Abstract
Methylene blue (MB) is a metabolic enhancer that has been demonstrated to improve memory retention when given post-training in low doses in a variety of tasks in rats, including inhibitory avoidance, spatial memory (in both normal and metabolically-impaired subjects), object recognition, and habituation to a familiar environment. MB has been also shown to improve memory retention of extinction of fear conditioning in the rat. No experiments have been conducted to determine the effects of MB on more complex learning such as in discrimination tasks that require repeated days of training. This study examined the effects of daily MB on spatial discrimination memory in a baited holeboard maze. Following three days of discrimination training, subjects treated daily with post-training MB (1 mg/kg) reliably discriminated between rewarded (baited) and non-rewarded (unbaited) trials as indicated by a greater number of correct responses on rewarded trials than non-rewarded trials during the last three days of discrimination training. No such discrimination effects were observed in the saline-treated control group during the same training period. To determine whether the memory-enhancing effects of MB are associated with an increase in metabolic energy capacity in the brain, cytochrome c oxidation was measured in brains from rats treated with 1 mg/kg MB or saline for three days. The number of daily injections was chosen based on the behavioral data which revealed group differences three days after the beginning of MB treatment. Brain cytochrome oxidase activity in the MB-treated group was approximately 70% higher than in saline-treated rats. The findings suggest that repeated post-training MB may improve memory consolidation between days of learning by an induction in the enzyme cytochrome oxidase, leading to increased metabolic capacity in brain regions requiring more energy during discrimination learning.
Keywords: methylene blue, memory retention, cytochrome oxidase, metabolic enhancer, spatial memory, discrimination learning
1. Introduction
In 1886, Paul Ehrlich first coined the term “magic bullet” to refer to methylene blue (MB) selective uptake by nerve cells after systemic injection into live rats. This MB property led Ehrlich to postulate that chemicals could be used in vivo to selectively target tissues in the body, which became the underlying principle of modern chemotherapy (Sorgel, 2004). Indeed, MB administration has been used as a selective supravital stain of nerve cells for over one hundred years. In particular, when injected intraperitoneally into live rats, MB crosses the blood-brain barrier and selectively stains brain tissue, which can be made visible after dissection when reoxidized because MB regains its blue color (O’Leary et al., 1968). Detailed pharmacokinetic studies of MB’s organ distribution have shown that MB is selectively trapped in the brain and that its concentration is 10-20 times higher in the brain than in the circulation one hour following systemic administration (Peter et al., 2000). Therefore, MB is a suitable candidate for selectively acting on the brain following systemic administration.
The memory enhancing effects of MB were first reported by Martinez, Jr. and colleagues (1978), who discovered that low dose post-training administration of MB improved memory retention in an inhibitory avoidance task in rats. They showed that 1 mg/kg MB given immediately post-training improved memory retention tested one day later; whereas retention was not affected by the same MB dose given 15 minutes before training, 6 hours after training, or 15 minutes before testing. These results suggested that MB could facilitate the memory consolidation occurring in the brain following a learning session. Independent studies in our laboratory have supported this interpretation by showing that post-training MB increases memory retention in appetitive, aversive and spatial learning tasks, such as in object recognition, between-days habituation to a familiar environment, and spatial memory retention and reversal learning in the baited holeboard maze (Callaway et al. 2002, 2004; Riha et al. 2005). Gonzalez-Lima & Bruchey (2004) also found that memory retention of extinction of Pavlovian fear conditioning was enhanced with post-extinction administration of MB, and that this effect was related to an increase in brain cytochrome oxidase activity.
However, no experiments have been conducted to determine the effects of MB on more complex learning such as in discrimination tasks that require repeated days of training. The previous holeboard tasks included food search in reward-only trials, which was a simple spatial memory task to find “where” the baited holes were located. This study used a non-reward trial that preceded reward trials. The goal was not simply to find where are the baited holes but to learn to discriminate “when” the non-reward trial was given, which involves “temporal” memory (“when” is baited) as well as “spatial” memory (“where” is baited). The goal of the first experiment was to investigate the memory retention effects of daily 1 mg/kg MB on a discrimination learning task in the holeboard maze, using an appetitive spatial memory task that requires repeated days of training. Since previous studies demonstrated an improvement in spatial memory retention in the holeboard maze food search task following MB administration (Callaway et al. 2002, 2004), we were interested in further investigating its effects on the more demanding discrimination learning of rewarded versus non-rewarded trials in the maze.
