Abstract
Some pathogens utilize unique routes to enter cells that may evade the intracellular barriers encountered by the typical clathrin-mediated endocytic pathway. Retrograde transport and caveolar uptake are among the better characterized pathways, as alternatives to clathrin-mediated endocytosis, that are known to facilitate entry of pathogens and potential delivery agents. Recent characterization of the trafficking mechanisms of prion proteins and certain bacteria may present new paradigms for strategizing improvements in therapeutic spread and retention of therapy. This review will provide an overview of such endocytic pathways, and discuss current and future possibilities in using these routes as a means to improve therapeutic delivery.
1. Introduction
Studies in gene therapy and drug targeting have brought to light the importance of identifying cellular and intracellular barriers to efficient delivery. Accordingly, a broad audience has been made aware in recent years of the characteristics of a typical trafficking pathway for many targeted therapeutics. Such a pathway is characterized by: receptor binding followed by cell entry via receptor-mediated endocytosis into clathrin-coated pits and vesicles, delivery to early endosomes, and passage through late endosomes/lysosomes where cargo degradation otherwise takes place [1], [2], [3]. Ligand–receptor pairs, viruses and other pathogens, as well as non-viral gene delivery vectors are known to enter cells by such routes. If a targeted therapeutic, such as a gene delivery vector, is to impart therapeutic efficacy, however, the degradative pathway must somehow be avoided.
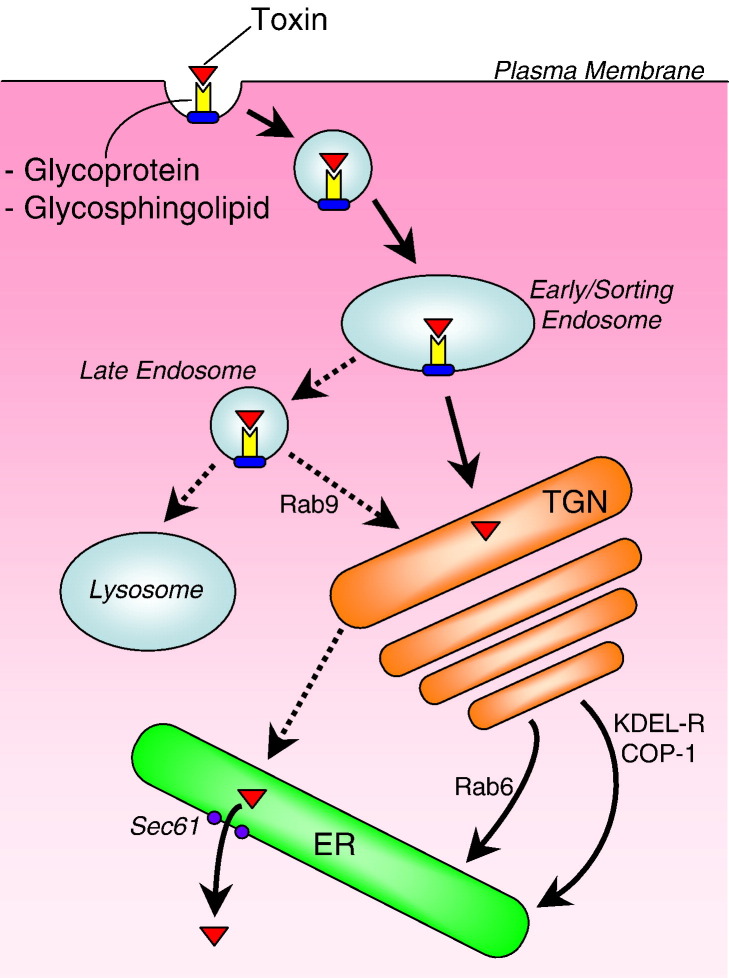
Endocytic pathways other than classical clathrin-mediated endocytosis targeted for the endosomal/lysosomal compartments have been better characterized in recent years. Such pathways may offer alternative uptake and trafficking routes for gene delivery vectors and targeted therapeutics that may avoid the barriers posed by the classical route. For example, the retrograde transport pathway, used by plant and bacterial toxins, facilitates endocytic trafficking from the cell surface to the Golgi, and from the Golgi to the endoplasmic reticulum (ER), in reverse of classical secretion [4], [5] (Fig. 1 ). These toxins can then make use of the cell's own protein auditing system to become transported to the cytoplasm where the toxic activity can take place.
Fig. 1.
Retrograde transport of plant and bacterial toxins. Initial binding to a cell-surface molecule triggers receptor-mediated endocytosis and delivery to early/sorting endosomes. Toxins may undergo delivery to the TGN from either early or late endosomes. From the TGN, toxins may transit through the Golgi cisternae and become transported to the ER through either a KDEL receptor-dependent or -independent pathway. Alternatively, transport may take place directly from the TGN to the ER. The toxin is displaced from the ER through a pore into the cytoplasm where the translation machinery can be accessed.
