Abstract
Rats emit ultrasonic vocalizations (USVs) as social signals in several situations. Lesion studies have shown that rat perirhinal cortex (PR), a polymodal sensory region that is reciprocally connected with the amygdala, is critical for normal fear conditioning to so-called “22 kHz USVs”. Here we evaluated single-unit responses in rat PR to 22 kHz USVs and other acoustic stimuli. One question was whether PR circuits are specifically and preferentially tuned, prior to fear conditioning, to respond to USVs and USV-like stimuli. Two 22 kHz USVs were pre-recorded from different conspecifics. Each USV consisted of a “bout” of several discrete calls. Using experimentally-naïve rats, single-unit responses to the USVs were compared with responses to continuous or discontinuous tones that had the same root frequency as the USVs (19 or 22 kHz). The on/off patterns of the discontinuous tones were temporally-matched to the call structure in the corresponding USVs. Compared to continuous tones, the USVs were no more likely to elicit single-unit firing changes in PR. On the other hand, the continuous tones and USVs clearly did elicit different firing patterns in many units. More specifically, the USVs sometimes elicited a transient increase in discharge frequency to each call in a bout of calls. Interestingly, the USVs and the temporally-matched tone segments usually elicited similar firing patterns. The USV-elicited firing pattern in PR thus appears to be controlled by the on/off temporal structure of the calls rather than by the frequency or amplitude modulations associated with each call in a bout of calls.
Keywords: species-specific behavior, biological preparedness, auditory social signals, alarm calls, rhinal cortex
1. Introduction
Rats emit 22 kHz ultrasonic vocalizations (USVs) under a variety of conditions [1,3–9,14,21,23,35,36,37,40]. These vocalizations, which commonly serve as alarm signals to warn conspecifics of danger, consist of a series of discrete “calls” that are organized into “bouts” (groups) of calls [4, 6]. Twenty-two kHz USVs are characterized by frequency and amplitude modulations, variable call and bout durations, and variable inter-call intervals [5–7]. Although referred to as “22 kHz USVs”, the principle frequency of these ethologically-important signals can actually range from 18 kHz to 32 kHz [6]. Twenty-two kHz USVs are easily distinguishable from so-called “50 kHz USVs”, which are typically associated with what might be termed positive affect and have been referred to as “rat laughter” [6,20].
To gain some insight into the neurophysiological processing of 22 kHz USVs, we recorded single-unit responses in rat cortex to three types of stimuli: pre-recorded USVs; frequency- and temporally-matched tone segments; and continuous tones with the same or lower principle frequencies. For this first neurophysiological study, single-unit responses were recorded in the perirhinal cortex (PR) of freely-behaving rats. PR is a polymodal association area that receives unimodal inputs from all sensory modalities as well as polymodal inputs from other association cortices [11,12]. In the rat, auditory information furnishes a major portion of the cortical afferents to PR [11].
Rat PR has extensive reciprocal connections with the entorhinal cortex, the hippocampal formation, and the amygdala [11,31,41]. The anatomical connectivity of PR places this periallocortical region at a pivotal intersection between perceptual processing in neocortex and the declarative and emotional memory systems associated with various medial temporal lobe structures [26,27,39]. Removal of rat PR impairs subsequent fear conditioning to 22 kHz USVs [24,29] but has no significant effect on conditioning to continuous tones cues at the same [24,29] or lower frequencies [10,24,34].
2. Materials and Methods
Single units were recorded in PR from 25 experimentally-naïve male Sprague-Dawley rats weighing 250 – 400 g (Charles-River Laboratories; Wilmington, MA). Each animal was singly housed on a 12 hr light/dark cycle with ad libitum access to food and water. All animals were handled 3 days prior to surgery and 1 day prior to experiments. Animal care and experimental procedures were conducted in accordance with Yale University’s Institutional Animal Care and Use Committee guidelines. Subjects were surgically implanted unilaterally with a custom-made microwire bundle into the right PR. Electrode microwire bundles were composed of 8 tungsten wires, each 25 μm in diameter (California Fine Wire Co.; Grover Beach, CA). The design of the electrode assembly allowed the microwire bundle tip to be secured to the lateral surface; whereas the pinset was attached to the dorsal surface of the skull.
Animals were anesthetized with ketamine (100 mg/kg, i.p.) and xylazine (20 mg/kg, i.p.) and then secured in a stereotaxic instrument (Stoelting, Wood Dale, IL). The skin on the dorsal surface of the head was cut to expose the skull. Four holes were drilled through the skull and threaded with stainless-steel anchor screws. A ground wire was attached to one of these screws and used as a reference electrode. The lateral surface of the skull was exposed by pulling the temporal muscle away from the skull with tissue spreaders. A 2-mm hole, drilled at the intersection of the zygomatic arch and the parietal bone, exposed temporal cortex just dorsal to PR. Using a micromanipulator, the electrode bundle tip was driven 1–2 mm into cortex toward PR at a 45° angle relative to the horizontal plane. Dental cement (Stoelting, Wood Dale, IL) permanently secured the electrode bundle in place on the lateral surface of the skull and secured the pin set on the dorsal surface of the skull.
