last decade, numerous studies have shown that medical treatment with misoprostol for early pregnancy failure is safe, effective, and convenient [3,6,9]. This treatment is gaining acceptance by physicians and patients [9,10]. However, confirmation of treatment success and the decision for further treatment are often based on ultrasonography findings. The widespread use of this treatment, especially in the developing world, may still be in part dependant on the availability of a reliable predictive tool that can be used in clinical settings where ultrasonography is not easily available. Using a CART analysis, we identified two factors – self-reported heavy bleeding and an open cervical os on day 3 – as potentially useful indicators of treatment success after the first dose of misoprostol. Approximately 84% of women who have either indicator positive have had a successful treatment. After a second dose, if a woman neither reports having passed tissue nor has old blood in the vagina, the treatment fails in 94% of cases.
Given the relatively low likelihood of treatment success (49%), if a patient has not had heavy bleeding and the cervical os is closed on day 3, giving a second dose of misoprostol may be an appropriate choice. These patients account for approximately one-third of our study population. However, women with either or both indicators positive still have a 16% chance of having incomplete expulsion at that time. Although expulsion may complete spontaneously in these women, patients should be counseled that there is a small possibility (< 1%) of infection or hemorrhage, and that further treatment may still be needed.
Clinical indicators on day 8 have a low positive predictive value (67%), i.e. presence of either or both indicators does not assure a treatment success. However, these indicators have a high negative predictive value (94%). Women who have neither passed tissue nor have old blood in the vagina are very likely to have had a failed treatment. Offering vacuum aspiration may be appropriate.
To our knowledge, this is the first study that examines clinical indicators for treatment success after misoprostol application for early pregnancy failure. However, several limitations in our study should be acknowledged. First, how well a clinical indicator can predict outcome (i.e. positive predictive value) partly depends on the success rate of the treatment. This relationship is a positive correlation. Since the success rate after the first dose of misoprostol was 71% in our study [3], the positive predictive value on day 3 (84%) appears reasonably good. Nonetheless, a higher predictive value would be more desirable.
Second, our study tries to identify individual indicators two days after the treatment. The short interval between the treatment and the follow-up visit could make prediction difficult. For instance, an open cervical os could indicate that the uterus has responded to the misoprostol treatment. It would also indicate that the expulsion may not be complete, is in the process of occurring, or has just been completed. Our data suggest that on day 3, an open os is more likely to be indicative of a positive uterine response and expulsion may have just been completed. However, this indicator may not be applicable for later dates.
Third, in the symptom diary, women were asked “how much bleeding have you had in the last 24 hours?” The following choices were given: none, spotting, light, moderate, heavy, and more than two pads per hour. Heavy bleeding in this study is defined as heavy or more than two pads per hour. Nonetheless, self-reported heavy bleeding is still a subjective measure. Non-differential misclassification could reduce the predictive value.
Finally, women who were found to have had complete expulsion on day 3 did not come back on day 8 in our study. In a setting without ultrasonography it is likely that more women would be evaluated on day 8, which would change the prevalence of success in the group being tested. This could change the performance of the indicators on day 8. CART analysis may even select different indictors in a new group of women. Unfortunately, data from the current study do not allow us to assess the impact of the above scenario.
Our study shows that clinical indicators can be predictive of success or failure of medical management with misoprostol for early pregnancy failure. However, these indicators are not definitive. Ultrasonography is still superior to clinical indictors in diagnosing complete expulsion of the products of conception. Still, among women who have positive clinical indicators after the first dose of misoprostol, it would take six ultrasonography examinations to identify one patient with incomplete expulsion. As most miscarriages can resolve spontaneously, it remains to be tested whether a precise diagnosis will be able to translate into substantial gain in resource poor areas. When access to ultrasonography is limited, these clinical indicators may provide some guidance in the physician’s decision-making for further treatment. More studies are needed to identify better clinical indicators.
Acknowledgments
The work of this paper is supported by the Intramural Research Program at the National Institute of Child Health and Human Development, National Institutes of Health, DHHS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alberman E. Spontaneous abortions: epidemiology. In: Stabile I, Grudzinskas G, Chard T, editors. Spontaneous abortion: diagnosis and treatment. London: Springer-Verlag; 1992. pp. 19–20. [Google Scholar]
- 2.Creinin M, Schwartz J, Guido R, Pymar H. Early pregnancy failure – current management concepts. Obstet Gynecol Surv. 2001;56:105–13. doi: 10.1097/00006254-200102000-00024. [DOI] [PubMed] [Google Scholar]
- 3.Zhang J, Gilles J, Barnhart K, Creinin M, Westhoff C, Frederick M. Comparison of medical management with misoprostol and surgical management for early pregnancy failure. N Engl J Med. 2005;358:761–9. doi: 10.1056/NEJMoa044064. [DOI] [PubMed] [Google Scholar]
- 4.Lee D, Cheung L, Haines C, Chan K, Chung T. Comparison of the psychologic impact and client satisfaction of surgical treatment with medical treatment of spontaneous abortion: a randomized controlled trial. Am J Obstet Gynecol. 2001;185:953–8. doi: 10.1067/mob.2001.117661. [DOI] [PubMed] [Google Scholar]
- 5.Graziosi G, Bruinse H, Reuwer P, Mol B. Women’s preferences for misoprostol in case of early pregnancy failure. Eur J Obstet Gynecol Reprod Biol. 2006;124:184–6. doi: 10.1016/j.ejogrb.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 6.Graziosi GC, Bruinse HW, Reuwer PJ, Mol BW. Women’s preferences for misoprostol in case of early pregnancy failure. Eur J Obstet Gynecol Reprod Bio. 2006;124:184–6. doi: 10.1016/j.ejogrb.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 7.Breiman L, Friedman JH, Olshen RA, Stone CJ. Classification and Regression Trees. 2. Pacific Grove, CA: Wadsworth; 1984. [Google Scholar]
- 8.Zhang H, Singer B. Recursive partitioning in the health sciences. New York: Springer-Verlag; 1999. [Google Scholar]
- 9.Graziosi GCM, Mol BW, Ankum WM, Bruinse HW. Management of early pregnancy loss. Intl J Gynecol Obstet. 2004;86:337–46. doi: 10.1016/j.ijgo.2004.04.038. [DOI] [PubMed] [Google Scholar]
- 10.Murchison A, Duff P. Misoprostol for uterine evacuation in patients with early pregnancy failures. Am J Obstet Gynecol. 2004;190:1445–6. doi: 10.1016/j.ajog.2004.02.028. [DOI] [PubMed] [Google Scholar]