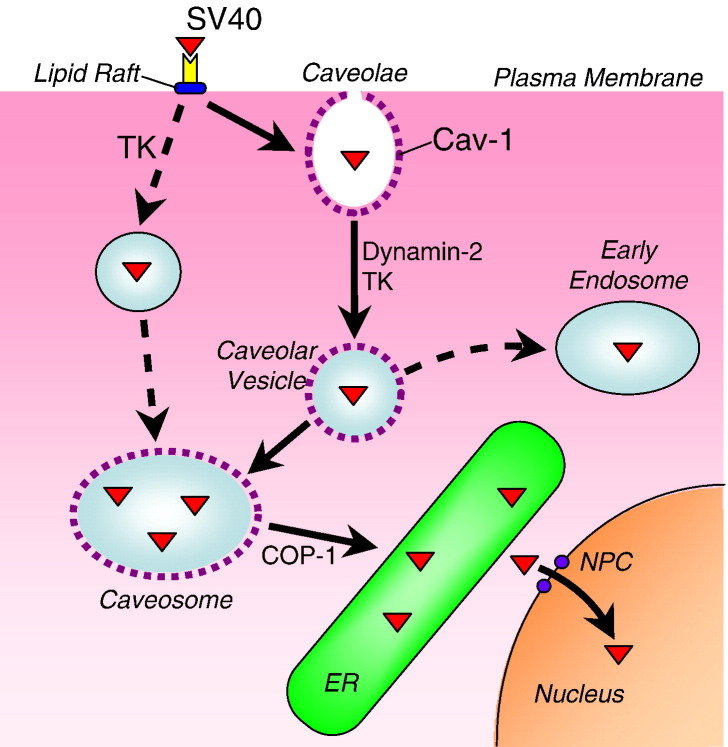
Endocytosis via caveolae has been well-studied, and the route by which SV40 and similar pathogens utilize caveolar uptake for infection has been characterized in recent years [6]. In this pathway, caveolar vesicles fuse with caveosomes, which facilitate prolonged survival of the pathogen in the cell before transit to the ER, from which nuclear entry can take place for viral replication (Fig. 3).
Fig. 3.
Trafficking via caveolae and caveosomes. SV40 bound to the cell surface can distribute to caveolae, which can pinch off to form a vesicle that is released from the cell surface and transported to Cav1-positive caveosomes. Alternatively, SV40 may directly internalize via lipid rafts and fuse with caveosomes. COP1-mediated transport facilitates delivery from caveosomes to the ER, from which SV40 possibly exits into the cytosol and enters the nucleus via nuclear pores.
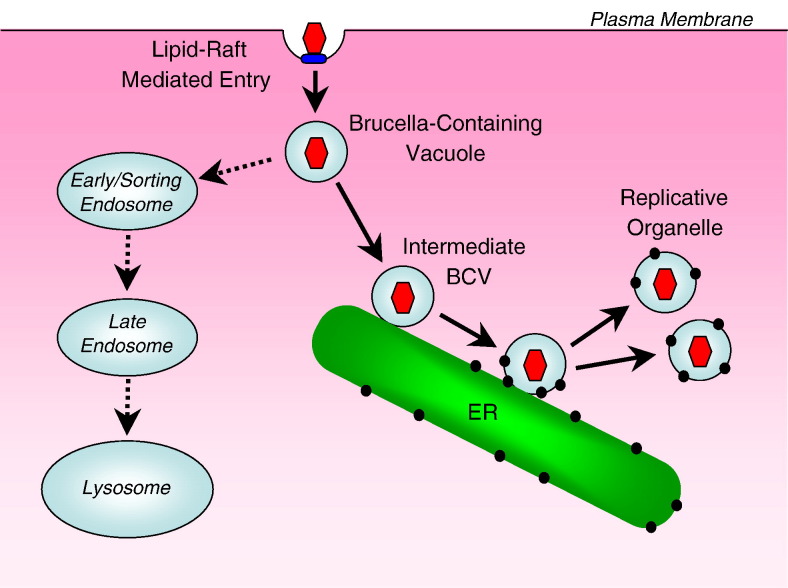
Formation of replication-competent vacuoles inside cells is a strategy used by Brucella and similar pathogens to gain long-term survival inside host cells [7]. Such a pathway is typified by endocytosis into pathogen-containing vacuoles, delivery to and interaction with the ER, followed by formation of an ER-derived replicative organelle (Fig. 4).
Fig. 4.
Vacuole and replicating organelle formation. Membrane-bound Brucella is internalized into a Brucella-containing vacuole (BCV), which can either fuse with early/sorting endosomes, or undergo maturation, which entails transit to the ER. Interactions with the ER can result in acquisition of ER markers, while the vacuole undergoes maturation to a replicative organelle.
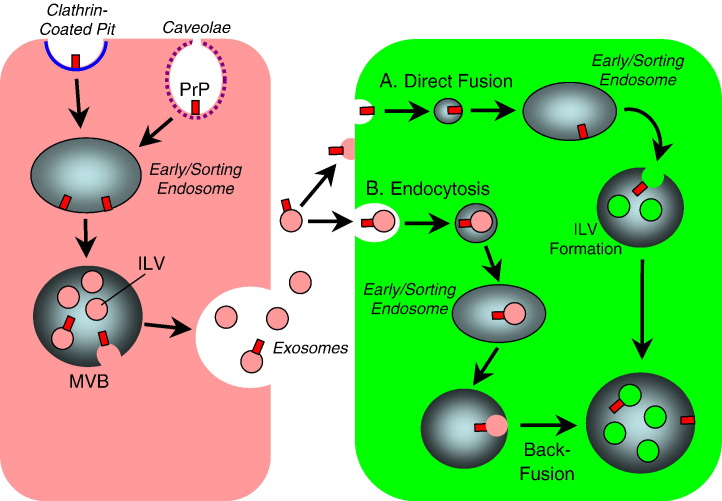
Endocytic pathways may also lead to effective delivery and spread to neighboring cells. The secretion of exosomes likely enables prion proteins to be transmitted from cell to cell [8], [9] (Fig. 2 ).