PR single-unit activity was monitored in awake, freely-behaving animals. A unity-gain headstage passed the signal to a high-gain amplifier (15,000X; band-pass filtered at 154 Hz and 13 kHz; Plexon, Inc.; Dallas, TX). The signal was processed by a Multichannel Acquisition Processor (Plexon), which allowed real-time thresholding and waveform shape discrimination. Electrical activity was also monitored with a digital oscilloscope (TDS 30334, Tektronix, Richardson, TX). Units that had a signal-to-noise ratio of at least 5:1 were stored for further offline analysis. Cluster cutting techniques were used with principle components, spike width, and spike amplitude to further isolate individual waveforms (Offline Sorter, Plexon). Cross-correlations between single units recorded on different wires were analyzed to reduce possible redundancy. These procedures were sufficient to isolate action potentials from individual PR neurons.
At the end of the recording sessions, subjects were given an overdose of sodium pentobarbital (100 mg/kg, i.p.). A small marking lesion was made by passing current through the tips of two of the wires in the electrode bundle (5 s, 50 μA DC; Lesion Maker, Grass Instruments, Quincy, MA). Subjects were transcardially perfused with 0.1 M phosphate-buffered-saline, followed by 4% paraformaldehyde. Brains were removed and placed in 4% paraformaldehyde for 24 hr and cryoprotected with 30% sucrose for 72 hr. Horizontal sections (70 μm), obtained with a freezing microtome, were Nissl-stained with cresyl violet. Brain sections were examined under a light microscope to determine the location of the marking lesion site.
Auditory stimuli were played with an Enhanced Real-Time Processor RP2.1 (Tucker-Davis Technologies, TDT, Alachua, FL) through high-frequency electrostatic loudspeakers (ES1, TDT) that were powered by an electrostatic loudspeaker driver (ED1, TDT). Speakers were located at the top of an octagonal-based Plexiglas® chamber (35 cm diameter × 50 cm height) enclosed in a larger sound-attenuating Faraday chamber (custom built). Loudness measurements were made at floor level using either an ultrasonic (Ultraprobe 9000, UE Systems Inc., Elmsford, NY) or audible decibel meter (RadioShack, Fort Worth, TX).
Experiment 1 evaluated single-unit responses to 3 types of auditory stimuli: a 4 kHz continuous tone (10 ms rise/fall time, 10 s duration, 70 dB SPL); a 22 kHz continuous tone (10 ms rise/fall time, 10 s duration, 70 dB SPL); and a pre-recorded 22 kHz USV (7.8 s duration, 60 dB SPL). Similar auditory stimuli have previously been used as cues to support Pavlovian fear conditioning [24]. Experiment 2 used 6 stimuli (including 4 new ones) that were designed to isolate certain features of USVs that may be responsible for eliciting single-unit responses: 2 pre-recorded USVs (~65 dB SPL), which differed markedly from each other in terms of call structure; 2 frequency- and temporally-matched tone segments (10 ms rise/fall time, 65 dB SPL); and 2 frequency-matched continuous tones (10 ms rise/fall time, 65 dB SPL). Two of these stimuli (one of the two USVs plus its frequency-matched continuous tone) were also used in Experiment 1. Figure 1 shows the spectrograms of 6 of the 7 different stimuli that were examined (the spectrogram of the continuous 4 kHz tone is not shown). Auditory stimuli were presented 20 times each, randomly interleaved.
Figure 1.
Spectrograms of six of the seven auditory stimuli. (A) A 19 kHz continuous tone. (B) A 19 kHz discontinuous tone that was temporally- and frequency-matched to the USV depicted in part C. (C) A bout of 11 calls centered at ~19 kHz (range: 19 22 kHz). The mean (± SE) call duration was 575 ± 44 ms and the mean inter-call interval was 132 ± 2 ms. (D) An oscilloscope trace of the amplitude modulations (voltage against time) of the USV depicted in part C. (E) A 22 kHz continuous tone. (F) A 22 kHz discontinuous tone that was temporally- and frequency-matched to the USV depicted in part G. (G) A bout composed of 4 calls centered at ~22 kHz (range: 22 – 24 kHz). The mean (± SE) call duration was 1853 ± 373 ms and the mean inter-call interval was 213 ± 2 ms. (H) An oscilloscope trace of the amplitude modulations (voltage against time) of the USV depicted in part G.
The two 22 kHz USVs were elicited from two naïve Sprague-Dawley rats by foot shocks delivered through a grid floor of a chamber (1 s, 1 mA; Coulbourn Instruments, Lehigh Valley, PA) that was placed inside a sound-attenuating enclosure. A high-frequency condenser microphone (Model 7012; ACO Pacific, Belmond, CA) was used to capture the USV waveforms. The output of the microphone was amplified by a MA2 preamplifier (TDT) and digitized at 100 kHz by an RP2.1 digital signal processor (TDT). Single bouts of calls were selected from 60 s samples. USV bouts were band-pass filtered between 18 – 26 kHz to reduce background noise.
Graphical and statistical analyses were performed with NEX software (Plexon) and Microsoft Excel. Single-unit responses to the auditory stimuli were plotted with perievent rasters and histograms (bin size = 50 ms). Spontaneous (baseline) single-unit activity was defined by the average firing rate during the 5 s period just before the presentation of the auditory stimulus. Ten planned t-tests were performed on each unit to classify the stimulus-elicited firing pattern. The classification scheme, which was based on firing patterns that are commonly elicited by auditory stimuli in other brain regions [13, 19], was intended to be as simple as possible and yet still capture some potentially-important differences among stimuli and among PR single units.