Previous experiments in our laboratory examined the behavior of rats given regular alternation or visual discrimination of rewarded and non-rewarded trials in order to determine reward related expectancies in appetitive memory tasks and associated brain cytochrome oxidase activity, but no prior study has examined the effect of MB on discrimination learning (Hu et al. 2005, 2006; Lilliquist et al. 1999; Nair and Gonzalez-Lima, 1999). We used a more difficult discrimination design than a simple discrimination based on a balanced alternation between reward and non-reward trials because the purpose of this study was to test the efficacy of MB on a more difficult learning task as opposed to a simpler learning task. It was hypothesized that subjects receiving daily MB would demonstrate improved discrimination between rewarded (baited) and non-rewarded (unbaited) trials, with more accuracy in rewarded than non-rewarded trails, as measured by reference memory scores.
The goal of the second experiment was to ascertain whether the memory-enhancing effects of MB are associated with an increase in brain metabolic energy capacity, as measured by cytochrome c oxidation (Wong-Riley 1989; Gonzalez-Lima and Cada, 1998) in brains from rats treated with 1 mg/kg MB or saline for three days. The number of daily injections was chosen based on the behavioral data from the first experiment which revealed group differences three days after the beginning of MB treatment. It was hypothesized that brain cytochrome oxidase activity in the MB-treated group would be higher than in saline-treated rats, leading to increased brain metabolic energy capacity (Callaway et al. 2004; Gonzalez-Lima and Bruchey, 2004).
2. Methods
2.1. Behavioral methods
2.1.1. Subjects
Subjects were 20 male Long-Evans hooded rats (Harlan, Indianapolis, IN) weighing between 148 and 165 g on the first day of the experiment. They were singly-housed under standard laboratory conditions with a 12 hr light/dark cycle. Rats were handled daily for 7 days prior to and throughout the experiment to habituate them to the experimenters. For motivational purposes in the food search task, subjects were food-restricted to half of their ad libitum intake by administering between 9-11 g of rat chow a day. However, not all of the food-restricted subjects become motivated enough to learn the food search maze task. Hence only subjects that demonstrated a learning curve during the first 4 days of food search training in the holeboard were used for further study. This exclusion criterion was done prior to the beginning of the discrimination training phase of the experiment and before any MB or saline treatment, leaving an N=16 for the discrimination study. Subjects were housed and handled according to protocols approved by the Institutional Animal Care and Use Committee. Experiments were carried out in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23).
2.1.2. Apparatus
Holeboard floor inserts (MED Associates Inc., St. Albans, VT) were placed in 2 automated MED Associates ENV-515 Test Environments, each measuring 31 × 45 × 45 cm with 16 beam infrared arrays to scan for activity counts. Test chambers were connected to a single computer (Dell Optiplex). The holeboard task floor inserts had 16 equidistant holes, with 4 rows of 4 holes, each measuring 3.2 cm in diameter. Inserts were placed on 7.6 cm risers with an underlying food tray so that reinforcers could be placed in the desired holes. Several food pellets were placed under a screen in each hole in order to control for olfactory cues coming from baited holes. Infrared beams detected entry to the task floor holes, via nose pokes initiated by the subjects. Software (MED-PC for Activity Boxes) recorded holes nose-poked, novel or repeat entries to holes, and reference memory scores (number of nose pokes to baited holes divided by the total number of nose pokes). Holeboards were placed in a dimly lit (100 lumens) sound-attenuated behavioral testing room.
2.1.3. Behavioral Training
Habituation Phase
Prior to habituation trials in the holeboard maze, rats were habituated to the novel bait used in the task. One Noyes 45 mg sucrose pellet (Research Diets, New Brunswick, NJ) was put in each subjects’ home cage daily following handling sessions. Subjects were habituated to the holeboard for 2 days with all 16 holes baited with one sucrose pellet. During habituation, one pellet was also placed on the holeboard surface. Subjects were run in pairs with a black partition dividing the area between the two mazes. Trials lasted 15 minutes or until all 16 holes were nose-poked.