Fig. 2.
Exosome-mediated spread of prion protein. Prion protein (PrP) may undergo either clathrin or caveolae-mediated endocytosis into early/sorting endosomes which can sort to multivesicular bodies (MVBs), into which intraluminal vesicles (ILVs) containing cytosolic material bud off into the endosomal lumen. ILV formation results in GPI-attached proteins remaining anchored at the outer surface of the ILV. MVBs can fuse with either lysosomes or with the plasma membrane, releasing the ILVs from the cell surface, which are now known as exosomes. Exosomes can either directly fuse with the plasma membrane of an uninfected cell (shown in green) or undergo endocytic uptake. Direct fusion may result in delivery of PrP to the membrane of endosomal vesicles, which may undergo ILV budding and result in the location of PrP on the ILV outer surface. Alternatively, endocytosed exosomes may undergo back-fusion with the endosomal limiting membrane, resulting in PrP localizing to the endosomal membrane.
Why is studying these pathways important for therapeutic delivery? Delivery to the lysosomal compartment poses one major barrier to gene and drug delivery. The appeal of using viruses or viral components in targeted therapeutics is partly due to the capacity of endosomal escape, and thus avoidance of lysosomal degradation, by penetrating the membrane of the maturing vesicle before cargo delivery to the lysosome. Peptides derived from several types of pathogens have been used to accomplish the same [10]. Such peptides are thought to change conformation in response to the acidifying environment of the endosomal lumen and as a result, interact with the endosomal membrane by forming pores or destabilizing the lipid bilayer, thus affording vesicle escape.
In the event that a gene delivery vector escapes the endocytic vesicle, cytosolic factors still pose additional barriers. The crowded cytosolic milieu can prevent rapid vector motility to the nucleus [11] while cytosolic nucleases can degrade the DNA cargo [12].
“Alternative” endocytic pathways such as those described above may contribute toward improvements in therapeutic delivery by facilitating: the avoidance of lysosomal delivery and degradation; enhanced delivery to a target organelle (such as the Golgi, ER, or nucleus) or compartment (such as the cytoplasm); and enhanced long-term therapy, such as the formation of an extranuclear replicating organelle.
It is clear that there is more than one route for entering a cell and studies on a variety of pathogens show that alternative endocytic pathways have been cleverly hijacked to avoid a degradative fate and evade the cell's defenses. Here we will examine some of these pathways, which may serve as possible routes for improving therapeutic delivery.
2. Retrograde trafficking of plant and bacterial toxins
Plant toxins, such as ricin and abrin, and bacterial toxins, such as Shiga toxin (STx), cholera toxin (CTx), and Pseudomonas exotoxin A (PEx), enter cells following a route in reverse of the classic secretory pathway [13]. Classical secretion is characterized by transport of newly synthesized proteins from the ER to the Golgi, followed by budding of vesiculated cargo from the trans-Golgi network, vesicle sorting in the cytoplasm, and fusion with the plasma membrane. While many types of viruses and similar pathogens can directly access the cytoplasm from the endocytic vesicle after endocytosis, toxins follow a retrograde route of classical secretion to accomplish the same.
Certain plant and bacterial toxins share a common structure comprised of two major domains or chains, termed A and B. The A chain forms the catalytic or toxic domain, whereas the B chain is responsible for cell binding [14]. The pro-form of the protein is non-toxic, whereas proteolytic cleavage inside the cell activates the toxin after cell entry. In PEx and ricin, this cleavage releases the A subunit, whereas in CTx and STx, the A subunit is separated into A1 and A2 chains.
The targets of toxic activity are specific components of the protein synthesis machinery. The A chains of Stx and ricin are RNA N-glycosidases that remove a conserved adenine residue from 28S rRNA that is the site of interaction with elongation factor (EF)-2 ternary complex [15], [16]. The effect is inhibition of protein synthesis, leading to cell death. The CTx A chain is an ADP-ribosyltransferase, that modifies the heterotrimeric G protein Gs-α to activate adenylyl cyclase [17], inducing intestinal chloride secretion [18]. The Pseudomonas exotoxin A chain ADP-ribosylates EF-2, preventing protein synthesis and leading to cell death [19]. As toxic activity requires interaction with protein synthesis molecules, these toxins depend on delivery to the cytoplasm to access this machinery.
2.1. Mechanism of toxin entry and trafficking
Consistent with most pathogen infection mechanisms, toxin cell entry is enabled by binding to a cell-surface molecule, thus triggering endocytic uptake. Infection by STx and CTx is initiated by B chain binding to host cell membrane glycolipids. STx binds the trisaccharide domain of globotriaosylceramide (Gb3/CD77) [20], [21], [22], whereas CTx binds the ganglioside, GM1 [23], [24]. The PEx B chain binds α2-macroglobulin receptor/low-density lipoprotein receptor-related protein [25]. The ricin B chain is a lectin that binds β1-4 linked galactosides, which are displayed on a wide range of cell-surface glycoproteins and glycolipids, thus owing to the promiscuity of ricin [26].