Stimulus-elicited firing changes were classified along three axes, according to whether they entailed (i) an increase or decrease in firing frequency, (ii) a phasic (transient) or a tonic (sustained) firing change, and (iii) firing in response to the stimulus onset or offset. Not all possible logical combinations were actually observed. The application of this classification scheme is further elucidated in the Results section. Phasic responses were tested in 50 ms bins and defined as a statistically-significant increase, compared to the average baseline firing rate, within the first 4 bins after the stimulus onset or offset. Tonic responses were tested for statistical significance by comparing the average firing rate throughout the stimulus duration with the average baseline firing rate. A Welsch-Satterwaite solution was used to correct the degrees of freedom for unequal sample sizes. A Bonferroni correction was used to ensure a family-wise α < 0.01. Perievent rasters and histograms were used for visual confirmation of response patterns. Maximum-likelihood ratio tests (G-tests) were used to test the significance of differences in the proportions of single units that exhibited various response patterns to the auditory stimuli [38].
3. Results
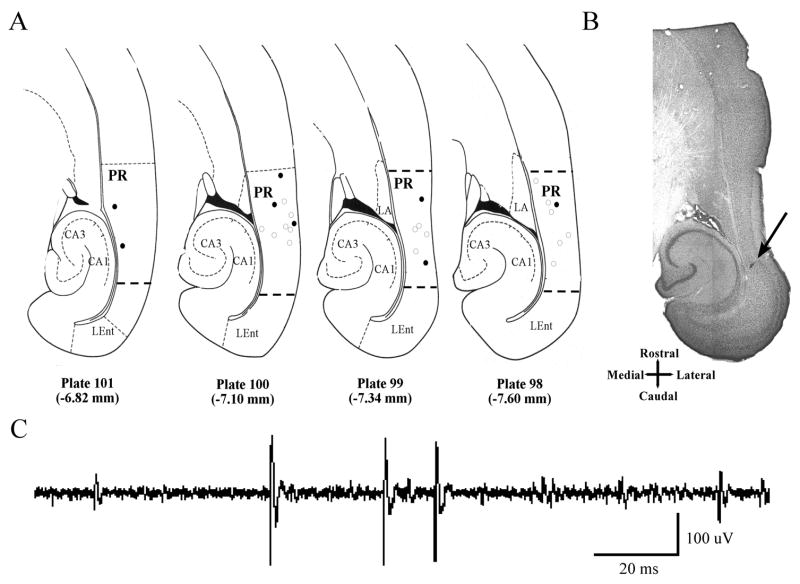
Combining Experiments 1 and 2, a total of 142 well-isolated single units were recorded from 25 animals. Figure 2C shows an example of a voltage trace of a single-unit recording. Overall, the mean (± SE) spontaneous firing rate, measured before the presentation of auditory stimuli, was 3.3 ± 0.3 Hz. Histological analysis verified that all of the electrode tips were in PR, most often in layers II/III and layer V (Fig. 2A,B). In the rostro-caudal axis, the most common locations were between −4 and −6 mm relative to Bregma. In the dorso-ventral direction, the locations were between −6.8 and −7.6 mm relative to Bregma, corresponding to plates 98 – 101 of the Paxinos and Watson atlas [30].
Figure 2.
An example of a PR single-unit recording and horizontal sections showing the location of recording sites within PR. (A) Twenty-five electrode placements are mapped onto stereotaxic plates modified from the Paxinos and Watson atlas [20]. Results are combined from Experiment 1 (marker = ○) and Experiment 2 (marker = ●). Dorso-ventral locations of plates 98–100 showing depth relative to Bregma. (B) A 70 μm horizontal section corresponding to plate 98 of the Paxinos and Watson atlas [20]. A representative marking lesion, indicated by the arrow, was made at the tip of the electrode bundle. (C) An example of a recording from a single unit in PR in a freely-behaving rat. Abbreviations: CA1, field CA1 of the hippocampus; CA3, field CA3 of the hippocampus; LA, lateral nucleus of the amygdala; LEnt, lateral entorhinal cortex; PR, perirhinal cortex.
Experiment 1
The first experiment compared PR single-unit responses (76 units from 17 rats) elicited by three auditory stimuli: a pre-recorded 22 kHz USV, a 22 kHz continuous tone, and a 4 kHz continuous tone. These particular types of stimuli were chosen because they have previously been used as cues to evaluate the role of PR in Pavlovian fear conditioning [24]. Forty-five units (59%) were responsive to one or more of the auditory stimuli (95% CI: 49–70%). There was no significant difference in the proportion of single units that were responsive to any one of the three stimuli (G = 3.11, p > 0.05). Of the 76 units, 45% were responsive to the 4 kHz tone, 28% were responsive to the 22 kHz tone, 36% were responsive to the 22 kHz USV, and 34% were responsive to more than one stimulus (Table 1).
Table 1.
Experiment 1: Single-Unit Responses to Three Auditory Stimuli
| Response Firing Pattern | Auditory Stimulus | ||
|---|---|---|---|
| 4 kHz tone | 22 kHz tone | 22 kHz USV | |
| Phasic onset (+) | 17/76 (22%) | 7/76 (8%) | 17/76 (23%) |
| Phasic offset (+) | 5/76 (7%) | 5/76 (7%) | 0% |
| Phasic onset (+)/offset (+) | 3/76 (4%) | 5/76 (7%) | 0% |
| Tonic (+) | 4/76 (5%) | 1/76 (1%) | 5/76 (7%) |
| Tonic (−) | 5/76 (7%) | 3/76 (4%) | 5/76 (7%) |
| Distribution of Auditory Responsiveness1 | 34/76 (45%) | 21/76 (28%) | 27/76 (36%) |
Twenty-six of the 76 units (34%) were responsive to more than one stimulus.