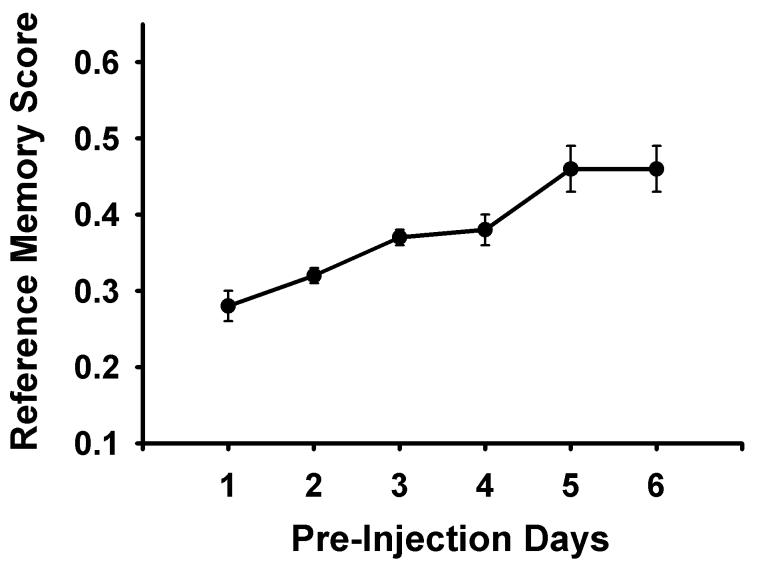
Food Search Training (Days 1-6)
Habituation was followed by 6 days of food search training with 4 holes baited in a fixed pattern (Figure 1). Each subject had 5 consecutive daily trials lasting 5 min each or until the fourth baited hole was nose-poked, with an average inter-trial interval of 2 minutes. In between trials, rats were put into a dark box and to control for any olfactory cues that could affect performance in subsequent trials, experimenters wiped down the test chambers with a mild detergent and rotated the holeboard insert, before rebaiting the maze. Holes were always baited in the same pattern. Bait left over from previous trials was discarded. The computer recorded reference memory score was calculated by adding the number of nose pokes to baited holes and divided by the total number of nose pokes.
Figure 1.
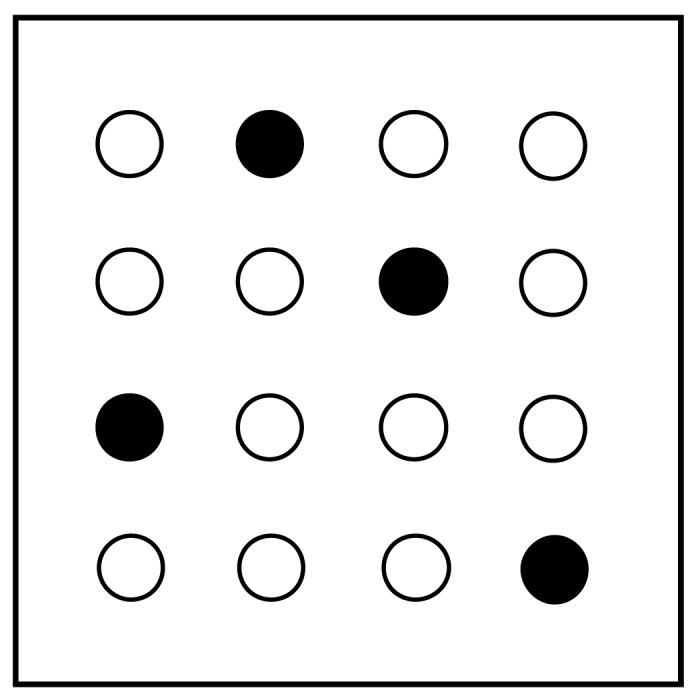
The baiting pattern in holeboard utilized for all trials. Dark circles are baited holes, and open circles are unbaited holes.
On the fifth day of training, experimenters matched subjects into two groups according to training performance and on the sixth day subjects were injected with either MB (n = 8, 1 mg/kg USP grade, Faulding Pharmaceuticals, Paramus, NJ) or saline (n = 8, 0.9% NaCl) intraperitoneally (i.p.) immediately following the last trial. Blue food coloring added to saline was used to blind experimenters to group assignment.