Cell-surface binding triggers internalization of the toxins into endocytic vesicles, which undergo lumen acidification as the vesicles mature to late endosomes (Fig. 1). Typically, cargo of late endosomes would become degraded due to vesicle fusion with lysosomes, which contain proteolytic enzymes. Vesiculated toxins evade this fate by transit to the TGN from either early or late endosomes (Fig. 1). These pathways are dependent, at least in part, on lipid association, certain Rabs, and specific vesicle and TGN receptors known as SNAREs (soluble N-ethylmaleimide-sensitive fusion attachment protein receptors).
Association with lipid rich membrane domains is a common mode of cell uptake, and accordingly, STx and CTx associate with detergent resistant membrane microdomains (DRMs) [27] and undergo transport from early endosomes. PEx, on the other hand, undergoes lipid-independent transport from late endosomes to the TGN [28]. While the late endosome pathway used by PEx is dependent upon the small GTPase, Rab9 [29], transport from early endosomes to the TGN is a Rab9-independent route used by CTx, STx, and ricin [30].
Transport of cargo from endocytic vesicles to target organelles entails the fusion of vesicle and target membranes, which requires interaction of specific vesicle and target membrane SNAREs (or v-SNAREs and t-SNAREs, respectively). Correspondingly, early endosome to TGN, and late endosome to TGN pathways depend on the contributing SNAREs, as each route is characterized by its own separate v-SNAREs and t-SNAREs [31], [32].
Once vesiculated toxins reach the TGN, the cargo is transported to the endoplasmic reticulum (ER) via several routes (Fig. 1). One is through the Golgi cisternae by interaction with the KDEL receptor, which cycles between the TGN and ER in a coatamer protein complex (COP-1)-dependent manner [33], [34], [35]. The COP1 protein complex, which coats vesicles budding from the Golgi apparatus, can sort vesiculated cargo based on interactions with the cytoplasmic domains of membrane proteins. The KDEL receptor recognizes and binds KDEL motifs on cargo proteins, and is responsible for retrieving escaped ER proteins from the Golgi. PEx contains a KDEL-like sequence that is exposed after A chain release by furin cleavage in early/recycling endosomes [28], [36], [37], [38], [39]. The lipid-sorted pathway, used by STx, is both KDEL receptor and COP1-independent, and controlled by Rab6 [40], [41], [42], [43]. PEx may also use this pathway [28]. A third poorly characterized pathway bypasses the Golgi cisternae and instead transports cargo directly from the TGN to the ER in a KDEL receptor and COP1-independent manner. CTx uses this pathway, despite the existence of a KDEL motif in its peptide sequence [44]. It is possible that this motif functions to retain CTx in the ER after delivery, and prevent possible anterograde transport to the Golgi.
Ricin may utilize all 3 pathways. While ricin lacks a KDEL sequence, evidence shows that it can bind the chaperone, calreticulin, which has a KDEL motif, and undergoes COP1-dependent trafficking to ER [45]. Ricin can elicit cell death when both the classical COP1-dependent and Rab6-dependent pathways are inhibited, suggesting that ricin can bypass Golgi stacks along a similar pathway as CTx [46]. As ricin can bind glycolipids containing a terminal galactose, it may also follow a lipid-sorting pathway.
Once the toxins have reached the ER, it is thought that the cytoplasm is accessed by taking advantage of the protein auditing system in the ER known as ERAD (ER-associated protein degradation). This mechanism eliminates misfolded proteins from the ER by discard into the cytoplasm through a pore known as the Sec61 translocon (now termed a dislocon) [47], [48] (Fig. 1). Typically, such proteins are ubiquitinated and targeted to the proteasome for degradation. However, toxins contain abnormally low lysine content, thus are poor substrates for ubiquitination [49], and hence are spared from degradation but survive in the cytoplasm to access the protein synthesis machinery.
2.2. Retrograde trafficking for cytosolic delivery of therapeutics?
Similar types of bacterial toxins, such as diphtheria toxin, can directly penetrate from the endosomal membrane into the cytosol, thus avoiding the retrograde route altogether [4]. This feature has been utilized for the delivery of exogenous genes [50] and peptides [51], [52] into the cytoplasm. Gene delivery conjugates have also been produced and tested that make use of components from toxins, like PEx, that undergo retrograde trafficking. For example, multidomain fusion proteins containing the translocation domain of PEx have been used to deliver genes into the cytosol [53], [54]. Whether these conjugates actually trafficked in similar fashion to wild-type PEx is unknown, though it appears that the intention of the molecular design was to breach the endosomal membrane. Shiga toxin and cholera toxin, which also enter the cytoplasm from the ER, have been tested as gene transfer agents [55], [56], [57], [58], though, like PEx derived vectors, it is not clear whether these agents traffic as the wild-type toxins when used for gene delivery.