The tone stimuli elicited one of five categories of firing patterns: phasic onset (+), a transient increase in the firing rate to the stimulus onset; phasic offset (+), a transient increase in firing rate to the stimulus offset; phasic onset (+)/offset (+), a transient increase in firing rate to both the stimulus onset and offset; tonic (+), a sustained increase in firing rate during the stimulus presentation; and tonic (−), a sustained decrease in firing rate during the stimulus presentation. Due to the limited sample of neurons and small number of stimuli, one cannot assume that these categories exhaust all possible auditory response patterns that can occur in PR. Figure 3 shows perievent raster plots and histograms associated with four common types of firing patterns elicited by the continuous tone stimuli: phasic onset (+); phasic offset (+); phasic onset (+)/offset (+); and tonic (+).
Figure 3.

Four common firing patterns elicited by continuous tones in Experiment 1 (presented for 10 s, shown by gray shading). Time bins are 50 ms wide. (A) A phasic onset (+) response. (B) A phasic offset (+) response. (C) A phasic onset (+)/offset (+) response. (D) A tonic (+) response.
Across all three stimuli, the phasic onset (+) response was the most commonly-elicited firing pattern (Table 1). The 22 kHz USV stimulus sometimes elicited (9/76 units) a phasic onset (+) response to each call in the bout of calls, as described below. We have termed this subtype of phasic onset (+) discharge a “call-related” firing pattern. Figure 4 shows an example of a call-related firing pattern. The raster plot and frequency histogram (Fig. 4A) indicate that the largest phasic response occurred to the onset of the first call. The histogram suggests a degree of periodicity in the firing frequency during the USV.
Figure 4.
An example of a PR single-unit recorded in Experiment 1 that exhibited a “call-related” firing pattern by a 22 kHz USV (shown in Fig. 1C, D). The time bins are 50 ms wide. (A) A perievent histogram and raster plot display of a single-unit response to the 11-call USV. The USV is present from 0 to 7.8 s (shaded area). The histogram suggests a phasic onset (+) and a tonic (+) response. There is also a hint of periodicity in the firing rate across the course of the stimulus. (B) A 3-D contour plot reveals that the apparent periodicity is associated with the onset of individual calls. The axis labeled “Time” aligns firing to the onset of each individual call. The axis labeled “Call Segment” shows the responses to each of the successive calls. The left edge of the contour begins 100 ms before the stimulus onset. Lines that are more or less perpendicular to the Time axis represent the beginning and ends of successive time bins. The first two time ribbons (first 100 ms) show firing levels before the stimulus onset (arrow). In addition to the large phasic increase in the firing rate to the initial call, there is also a phasic increase in the firing rate to each of the subsequent calls. This repeating increase in the firing rate has been termed a “call-related” firing pattern. The ridge of the initial part of the contour occurs at a latency (from the call onsets) in the bin that spans the interval from 50 to 100 ms.
The source of this apparent periodicity is revealed in the three-dimensional (3-D) contour shown in Figure 4B, which organizes the time course of firing on a call-by-call basis. The contour plot shows that each call in the series is associated with a transient increase in the firing rate. The ridge of the contour reveals that the increase in firing was greatest to the first call of the series, after which there was a smaller but obvious phasic increase in the firing rate to the onset of each subsequent call in the series. The response latency was determined as first 10 ms time bin, after the onset of each call, to show a statistically-significant change in firing rate. For this unit, the first significant change in firing occurred in the time interval from 50 to 60 ms after the onset of each call. The peak response occurred in the time interval from 60 to 70 ms. The ridge of the contour plot (shown in orange/light blue) suggests a peak response in the bin that occurs from 50 to 100 ms.
Experiment 2
The second experiment compared PR single-unit responses (66 units from 8 rats) elicited by six types of auditory stimuli: 2 pre-recorded USVs (the one used in Experiment 1 plus a new one); 2 temporally- and frequency-matched tone segments (discontinuous tones); and 2 frequency-matched continuous tones (Fig. 1). These stimuli were selected to evaluate which features of the USVs are important in eliciting the call-related firing pattern. Twenty-nine of the 66 units (44%) were responsive to one or more of the auditory stimuli. Twenty-eight of the 29 units responded to more than one of the six auditory stimuli. There was no significant difference in the proportion of units that were responsive to any one of the six stimuli (G = 0.55, p > 0.05).
The majority of auditory-responsive units (20/29; 69%) responded to both USVs with a call-related firing pattern of either of two types. In 55% (11/20) of these units, the USVs elicited a phasic onset (+) response to each call in the bout of calls (Fig. 5A, D), as observed in Experiment 1 (compare with Fig. 4). In 45% (9/20) of these units, the USV elicited a phasic offset (+) response to each call in the bout of calls (Fig. 6A,D), a firing pattern that was not observed in Experiment 1. This phasic offset (+) response to each call occurred in response to both of the USVs (Fig. 6A, D). The most prominent difference in the firing patterns elicited by the two USVs (Fig. 1C, G) was in the number of phasic (+) responses (Fig. 5A, D and Fig. 6A, D), which was determined by the number of calls in each USV.
Figure 5.