Discrimination Training (Days 7-12)
The discrimination training consisted of a memory-based daily alternation between rewarded and non-rewarded trials (Lilliquist et al. 1999). Beginning on the seventh day, discrimination training sessions began with one non-rewarded/unbaited trial lasting 5 min or until the fourth hole normally baited was nose-poked. This was followed by four rewarded/baited training trials (baited in the same pattern as on Days 1-6). The first trial of each day was the non-rewarded trial, thus performance depended on the memory-based discrimination of the daily alternation schedule of reward versus non-reward. Training consisted of more rewarded trials (4) than non-rewarded (1) trials per day to prevent the rapid extinction of reward-seeking performance produced by the presentation of the non-rewarded trials (Lilliquist et al. 1999). Discrimination training sessions lasted for 6 days. Immediately following the last daily trial in the maze, subjects were injected with either MB (1 mg/kg) or saline (0.9% NaCl). MB and control subjects were run in pairs to rule out any confounds that time of day may have caused on performance.
2.2. Biochemical Methods
2.2.1 Subjects
All animal procedures were approved by the institutional care and use committee at the University of Texas at Austin, and conform to all NIH and USDA guidelines. To determine whether the memory-enhancing effects of MB are associated with an increase in brain metabolic energy capacity, cytochrome c oxidation was measured in rats treated with 1 mg/kg MB (n = 5) or saline (n = 5). Ten Sprague-Dawley rats (193-229g) were standard facility reared on a 12:12 light:dark cycle, handled and provided food and water ad libitum. Three daily injections were administered I.P. during the light cycle, 24 hours between each injection. The number of daily injections was chosen based on the behavioral data in the first study which revealed group differences three days after the beginning of MB treatment. One rat injected with saline was excluded from statistical analysis because cytochrome c oxidation was more than 2 standard deviations away from the group mean.
Twenty-four hours following the third injection, animals were killed by decapitation. The brains were rapidly removed and one hemisphere was placed in a chilled glass homogenizer. Homogenization was performed by hand, keeping the homogenizer in ice, until the tissue was uniform. The tissue was transferred to chilled centrifuge tubes, spun briefly to remove air bubbles, and slowly lowered in cold isopentane (-40°C), and stored at -40°C. A detailed explanation of the biochemical methods is found in Gonzalez-Lima and Cada (1998).
2.2.2. Materials
The following solutions were prepared for the spectrophotometric procedure: (1) isolation buffer (pH 7.4) in distilled water consisting of 0.32M sucrose, 0.99mM EDTA, 8.4mM Trizma HCl, and 1.6mM Trizma base; (2) dialysis buffer (pH 7.0) in distilled water that included 20.7mM potassium phosphate monobasic and 29.3mM sodium phosphate dibasic; (3) 10% sodium deoxycholate in distilled water; and (4) 3 ml of 5 mM cytochrome c solution (95% purity from horse heart, Sigma) in dialysis buffer.
2.2.3. Spectrophotometry Procedure
Preparation and reduction of cytochrome c
(1) Reduction was achieved by adding sodium ascorbate (0.3 g) to the 5 mM cytochrome c solution, slowly inverting and allowing to stand for 5 min.
(2) The reduced cytochrome c solution was added to an equilibrated Sephadex PD-10 column (Amersham, Uppsala, Sweden) in order to remove the sodium ascorbate. The eluent was collected and stored on ice.
(3) To verify a minimum 95% reduction, 10 μl of cytochrome c solution was added to a warmed cuvette containing 990 μL dialysis buffer (37°C), inverted slowly to mix and absorbance at 550 nm was measured. Subsequently, sodium hydrosulfite was added to the cuvette, inverted again, and the absorbance was checked again at 550 nm. The first reading should be no less than 95% of the second reading. In addition, absorbance of the diluted solution (without sodium hydrosulfite) was measured at 550 nm (absorbance of reduced cytochrome c) and 565 nm (absorbance of oxidized cytochrome c). The ratio of 550/565 nm should be greater than 6. Both verifications were performed in duplicate.
(4) Using the averaged absorbance at 550 nm (without sodium hydrosulfite), the concentration of cytochrome c in the eluent was calculated using Beer’s law: concentration = Absorbance/Extinction coefficient (c = ABS/ε). The extinction coefficient of reduced cytochrome c was measured in our spectrophotometer (Shimadzu model UV-1201V) and determined to be 19.6 l/mmol. The concentration of reduced cytochrome c in the eluent was used to make a 0.07% cytochrome c solution for tissue assay.