Protein toxins have also been used to generate specific cytotoxic T lymphocyte (CTL) activation against certain epitopes [59], [60]. Delivery of antigenic peptides by fusion to modified toxins has facilitated cytosolic entry of antigens, which can then be degraded in the proteasome and displayed on MHC class I molecules to prime a CTL response. This approach to generating a new type of vaccine has been used to deliver epitopes via PEx [61], STx [62], and other types of toxins [14]. As the molecular players of the toxin/retrograde trafficking pathway are better characterized, it may be possible to utilize this route as a means to target compounds to the other specific organelles contributing to this pathway. One could envisage the design of new molecules that can interact with target cells similarly to the toxins discussed here, and mimic the toxin trafficking pathway to deliver peptides specifically to the Golgi or ER to, perhaps, correct a defect, or elicit toxicity for the treatment of cancer. Moreover, given its avoidance of the lysosome, perhaps this route could be of better use for gene delivery and enable greater survival of gene therapy vectors after target cell entry.
3. Prion protein trafficking and intercellular delivery
The cellular prion protein (PrPc) is a glycosylphosphatidylinositol (GPI)-anchored protein that is ubiquitously expressed, though found at higher levels in neurons, some non-neuronal tissue, and immune cells [63], [64]. The function of PrPc remains unclear, but is thought to contribute to: copper and/or zinc ion transport or metabolism, protection from oxidative stress, cellular signaling, membrane excitability and synaptic transmission, apoptosis, and neurite outgrowth. The diseases associated with prions occur when PrPc undergoes conversion to a scrapie form (PrPsc) [65], [66], resulting from the transconformation of an α-helix to β-sheet-rich structure. PrPsc can seed further conversion reactions, thus greatly increasing the rate of transconformation [67]. The resulting molecules can oligomerize into an amyloid fibril, and acquire the tendency to form amyloid deposits in brain tissue.
In humans, 15% of prion diseases are inherited, due to mutation in the prion protein gene. The infectious form, which causes Kuru and Creutzfelt–Jakob disease in humans, scrapie in sheep, and bovine spongiform encephalopathy in cattle, is thought to enter the host through the gastrointestinal tract and become acquired by peripheral nerves and lymphoid tissue where replication takes place [68], [69]. Invasion of the central nervous system is likely due to transfer via phagocytic mononuclear cells [70], [71]. The transfer of the infectious agent from cell to cell is hypothesized to occur via exosomes [72], which are vesicles that are secreted from the cell surface.
3.1. Mechanism of intracellular and intercellular transport
After synthesis, PrPc is secreted to the cell surface where its GPI anchor is inserted at plasma membrane lipid rafts [73], [74], which are domains within the lipid bilayer exhibiting a more ordered assembly of specific lipids (usually glycosphingolipids and cholesterol) compared to surrounding plasma membrane [75]. PrPc can constitutively endocytose via either clathrin-coated vesicles or caveolae (Fig. 2), likely depending on cell type or lipid microenvironment [74]. Once internalized, PrPc traffics through late endosomes/lysosomes, with a steady state fraction localized to multivesicular bodies (MVBs) in neurons, brain, and non-neuronal cells [76], [77], [78]. MVBs are formed by the pinching off of cytosol-filled, or intraluminal vesicles (ILVs) into the endosomal lumen [79], [80] (Fig. 2). The sorting of cargo into ILVs involves at least 18 proteins and is a tightly regulated process [81]. Among the cellular factors contributing to this process is the Endosomal Sorting Complex Required for Transport (ESCRT) machinery, which are cytosolic proteins that selectively sort cargo to ILVs [81]. In this process, ubiquitinated cargo proteins are recognized by Hrs complex (or ESCRT-0) proteins, which then recruit Tsg101 and the ESCRT-I complex, that also recognize ubiquitinated cargo. Tsg101 then recruits ESCRT-III via ESCRT-II or Alix, which together sequester cargo proteins into the inward-budding ILVs.
MVBs can either fuse with the lysosome or with the plasma membrane. Plasma membrane fusion enables the release of ILVs (now known as exosomes) extracellularly (Fig. 2). Exosomes may serve as a mode of intercellular communication, a means to discard proteins, and may be exploited by pathogens for disease transmission [82]. Both PrPsc and PrPc have been detected in cell culture supernatants, associated with secreted exosomes [9]. These exosomes elicited conversion of endogenous PrPc to PrPsc when incubated with naïve host cells [72], and caused acute typical neuropathology when inoculated into mice [9]. These findings suggest that exosomes may facilitate transfer of infectious prion agent.
Once released, exosomes may transport associated cargo to remote as well as neighboring cells, which likely explains how prions may be delivered to sites distant from the site of introduction. Transfer of the protein to recipient cells is thought to take place by interaction of exosomes with recipient cell membranes through two possible types of mechanisms (Fig. 2). One mechanism is the direct fusion of exosomes with the plasma membrane, thus transferring contents of exosomal membranes to the plasma membrane. This has been suggested by the observation that recipient cells receive incoming PrPsc into recipient raft domains, and exchange of membrane components can take place between exosomes and recipient cells [83]. A more likely mechanism, however, is that exosomes undergo endocytic uptake into recipient cells, then back-fuse with the limiting membrane of the endocytic vesicle, thus transferring exosomal membrane contents. Exosomal delivery of transconformation activity is supported by this mechanism, as the conversion process is thought to occur at low pH [84], [85].