An example of phasic onset (+) firing patterns elicited by 6 stimuli in a single unit from Experiment 2. (A) Perievent raster and histogram plots (bin size = 50 ms) are depicted with the spectrograms of the eliciting 11-call USV (shown in Fig. 1 C, D) directly underneath. The 3-D contour plot (bin size = 25 ms) reveals a “call-related” firing pattern. The ridge of the contour occurred at a latency (from the call onsets) of 25 – 50 ms. (B) The same plots are shown for the temporally- and frequency-matched discontinuous tone (shown in Fig. 1 B). The ridge of the contour occurred at a latency of 25 – 50 ms. (C) The analogous plots are shown for the frequency-matched continuous tone (shown in Fig. 1 E). The peak occurred at a latency of 50 – 75 ms. The histogram suggests that the phasic onset (+) response might be followed by a tonic (−) response. However, the apparent tonic (−) component was not statistically significant (p > 0.05). (D) Single-unit responses to a 4-call USV (shown in Fig. 1 G, H) plotted as in part A. The ridge of the contour occurred at a latency of 25 – 50 ms. (E) The same plots are shown for the matched discontinuous tone (shown in Fig. 1 F). The ridge of the contour occurred at a latency of 25 – 50 ms. (F) The analogous plots are shown for the frequency-matched continuous tone (shown in Fig. 1 E). The peak occurred at a latency of 25 – 50 ms.
Figure 6.
An example of phasic offset (+) firing patterns elicited by 6 stimuli in a single unit from Experiment 2. (A) Perievent raster and histogram plots (bin size = 50 ms) are depicted with the spectrograms of the eliciting 11-call USV (shown in Fig. 1 C, D) directly underneath. The 3-D contour plot (bin size = 25 ms) reveals a “call-related” firing pattern. The ridge of the contour occurred at a latency (from the call offsets) of 75 – 100 ms. (B) The same plots are shown for the temporally- and frequency-matched discontinuous tone. The ridge of the contour occurred at a latency of 75 – 100 ms. (C) The analogous plots are shown for the frequency-matched continuous tone. The peak response occurred at a latency of 75 – 100 ms. (D) Single-unit responses to a 4-call USV (shown in Fig. 1 G, H) plotted as in part A. The ridge of the contour occurred at a latency of 50 – 75 ms. (E) The same plots are shown for the matched discontinuous tone (shown in Fig. 1 F). The ridge of the contour occurred at a latency of 75 – 100 ms. (F) The analogous plots are shown for the frequency-matched continuous tone (shown in Fig. 1 E). The peak occurred at a latency of 75 – 100 ms.
Single-unit responses to the two USVs and the two temporally-matched tone segments were always congruent with respect to the elicitation of onset or offset responses (see Fig. 5B, E and 6B, E). The most notable difference in the firing pattern elicited by the two discontinuous tones (Fig. 1B, F) was in the number of phasic (+) responses (Fig. 5B, E), which was determined by the number of tone segments. Thus the call-related firing pattern elicited by the USVs was closely mimicked by presentations of temporally- and frequency-matched tone segments.
In general, single-unit responses to USVs were predictable based on responses to the frequency-matched continuous tones. In the majority of units that responded to the USVs with a call-related firing pattern (18/20; 90%), the frequency-matched continuous tones also elicited corresponding phasic onset or offset (+) responses (see Fig. 5C, F and Fig. 6C, F) However, in 2 of the 20 units that responded to USVs with call-related phasic (+) responses, the continuous tones produced a tonic (+) response. In these two units, responses to continuous tones clearly did not predict the USV call-related firing pattern.
In 9 of the 29 auditory-responsive units, the USVs did not elicit a call-related firing pattern. Six of these 9 units exhibited almost identical firing patterns to all six types of stimuli. For example, one unit produced a single phasic onset (+) response at the beginning of all six stimuli. Given the phasic onset (+) response to the continuous tone, one might have predicted that the USV would elicit a call-related firing pattern, but this did not occur. Another unit exhibited a significant onset phasic (+) response to the 19 kHz continuous tone, but was unresponsive to the other stimuli. Two of 9 auditory-responsive units showed statistically-significant firing changes to the USVs, but were unresponsive to the continuous and discontinuous tones. One of these two units showed a single phasic onset (+) response to the beginning of both USVs, but was unresponsive to the continuous and discontinuous tones. The other unit showed a single phasic offset (+) response to both USVs, but was unresponsive to the continuous and discontinuous tones. These two units suggest that something more than root frequency and temporal discontinuity may control the firing of some PR units.
4. Discussion
Single-unit responses to auditory social signals have been previously studied in non-human primates [32,33], but not in rodents, which offer certain experimental advantages. The two experiments described here examined single-unit responses to 22 kHz USVs in rats. Experiment 1 was designed to explore whether, in experimentally-naïve rats, PR neurons show preferential responsiveness or distinguishable firing patterns to USVs versus other auditory stimuli that have been used as cues in studies of Pavlovian fear conditioning. This inquiry was partly motivated by the theoretical possibility that auditory stimulus processing in cortical circuits may have evolved to be selectively “tuned” to certain types of ethologically-important natural stimuli. The results describe single-unit responses to three types of stimuli that have been used as cues in studies of Pavlovian fear conditioning: a 4 kHz continuous tone; a 22 kHz continuous tone; and a 22 kHz USV.
A large proportion of PR units (59%) were responsive to one or more of the three auditory stimuli (see Table 1). The high level of auditory responsiveness is consistent with the anatomical fact that rat PR receives a large afferent input from auditory cortex [11] as well as projections from auditory parts of the thalamus [16]. Unlike the continuous tones, the 22 kHz USV sometimes elicited a clearly-distinguishable “call-related” firing pattern (Figs. 4 – 6). This pattern consisted of a transient increase in the firing frequency in response to each of the successive calls in a bout of calls; that is, a “phasic (+)” response to each call. From a neurophysiological perspective, this repeating increase in the firing frequency seemed potentially important for synaptic transmission, neuronal plasticity, and stimulus coding.