Tissue preparation and assay
(5) An aliquot of brain tissue homogenate was further homogenized in cold isolation buffer to yield 20% tissue homogenate (1 g per 4 ml buffer).
(6) Another solution containing 50 μl of 20% tissue homogenate, 3.75 ml isolation buffer and 200 μl of the 10% deoxycholate stock was prepared, vortexed briefly, and incubated for 5-10 min at room temperature. Following incubation, the solution was kept on ice and vortexed intermittently. This solution contained 0.25% tissue and was used within 30 min.
(7) A cuvette containing 0.07% cytochrome c solution (990 μl) was heated to 37°C. Next, 10 μl of the 0.25% tissue solution was added, slowly inverted twice, and placed in the spectrophotometer.
(8) Change in the absorbance at 550 nm was recorded for 3 minutes.
(9) Steps 7 and 8 were performed at least in triplicate. For each subject, the greatest change in absorbance over the first minute was used for statistical analysis.
(10) Cytochrome c oxidation was calculated by dividing the change in the absorbance in the first min by the extinction coefficient. This number was then divided by 0.000025, to determine cytochrome oxidase activity in the brain homogenate. Enzyme activity is expressed as μmol of reduced cytochrome c per min per gram of wet tissue weight (μmol/min/g).
3. Results
3.1. Behavioral results
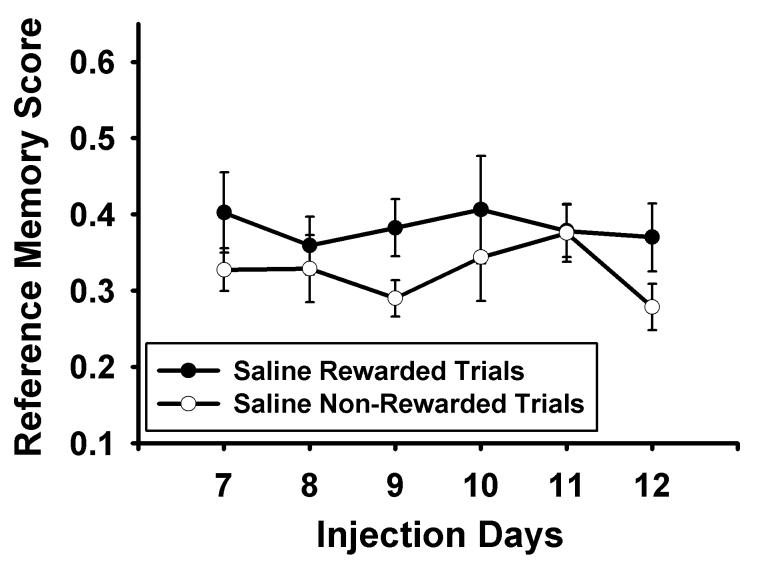
An acquisition curve (Days 1-6) was utilized to verify that subjects were learning the food search task, F(5,75) = 17.469, p < 0.001 (Figure 2). During the discrimination phase (Days 7-12), the saline group showed a better overall mean in rewarded trials than non-rewarded trials, F(1,13) = 8.616, p = 0.012; however, they did not reliably increase their discrimination learning over the six days, F(1,5) = 0.635, p = 0.674 (Figure 3).
Figure 2.
Acquisition curve for all subjects showing means ± standard errors for reference memory scores (number of nose pokes to baited holes/total number of nose pokes) for the training phase of the experiment, prior to injections, in the holeboard. During this phase there were 5 daily trials with 4 of the 16 holes baited in a fixed baiting pattern (Figure 1).
Figure 3.
Means ± standard errors for reference memory scores (number of nose pokes to baited holes/total number of nose pokes) in rewarded versus non-rewarded trials in saline administered subjects for the discrimination training phase of the experiment. Saline-administered rats did not demonstrate a strong discrimination between rewarded and non-rewarded trials.