3.2. Exosomes for therapeutic spread?
Gene therapy treatment of solid tumors relies on efficient delivery to as many cells as possible to obtain the most potent level of gene expression and potential tumor ablation. However, delivery of a gene to 100% of cells in a tumor may be an improbable task. Expression of a gene product that can somehow spread a therapeutic effect to neighboring cells is a more feasible approach. Thus, the use of the herpes simplex virus thymidine kinase (HSV-TK) gene as a therapeutic transgene has sustained great appeal in gene therapy due to the ‘bystander effect’ caused by the toxic enzyme product [86]. This effect describes the ability of the HSV-TK enzyme product, triphosphorylated ganciclovir, to spread to neighboring cells via gap junctions within the solid tumor, whereas the substrate has no such effect on cells. More recently, attempts have been made to enhance this effect by producing TK as a recombinant fusion to the VP22 protein [87], which has an ability to become secreted from the expressing cell and taken up by neighboring cells through a mechanism that remains unclear [88], [89]. The capacity of secreted exosomes from a ‘producer’ cell to be taken up by neighboring as well as remote cell targets could be a potent means of delivering the most efficient levels of a therapeutic to cell targets. One could envisage the ability to modify such exosomes with a membrane bound targeting ligand to perhaps limit the spread of a therapeutic to surrounding tumor tissue while at the same time target remote, metastatic tumors. Such modified exosomes containing a therapeutic protein or compound could efficiently deliver the therapy into recipient cells by fusion of the exosomal membrane with recipient cell membranes, thus releasing the product directly into the target cells. Further characterization of the pathogenic and cellular factors contributing to this process would determine whether this approach to therapeutic dissemination could be a feasible option in the future.
4. SV40 trafficking via caveolae and caveosomes
Caveolae are flask-shaped invaginations at the plasma membrane [90] characterized by a coat comprised mostly of caveolin-1 (Cav1) [91], [92], which is a palmitylated, cholesterol-binding protein [93], [94]. Caveolae regulate several different signaling cascades, thus caveolin defects can contribute to a broad range of diseases, including cancer, cardiovascular disease, diabetes, atherosclerosis, pulmonary fibrosis, and muscular dystrophies [95]. Certain mammalian viruses, such as polyomaviruses, influenza viruses, coronaviruses, and echovirus, use caveolae-mediated transport from the cell surface to enter cells [96], [97], [98], [99], [100]. The trafficking of simian virus 40 (SV40), a non-enveloped DNA virus of the papovavirus family, has been most extensively studied, and follows a pathway of caveolae-mediated transport that facilitates nuclear delivery of the virus while avoiding the endosomal/lysosomal degradation route [101], [102], [103], [104].
4.1. Trafficking via caveolae and caveosomes
SV40 binds two receptors. One is ganglioside GM1, located in lipid rafts [105], and the other is major histocompatibility (MHC) class I [106]. Virus binding induces receptor clustering, and sequestration of lipid rafts and associated receptor/virus complexes into caveolae [107], [108] (Fig. 3 ). Binding also induces a cascade of signaling events, including tyrosine kinase phosphorylation and protein kinase C activation [101], [109], which contribute to caveolar formation and endocytosis. One downstream effect of signaling is actin depolymerization, which facilitates caveolar internalization, and recruitment of dynamin-2 to pinch off the caveolar neck and release caveolar vesicles [104], which some have termed ‘cavicles’ [110]. MHC class I molecule is not endocytosed with the virus [107].
After release from the cell surface, caveolar vesicles require intact microtubules to traffic within the cell [110], suggesting that dynein motors may contribute to motility. These vesicles may fuse with endosomes from the clathrin-mediated pathway [111], or with caveosomes [101], which are Cav1-positive, pH neutral compartments rich in cholesterol and glycosphingolipids [101] (Fig. 3). In non-infected cells, these compartments are likely to serve as intermediate depots for transport of sphingolipids and GPI-linked proteins from the plasma membrane to the Golgi apparatus [112], [113]. After delivery to caveosomes, SV40 can be retained in these compartments for several hours. This contrasts with findings from uninfected cells showing that caveosomes may mediate receptor turnover [114], [115], and suggests that delivery to and function of caveosomes depends on the protein cargo.
SV40-containing caveosomes are sorted by Cav1-negative carriers that traffic along microtubules to the ER [101], [116] (Fig. 3). COPI and COPII-coated carrier vesicles may contribute to this process. It is thought that SV40 somehow penetrates into the cytosol after delivery to the ER, from which nuclear entry is gained via the nuclear pore complex [117] (Fig. 3). In an alternative and more rapid endocytic pathway, SV40 may be directly internalized via lipid rafts in a dynamin-2-independent manner and vesiculate into Cav1-negative organelles [102]. This pathway was identified because SV40 could infect caveolin-1 knock-out mouse cells.
The molecular mechanisms contributing to caveolar-mediated trafficking remain to be fully characterized. Even the role of caveolin is unclear. Expression of Cav1 in cells lacking caveolae is sufficient to generate caveolar formation [118], whereas overexpression of Cav1 can inhibit this [119]. It has been proposed that Cav1 stabilizes caveolar invaginations whereas Cav1-negative intermediates can rapidly bud from the cell membrane and endocytose [115]. This may partly explain findings using green fluorescent protein (GFP)-tagged Cav1 showing that a large pool of immobile caveolin exists in cultured cells [120]. Such reports in addition to the SV40 trafficking pathway have suggested that caveolae mediate slow (up to 1–2h) endocytosis that requires sequestration of cargo at the cell surface before internalization [101]. This contrasts with the rapid endothelial transcytosis mediated by caveolae in vivo that has been observed using specific targeted nanoparticles [121]. These studies demonstrated that in vivo circulating particles targeted to caveolae rapidly crossed the endothelium and underlying basement membrane, and accumulated into the interstitial space at lung tissues within minutes. The contrasting findings of the latter and former studies may depend on whether the studies are being performed in vivo or in cultured cells, as well as the type of probe being used.