Experiment 2 therefore focused on the stimulus requirements for eliciting this repeating call-related firing pattern. Single-unit responses were examined to six different stimuli: two different 22 kHz USVs; two discontinuous tones that were matched to the corresponding USVs in terms of their on/off patterns, root frequency and total duration; and two continuous tones that were matched to the corresponding USVs in terms of root frequency and total duration (Fig. 1). One USV stimulus was identical to that used in Experiment 1 except that the loudness was increased slightly to be more comparable to the continuous and discontinuous tones. The second USV stimulus was specifically chosen to differ from the first one in terms of root frequency (19 versus 22 kHz), mean call duration (575 ms versus 1853 ms), mean inter-call interval (132 ms versus 213 ms), and mean call repetition rate (1.50 Hz versus 0.55 Hz). There were also obvious differences between the two USVs in terms of frequency and amplitude modulations (Fig. 1).
Overall, 44% of the units responded to one or more of the six auditory stimuli. Both USVs elicited a call-related firing pattern in 69% of the auditory-responsive units. The higher percentage of call-related firing patterns in the second experiment may reflect the fact that the loudness of the USVs was increased. Alternatively, there may have been systematic differences in the exact recording sites within PR. Most often, the call-related firing pattern consisted of a transient increase in the firing frequency that was triggered by the onset of each call in the series of calls (Fig. 5A, D). This firing pattern was classified as a “phasic onset (+)” response to each call. Less often, both USVs elicited an increase in the firing frequency to the offset of each call (Fig. 6 A, D). This firing pattern was classified as a “phasic offset (+)” response to each call. In general, the peak offset response occurred at a longer latency than the peak onset response (compare Figs. 5 and 6). Thus the offset responses may be driven by more complex or possibly different circuitry. In either case, the discontinuous tones always elicited a very similar firing pattern (Fig. 5 B, E; 6 B, E). When the USVs and discontinuous tones elicited onset responses, the continuous tones also tended (in 10 of 11 cases) to elicit onset responses (Fig. 5 C, F). Similarly, when the USVs and discontinuous tones elicited offset responses, the continuous tones also tended (in 8 if 9 cases) to elicit offset responses (Fig. 6).
The results suggest that the call-related firing pattern to 22 kHz USVs may emerge from a more basic tendency of PR neurons to respond to auditory stimulus onsets or offsets. Responsiveness to stimulus onsets or offsets appears to be a general characteristic of many auditory-responsive neurons [13,19]. Perhaps not surprisingly, therefore, the single-unit responses to continuous tones were often predictive of responses to USVs. However, there were clear cases in which tone-elicited firing was not predictive of USV-elicited firing. For example, among units that responded to continuous tones with phasic (+) responses, some responded to USVs with a call-related firing pattern and others did not. Furthermore, 2 units responded to both of the USVs, but were unresponsive to the other 4 stimuli.
The fact that the USVs and discontinuous tones usually (26/29; 89%) elicited similar firing patterns suggests that the frequency modulations associated with individual calls do not critically determine the firing pattern or the overall level of responsiveness. Although USVs clearly do contain frequency modulations (Fig. 1 C, D), some investigators have emphasized the relative constancy of the principle frequency within a bout of calls [6]. At the present, essentially nothing is known about which stimulus features are most important for rats in discriminating among different USVs or for the recognition of particular USVs. Depending on the number of training trials and other acquisition conditions, the answer may turn out to involve different combinations of the root frequency, the mean call duration, and the mean call repetition rate [2, 6].
The selection of PR as an initial region of interest was partly based on the discovery that damage to this structure impairs fear conditioning to USVs [24, 29] but has no significant effect on conditioning to continuous tones [10, 24, 29, 34]. The fact that cortical processing is not necessary for conditioning to continuous tones is commonly interpreted to mean that activity in the subcortical projection from the auditory thalamus to the amygdala is sufficient to represent these relatively simple stimuli [22,34,42,43]. Our working hypothesis has been that the discontinuous nature of the USV is at least part of the reason why normal fear conditioning to this stimulus does require cortical processing.
Consistent with this hypothesis, we recently found that neurotoxic PR lesions [29] also impair fear conditioning to a discontinuous tone (the one shown in Figure 1B). Cortical processing may be required to integrate these discontinuous auditory stimuli across time. Perhaps the auditory segments must be bound together into a unitary representation, sometimes referred to as an “auditory object” [18, 32], in order for normal fear conditioning to occur. The obvious next step is to examine rat PR single-unit responses before and after conditioning to USVs and other auditory stimuli. Ultimately, learning-related changes in elicited firing might be best understood by comparing single-unit responses in auditory cortex, perirhinal cortex, and the amygdala.
One recent experiment by Anders and Fendt [17] suggests that reactivity to 22 kHz USVs may depend on previous learning. More specifically, this study found no differences among experimentally-naïve rats in the levels of freezing elicited by 22 kHz USVs and continuous tones. Of course, it is possible that differences would have been observed in the presence of conspecifics or certain other critical stimuli [4]. Any innate defensive responses to 22 kHz USVs could theoretically depend on the simultaneous presence of multiple stimuli and certain environmental conditions [4]. Regardless of the interpretation of the preceding results, there may be some degree of biological “preparedness” [15,25,28,36] for forming or maintaining associations with USVs. In support of the latter possibility, the same study by Andres and Fendt [17] found that fear conditioning to 22 kHz USVs is more resistant to extinction than fear conditioning to continuous tones. It will be interesting and important to understand what types of experiences are necessary for forming and maintaining ethologically-appropriate responses to these and other types of USVs.