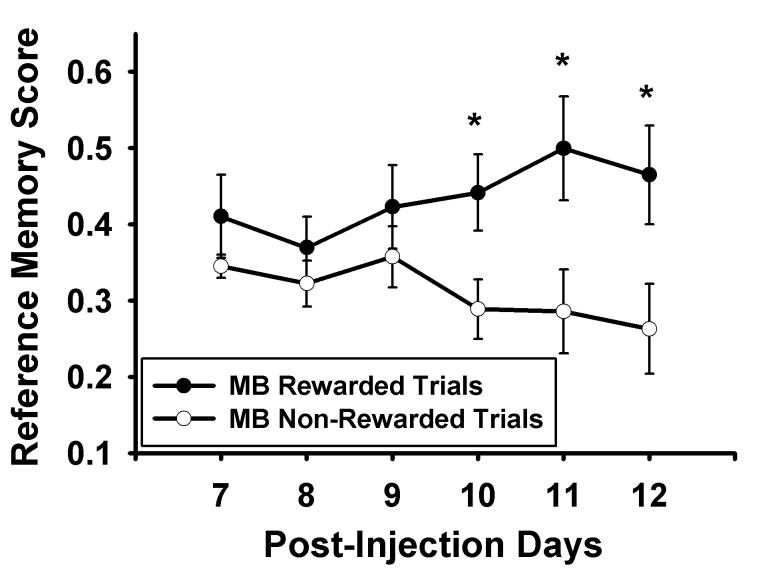
In contrast, MB-treated rats had better overall mean performance, F(1,14) = 8.707, p = 0.011, as well as reliably increased discrimination between rewarded and non-rewarded trials on Days 10-12 (Figure 4). A within-subjects repeated measures ANOVA found a significant interaction between rewarded and non-rewarded trials by day in the MB group, F(1,5) = 2.680; p = 0.028. On Days 10, 11, and 12, independent t-tests followed by the sharper Bonferroni correction (Hochberg 1988) were used to compare rewarded to non-rewarded reference memory scores in MB-treated subjects, and they revealed a significant difference on Day 10 (t(14) = 3.038, corrected p = 0.018), Day 11 (t(14) = 3.080, corrected p = 0.024), and Day 12 (t(14) = 2.585, corrected p = 0.022). The MB effect was specific to the discrimination learning rather than non-specific activity because there were no significant differences found in total number of nose pokes between MB-treated and control groups in rewarded or non-rewarded trials.
Figure 4.
Means ± standard errors for reference memory scores (number of nose pokes to baited holes/total number of nose pokes) in rewarded versus non-rewarded trials in methylene blue-treated subjects for the discrimination training phase of the experiment. Methylene blue-administered rats reliably discriminated between rewarded and non-rewarded trials on days 10-12 of the experiment. *p < .05
3.2. Biochemical results
Following 3 daily injections of 1 mg/kg MB, cytochrome c oxidation was significantly different in brain homogenates from MB-treated vs. saline-treated rats (t(1,7) = 5.976, p = 0.044). Rats given MB showed a higher rate in cytochrome c oxidation (111.84 ± 15.5 μmol/min/g) than saline-injected animals (65.82 ± 7.8 μmol/min/g). The increase in activity in the MB group was approximately 70% higher than saline-treated rats.
4. Discussion
Methylene blue (MB) is a brain metabolic enhancer that has been demonstrated to improve memory retention when given post-training in low doses in a variety of simple appetitive and aversive learning tasks in rats (Callaway et al. 2002, 2004; Gonzalez-Lima and Bruchey, 2004; Martinez Jr et al. 1978; Riha et al. 2005). The present results indicate that MB could also facilitate more complex learning such as in discrimination tasks that require repeated days of training. MB enhanced memory retention of discrimination learning of rewarded versus non-rewarded trials in the holeboard maze food search task. During discrimination training, subjects treated with MB reliably discriminated between rewarded and non-rewarded trials while the saline-administered subjects did not. This was indicated by a greater number of correct responses on rewarded trials than non-rewarded trials in the MB group. These effects were seen in the last three days of the study, which is consistent with other behavioral data conducted in our laboratory showing that repeated memory consolidation periods with MB treatment may improve memory retention (Gonzalez-Lima and Bruchey, 2004). These results agree with Callaway et al.’s (2002, 2004) findings that MB improves spatial memory in the holeboard, and extend these findings to a more demanding between-days discrimination paradigm. We concluded that MB has positive effects on discrimination learning, which is consistent with previous reports in simpler learning tasks, and suggest that MB is a memory enhancing compound with applications for both simple and discriminative learning tasks.