4.2. Caveolar uptake to avoid lysosomal degradation of therapeutics?
Trafficking studies on non-viral gene delivery vectors have shown that such vectors may enter the same cells using multiple cell entry routes [122], [123]. Some of these routes may support delivery to the degradative pathway, while the caveolar route used by SV40 may not only spare cargo from degradation but enable prolonged residence in the cell for a period of time before routing to the ER. Given that cellular proteins, such as transforming growth factor β receptor (TGFβ-R), elicit receptor signaling when internalized via clathrin-coated pits whereas caveosome uptake promotes receptor turnover [114], it appears that using caveosomes as a means to evade degradation would depend on modulation by the vesicle cargo itself. In this regard, further studies characterizing the molecular interactions between pathogens like SV40 and the host cell that enable pathogen survival in caveosomes would be useful.
It has been shown that particles resembling non-viral gene delivery vectors can utilize either clathrin or caveolar-mediated uptake into cells, depending on particle size [124]. While the same vector or protein may enter the same cells via different routes, some routes appear to support gene transfer while others do not. Accumulating studies on vector trafficking do not show, however, that one route consistently supports gene transfer. For example, recent studies have shown that certain polyplexes entered cultured cells by both clathrin and caveolar-mediated pathways, yet the latter pathway preferentially led to gene expression whereas inhibition of the former pathway had no effect [125]. The reverse was observed for histidinylated polyplexes, whereby clathrin-mediated endocytosis appeared to preferentially support gene expression even though the vector entered cells by both clathrin and non-clathrin-mediated pathways [126].
Recent studies showing that caveolae mediate the rapid transcytosis of targeted particles across the endothelium, thus enabling access to deep tissue cells in vivo [121], demonstrate that it is possible to overcome the endothelial barrier and target tissue from the circulation. While it has been suggested that tumor vasculature can be ‘leaky’, and thus facilitate accumulation of therapeutics into a solid tumor, normal tissue, containing different types of endothelium, may not be as easily accessible. Thus, targeting to endothelial cell-surface proteins for the transport of molecules across the vessel wall would be an important strategy for drug and gene delivery.
5. Vacuole and replicative organelle formation by Brucella
Some pathogens acquire prolonged survival in a host cell by forming a vacuole in which the pathogen resides and evades the host defenses. Among the bacterial pathogens that share a similar vacuole trafficking mechanism are: Shigella, Listeria, Mycobacterium, Salmonella, Legionella, Francisella, and Brucella [127], [128]. The trafficking of Brucella is among the most extensively studied, and characterized by endocytosis into a special vacuole that interacts with the ER and forms a replicative organelle in which the bacterium establishes long-term survival. Brucella replicates inside infected host cells, such as macrophages [129], and causes brucellosis, which can affect a broad range of mammals, including livestock and humans [130]. Human brucellosis can be a chronic and debilitating disease, and the long-term survival of Brucella in host cells contributes to disease chronicity. Chlamydia also forms pathogen-containing vacuoles after cell entry. C. pneunomiae (Cpn) is associated with respiratory tract infections, including pneumonia, asthma, bronchitis, sinusitis, and sarcoidosis [131].
5.1. Brucella trafficking
Brucella infection is initiated by binding of bacterial surface-exposed Hsp60 to the cellular prion protein, PrPc [132]. While PrPc may typically undergo clathrin-mediated endocytosis, Brucella is somehow able to modulate its own endocytic uptake and avoid targeting to the lysosomal degradation pathway [133]. Instead, it is thought that uptake occurs via lipid raft endocytosis, as lipid raft disruption affects cell entry, short-term survival and replication of Brucella suis and Brucella abortus [134].
Endocytic uptake results in the formation of a Brucella-containing vacuole (BCV) (Fig. 4 ), which can interact with early endosomes but avoids fusion with late endosomes/lysosomes, as observed by the absence of GTPase Rab7 and other late endosomal markers from the vacuole [135], [136]. While BCV can acquire LAMP-1, it is argued that other compartments in addition to late endosomes/lysosomes can harbor the LAMP-1 marker [137]. As the BCV vacuole matures, it undergoes acidification [138], and comes in close contact with the ER [129] (Fig. 4). The BCV interacts with the ER and forms an ER-derived replicative organelle, establishing long-term survival (Fig. 4). In epithelial cells, the replicative organelle is ER-derived, whereas in phagocytic cells, BCVs dock at the ER and acquire ER-specific markers via limited fusion events [129]. At this stage, the organelle becomes permissive for bacterial replication. Requirement for additional membrane output for replication is likely provided by further fusion interactions with the ER membrane. Studies on L. pneumophila show that biogenesis of the ER-derived organelle requires interception of COPI vesicular trafficking from the ER [139]. This is not true for Brucella, however, as blocking such trafficking has no effect on BCV maturation or replication [129].