Acknowledgments
This work was supported by the National Institute of Health research grant MH058405 and Yale University. We thank Ashwini Tankhiwale for reading the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Anderson JW. The production of ultrasonic sounds by laboratory rats and other mammals. Science. 1954;119:808–9. doi: 10.1126/science.119.3101.808. [DOI] [PubMed] [Google Scholar]
- 2.Bang S, Allen TA, Jones LK, Boguszewski P, Brown TH. Asymmetrical generalization toward social alarm calls in rats given differential fear conditioning. Society for Neuroscience Abstracts. 2006 Program Number 67.15. [Google Scholar]
- 3.Barfield RJ, Geyer LA. Sexual behavior: ultrasonic postejaculatory song of the male rat. Science. 1972;176:1349–50. doi: 10.1126/science.176.4041.1349. [DOI] [PubMed] [Google Scholar]
- 4.Blanchard RJ, Blanchard DC, Agullana R, Weiss SM. Twenty-two kHz alarm cries to presentation of a predator, by laboratory rats living in visible burrow systems. Physiol Behav. 1991;50:967–72. doi: 10.1016/0031-9384(91)90423-l. [DOI] [PubMed] [Google Scholar]
- 5.Brudzynski SM. Ultrasonic vocalization induced by intracerebral carbachol in rats: localization and a dose-response study. Behav Brain Res. 1994;63:133–43. doi: 10.1016/0166-4328(94)90084-1. [DOI] [PubMed] [Google Scholar]
- 6.Brudzynski SM. Principles of rat communication: Quantitative parameters of ultrasonic calls in rats. Behav Genet. 2005;35(1):85–92. doi: 10.1007/s10519-004-0858-3. [DOI] [PubMed] [Google Scholar]
- 7.Brudzynski SM, Bihari F, Ociepa D, Fu XW. Analysis of 22 kHz ultrasonic vocalization in laboratory rats: long and short calls. Physiol Behav. 1993;54:215–21. doi: 10.1016/0031-9384(93)90102-l. [DOI] [PubMed] [Google Scholar]
- 8.Brudzynski SM, Holland G. Acoustic characteristics of air puff-induced 22-kHz alarm calls in direct recordings. Neurosci Biobehav Rev. 2005;29:1169–80. doi: 10.1016/j.neubiorev.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 9.Brudzynski SM, Ociepa D. Ultrasonic vocalization of laboratory rats in response to handling and touch. Physiol Behav. 1992;52:655–60. doi: 10.1016/0031-9384(92)90393-g. [DOI] [PubMed] [Google Scholar]
- 10.Bucci DJ, Phillips RG, Burwell RD. Contributions of postrhinal and perirhinal cortex to contextual information processing. Behav Neurosci. 2000;114(5):882–94. doi: 10.1037//0735-7044.114.5.882. [DOI] [PubMed] [Google Scholar]
- 11.Burwell RD. The parahippocampal region: corticocortical connectivity. Ann N Y Acad Sci. 2000;911:25–42. doi: 10.1111/j.1749-6632.2000.tb06717.x. [DOI] [PubMed] [Google Scholar]
- 12.Burwell RD. Borders and cytoarchitecture of the perirhinal and postrhinal cortices in the rat. J Comp Neurol. 2001;437:17–41. doi: 10.1002/cne.1267. [DOI] [PubMed] [Google Scholar]
- 13.Chimoto S, Kitama T, Qin L, Sakayori S, Sato Y. Tonal response patterns of primary auditory cortex neurons in alert cats. Brain Res. 2002;934:34–42. doi: 10.1016/s0006-8993(02)02316-8. [DOI] [PubMed] [Google Scholar]
- 14.Choi J-S, Brown TH. Central amygdala lesions block ultrasonic vocalization and freezing as conditional but not unconditional responses. J Neurosci. 2003;23:8713–21. doi: 10.1523/JNEUROSCI.23-25-08713.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Domjan M, Cusato B, Krause M. Learning with arbitrary versus ecological conditioned stimuli: Evidence from sexual conditioning. Psychon Bull Rev. 2004;11(2):232–46. doi: 10.3758/bf03196565. [DOI] [PubMed] [Google Scholar]
- 16.Doron NN, LeDoux JE. Cells in the posterior thalamus project to both amygdala and temporal cortex: a quantitative retrograde double-labeling study in the rat. J Comp Neurol. 2000;425(2):257–74. [PubMed] [Google Scholar]
- 17.Endres T, Fendt M. Learning and extinction of natural fear-inducing stimuli. Society for Neurosciences Abstracts. 2006 Program Number 215.1. [Google Scholar]
- 18.Griffiths TD, Warren JD. What is an auditory object? Nat Rev Neurosci. 2004;5:887–92. doi: 10.1038/nrn1538. [DOI] [PubMed] [Google Scholar]
- 19.Heil P. Auditory cortical onset responses revisited I. First-Spike Timing. J Neurophysiol. 1997;77:2616–41. doi: 10.1152/jn.1997.77.5.2616. [DOI] [PubMed] [Google Scholar]
- 20.Knutson B, Burgdorf J, Panksepp J. Ultrasonic vocalizations as indices of affective states in rats. Psycol Bull. 2002;128(6):961–77. doi: 10.1037/0033-2909.128.6.961. [DOI] [PubMed] [Google Scholar]