Since no significant differences were found in the total number of nose pokes between MB-treated and saline-administered groups in either rewarded or non-rewarded trials, the effects of MB observed in this study were due to greater accuracy in discrimination learning in the MB-treated group and were not a confound from effects on motivation or general activity during the task. Studies show that the memory retention effects of low dose MB cannot be attributed to alterations in locomotor activity, motivation, reward value, feeding, or fearfulness (Gonzalez-Lima and Bruchey, 2004; Riha et al. 2005). Despite these reports, and in order to control for any state-dependent learning or side-effects that may occur by administering MB pre-training, administration occurred following training in these behavioral studies. MB enhances memory of the events preceding its administration (Martinez Jr et al. 1978), so the time of injection must follow the target memory task.
The results from the biochemical brain study indicated that repeated MB administration enhanced cytochrome oxidase activity very effectively, a finding that supports previous studies indicating that MB appears to work as a brain metabolic enhancer by increasing cytochrome oxidase activity (Callaway et al. 2002, 2004; Gonzalez-Lima and Bruchey, 2004). Cytochrome oxidase is the terminal enzyme of the electron transport chain, and is tightly coupled to neuronal metabolism and ATP production (Wong-Riley 1989). Electrons can be donated from reduced MB to enter the electron transport chain, resulting in enzyme induction of cytochrome oxidase (Scott and Hunter Jr., 1966; Visarius et al. 1997). By increasing cytochrome oxidase activity after three days of administration, MB can enhance the amount of ATP available in brain cells in order to improve their overall mnemonic capacity during discrimination learning.
MB has a half-life of 5-6.5 hours (Peter et al. 2000), so it is unlikely that the increase in memory for the non-rewarded versus rewarded alternation observed more than 24 hours after the last injection of MB reflects a continued direct action of the drug. It is most likely due to secondary brain metabolic effects occurring at a critical time in memory consolidation when MB was on board. MB administration leads to cytochrome oxidase enzymatic induction, enhancing oxidative metabolic capacity in the brain during the post-training period of memory processing (Callaway et al. 2004). MB increases cytochrome oxidase enzymatic activity in a use-dependent manner. Brain regions with the highest metabolic demand during memory consolidation in a particular task show the largest increases in cytochrome oxidase activity (Gonzalez-Lima and Bruchey 2004). Through induction of cytochrome oxidase, post-training MB administration may increase the metabolic capacity of memory-related brain regions making them more functional when cytochrome oxidase has the highest demand. In this way memory facilitation may build up over days even though pharmacokinetic studies show that MB is no longer present in the circulation from one day to the next (Peter et al. 2000). Together, these and previous studies indicate that MB is a promising drug for memory improvement when administered post-training and in low doses, and it could provide a potent pharmacologic resource for those looking for memory-enhancing compounds with little to no side effects (Naylor et al. 1986, 1987).
Acknowledgments
Dr. K. M. Wrubel was supported by a Society for Neuroscience Minority Fellowship, M. A. Maldonado by NIH training grant T32 MH65728 and Prof. F. Gonzalez-Lima by NIH grant R01 NS37755. We thank Dr. Douglas Barrett for his programming assistance. Part of this work was submitted by Dr. K. M. Wrubel in partial fulfillment of the requirements for a Ph.D. degree at the University of Texas at Austin.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Callaway NL, Riha PD, Wrubel KM, McCollum D, Gonzalez-Lima F. Methylene blue restores spatial memory retention impaired by an inhibitor of cytochrome oxidase in rats. Neurosci Lett. 2002;332:83–86. doi: 10.1016/s0304-3940(02)00827-3. [DOI] [PubMed] [Google Scholar]
- Callaway NL, Riha PD, Bruchey AK, Munshi Z, Gonzalez-Lima F. Methylene blue improves brain oxidative metabolism and memory retention in rats. Pharmacol Biochem Behav. 2004;77:175–181. doi: 10.1016/j.pbb.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Lima F, Bruchey AK. Extinction memory improvement by the metabolic enhancer methylene blue. Learn Mem. 2004;11:633–640. doi: 10.1101/lm.82404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Lima F, Cada A. Quantitative histochemistry of cytochrome oxidase activity: Theory, methods, and regional brain vulnerability. In: Gonzalez-Lima F, editor. Cytochrome oxidase in neuronal metabolism and Alzheimer’s disease. Plenum; New York: 1998. pp. 55–90. [Google Scholar]
- Hochberg Y. A sharper bonferroni procedure for multiple tests of significance. Biometrika. 1988;75:800–802. [Google Scholar]
- Hu D, Xu X, Gonzalez-Lima F. Hippocampal cytochrome oxidase activity of rats in easy and difficult visual discrimination learning. Int J Neurosci. 2005;115:595–611. doi: 10.1080/00207450590523440. [DOI] [PubMed] [Google Scholar]
- Hu D, Xu X, Gonzalez-Lima F. Vicarious trial-and-error behavior and hippocampal cytochrome oxidase activity during Y-maze discrimination learning in the rat. Int J Neurosci. 2006;116:265–80. doi: 10.1080/00207450500403108. [DOI] [PubMed] [Google Scholar]
- Lilliquist MW, Nair HP, Gonzalez-Lima F, Amsel A. Extinction after regular and irregular reward schedules in the infant rat: influence of age and training duration. Dev Psychobiol. 1999;34:57–70. [PubMed] [Google Scholar]
- Martinez JL, Jr, Jensen RA, Vasquez BJ, McGuinness T, McGaugh JL. Methylene blue alters retention of inhibitory avoidance responses. Physiol Psychol. 1978;6:387–390. [Google Scholar]
- Nair HP, Gonzalez-Lima F. Extinction of behavior in infant rats: development of functional coupling between septal, hippocampal, and ventral tegmental regions. J Neurosci. 1999;19:8646–8655. doi: 10.1523/JNEUROSCI.19-19-08646.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naylor GJ, Martin B, Hopwood SE, Watson T. A two-year double-blind crossover trial of the prophylactic effect of methylene blue in manic-depressive psychosis. Biol Psychiatry. 1986;21:915–920. doi: 10.1016/0006-3223(86)90265-9. [DOI] [PubMed] [Google Scholar]
- Naylor GJ, Smith AHW, Connelly P. A controlled trial of methylene blue in severe depressive illness. Biol Psychiatry. 1987;22:657–659. doi: 10.1016/0006-3223(87)90194-6. [DOI] [PubMed] [Google Scholar]
- O’Leary JL, Petty J, Harris AB, Inukai J. Supravital staining of mammalian brain with intra-arterial methylene blue followed by pressurized oxygen. Stain Tech. 1968;43:197–201. doi: 10.3109/10520296809115068. [DOI] [PubMed] [Google Scholar]
- Peter C, Hongwan D, Kupfer A, Lauterburg BH. Pharmacokinetics and organ distribution of intravenous and oral methylene blue. Eur J Clin Pharmacol. 2000;56:247–250. doi: 10.1007/s002280000124. [DOI] [PubMed] [Google Scholar]
- Riha PD, Bruchey AK, Echevarria DJ, Gonzalez-Lima F. Memory facilitation by methylene blue: dose-dependent effect on behavior and brain oxygen consumption. Eur J Pharmacol. 2005;511:151–158. doi: 10.1016/j.ejphar.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Scott A, Hunter FE., Jr Support of thyroxine-induced swelling of liver mitochondria by generation of high energy intermediates at any one of three sites in electron transport. J Biol Chem. 1966;241:1060–1066. [PubMed] [Google Scholar]
- Sorgel F. The return of Ehrlich’s ‘Therapia magna sterilisans’ and other Ehrlich concepts?. Series of papers honoring Paul Ehrlich on the occasion of his 150th birthday. Chemother. 2004;50:6–10. doi: 10.1159/000077277. [DOI] [PubMed] [Google Scholar]
- Visarius TM, Stucki JW, Lauterburg BH. Stimulation of respiration by methylene blue in rat liver mitochondria. FEBS Lett. 1997;412:157–160. doi: 10.1016/s0014-5793(97)00767-9. [DOI] [PubMed] [Google Scholar]
- Wong-Riley MT. Cytochrome oxidase: an endogenous metabolic marker for neuronal activity. Trends Neurosci. 1989;12:94–101. doi: 10.1016/0166-2236(89)90165-3. [DOI] [PubMed] [Google Scholar]