- 21.Koo JW, Han JS, Kim JJ. Selective neurotoxic lesions of basolateral and central nuclei of the amygdala produce differential effects on fear conditioning. J Neurosci. 2004;24:7654–62. doi: 10.1523/JNEUROSCI.1644-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.LeDoux Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 23.Lee HJ, Choi J-S, Brown TH, Kim JJ. Amygdalar NMDA receptors are critical for the expression of multiple conditioned fear responses. J Neurosci. 2001;21:4116–24. doi: 10.1523/JNEUROSCI.21-11-04116.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lindquist D, Jarrard LE, Brown TH. Perirhinal cortex supports delay fear conditioning to rat ultrasonic social signals. J Neurosci. 2004;24:3610–17. doi: 10.1523/JNEUROSCI.4839-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marler P. Innateness and the instinct to learn. An Acad Bras Cinec. 2004;76:189–200. doi: 10.1590/s0001-37652004000200002. [DOI] [PubMed] [Google Scholar]
- 26.Murray EA, Bussey TJ. Perceptual-mnemonic functions of the perirhinal cortex. Trends Cogn Sci. 1999;3:142–51. doi: 10.1016/s1364-6613(99)01303-0. [DOI] [PubMed] [Google Scholar]
- 27.Murray EA, Graham KS, Gaffan D. Perirhinal cortex and its neighbors in the medial temporal lobe: contributions to memory and perception. Q J Exp Psychol B. 2005;58:378–96. doi: 10.1080/02724990544000077. [DOI] [PubMed] [Google Scholar]
- 28.Ohman A, Mineka S. Fears, phobias, and preparedness: Toward an evolved module of fear and fear learning. Psychol Rev. 2001;108(3):483–522. doi: 10.1037/0033-295x.108.3.483. [DOI] [PubMed] [Google Scholar]
- 29.Padlubnaya DB, Allen TA, Brown TH. Perirhinal lesion effects on delay fear conditioning depend on stimulus features of the conditional stimulus. Society for Neuroscience Abstracts. 2006 Program Number 67.14. [Google Scholar]
- 30.Paxinos G, Watson C. The rat brain. 4. San Diego, CA: Academic Press; 1998. [Google Scholar]
- 31.Pitkänen A, Pikkarainen M, Nurminen N, Ylinen A. Reciprocal connections between the amygdala and the hippocampal formation, perirhinal cortex, and postrhinal cortex in rat. Ann N Y Acad Sci. 2000;911:369–91. doi: 10.1111/j.1749-6632.2000.tb06738.x. [DOI] [PubMed] [Google Scholar]
- 32.Rauschecker JP, Tian B. Mechanisms and steams for processing of “what” and “where” in auditory cortex. Proc Natl Acad Sci USA. 2000;97(22):11800–6. doi: 10.1073/pnas.97.22.11800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Romanski LM, Averbeck BB, Diltz M. Neural representation of vocalization in the primate ventrolateral prefrontal cortex. J Neurophysiol. 2004;93:734–47. doi: 10.1152/jn.00675.2004. [DOI] [PubMed] [Google Scholar]
- 34.Romanski LM, LeDoux JE. Equipotentiality of thalamo-amygdala and thalamo-cortico-amygdala circuits in auditory fear conditioning. J Neurosci. 1992;12:4501–9. doi: 10.1523/JNEUROSCI.12-11-04501.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sales GD. Ultrasound and aggressive behaviour in rats and other small mammals. Anim Behav. 1972;20:88–100. doi: 10.1016/s0003-3472(72)80177-5. [DOI] [PubMed] [Google Scholar]
- 36.Seligman MEP. On the generality of the laws of learning. Psychol Rev. 1970;77(5):406–18. [Google Scholar]
- 37.Sewell GD. Ultrasound in adult rodents. Science. 1967;215:512. doi: 10.1038/215512a0. [DOI] [PubMed] [Google Scholar]
- 38.Sokal RR, Rohlf FJ. Biometry: the principles and practice of statistics in biological research. New York: W.H. Freeman; 1995. p. 887. [Google Scholar]
- 39.Squire LR, Stark CE, Clark RE. The medial temporal lobe. Annu Rev Neurosci. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- 40.van der Poel AM, Miczek KA. Long ultrasonic calls in male rats following mating, defeat and aversive stimulation: frequency modulation and bout structure. Behavior. 1991;19:127–42. [Google Scholar]
- 41.Witter MP, Naber PA, van Haeften T, Machielsen WC, Rombouts SA, Barkhof F, Scheltens P, Lopez de Silva FH. Cortico-hippocampal communication by way of parallel parahippocampal-subicular pathways. Hippocampus. 2000;10(4):398–410. doi: 10.1002/1098-1063(2000)10:4<398::AID-HIPO6>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 42.Yaniv D, Schafe GE, LeDoux JE, Richter-Levin G. A gradient of plasticity in the amygdala revealed by cortical and subcortical stimulation, in vivo. Neurosci. 2001;106(3):613–20. doi: 10.1016/s0306-4522(01)00312-8. [DOI] [PubMed] [Google Scholar]
- 43.Yaniv D, Desmedt A, Jaffard R, Richter-Levin G. The amygdala and appraisal processes: stimulus and response complexity as an organizing factor. Brain Res Rev. 2004;44(2–3):179–86. doi: 10.1016/j.brainresrev.2003.08.008. [DOI] [PubMed] [Google Scholar]