The molecular mechanism of entry and survival is still unclear, though VirB protein has received much attention. The screening of Brucella survival mutants generated by transposon insertion identified the VirB operon [140], which encodes a type IV-related secretion system that typically secretes/exports nucleoprotein complexes or proteins [129], [141]. In contrast to wild-type Brucella, the VirB mutants undergo lipid raft-independent endocytosis, remain in immature vacuoles, can dock with the ER but do not sustain fusion with the ER, and are ultimately targeted to the degradative pathway (thus failing to reach a replicative niche). The relevance of VirB in endocytic entry is unclear, as a minority of ingested wild-type bacteria survive in the host cell, suggesting that the majority of internalized bacteria do not escape the degradative pathway [129], [142], [143]. Furthermore, studies in B. suis show that VirB expression takes place after cell entry [144]. VirB is likely involved in late events corresponding with ER fusion [129]. It is possible that VirB mediates the transport of bacterial effector molecules into the host cell that may modulate BCV maturation.
5.2. Vacuologenesis for extranuclear replication and retention of therapeutics?
Gene therapy may be administered as a temporary, short-term treatment for a disease, such as cancer, or as a long-term or permanent solution to replace or correct a defective gene. Long-term approaches have typically entailed the use of a viral vector with the capacity to integrate a gene of interest into the host genome, which can pose serious concerns, including the possibility that gene insertion can activate an oncogenic effect [145], [146], [147]. Alternatively, DNA vectors containing elements for extrachromosomal retention in host cells may support a long-term, though not permanent, solution for transgene delivery [148]. In the same vein, perhaps an extranuclear organelle, such as the type of vacuole providing a haven for pathogens like Brucella, may enable the long-term residence and retention of a gene therapy vector or drug in a target cell. Bacterial-like vectors have been considered for cancer therapy and gene therapy [149], [150], [151]. While the mechanism for the formation of pathogen-containing vacuoles and replicative organelles needs further characterization, studies so far appear to suggest that these intracellular bodies occur as a result of interaction between pathogen gene products and host cell factors. Further studies identifying the players in these interactions and associated functions may direct future efforts to design molecules that can mimic these dynamics and induce the formation of similar intracellular organelles. One could envisage such organelles as being useful in the long-term intracellular retention of therapeutic compounds or genes, whose release over time could be of more benefit in comparison to large, potentially toxic, bolus doses of therapeutic that may require numerous repeat doses to be effective.
6. Conclusion
In the development of drug and gene delivery, directing the entry and trafficking of therapeutics to specific intracellular pathways is a worthwhile consideration. While the typical clathrin-mediated endocytic pathway used by many gene therapy vectors and targeted therapeutics introduces common cellular barriers, alternative pathways such as those discussed here may provide a means to evade such barriers. Moreover, such pathways may facilitate delivery to specific organelles and cellular compartments. Future studies on the molecular mechanisms mediating pathogen trafficking through these pathways may enable design of new vectors and delivery agents with improved ability to direct therapy to desired intracellular targets.
Acknowledgments
Work in the author's laboratory is supported by grants from the NIH (RO1 CA102126 and R21 CA116014), the Susan G. Komen Breast Cancer Foundation (BCTR02-1194), the Department of Defense (BC050662), and the Donna and Jesse Garber Award. Many thanks to Hasmik Agadjanian, Jun Ma, Altan Rentsendorj, and Vinod Valluripalli for helpful discussions on this work, and to JC, DR, and MM-K for ongoing support.
Footnotes
This review is part of the Advanced Drug Delivery Reviews theme issue on “Organelle-Specific Targeting in Drug Delivery and Design".
References
- 1.Medina-Kauwe L.K., Xie J., Hamm-Alvarez S. Intracellular trafficking of nonviral vectors. Gene Ther. 2005;12:1734–1751. doi: 10.1038/sj.gt.3302592. [DOI] [PubMed] [Google Scholar]
- 2.Khalil I.A., Kogure K., Akita H., Harashima H. Uptake pathways and subsequent intracellular trafficking in nonviral gene delivery. Pharmacol. Rev. 2006;58:32–45. doi: 10.1124/pr.58.1.8. [DOI] [PubMed] [Google Scholar]
- 3.Mukherjee S., Ghosh R.N., Maxfield F.R. Endocytosis. Physiol. Rev. 1997;77:759–803. doi: 10.1152/physrev.1997.77.3.759. [DOI] [PubMed] [Google Scholar]
- 4.Sandvig K., van Deurs B. Delivery into cells: lessons learned from plant and bacterial toxins. Gene Ther. 2005;12:865–872. doi: 10.1038/sj.gt.3302525. [DOI] [PubMed] [Google Scholar]
- 5.Johannes L., Decaudin D. Protein toxins: intracellular trafficking for targeted therapy. Gene Ther. 2005;12:1360–1368. doi: 10.1038/sj.gt.3302557. [DOI] [PubMed] [Google Scholar]
- 6.Pelkmans L. Secrets of caveolae- and lipid raft-mediated endocytosis revealed by mammalian viruses. Biochim. Biophys Acta. 2005;1746:295–304. doi: 10.1016/j.bbamcr.2005.06.009. (Electronic publication 2005 Jul 2005) [DOI] [PubMed] [Google Scholar]
- 7.Celli J. Surviving inside a macrophage: the many ways of Brucella. Res. Microbiol. 2006;157:93–98. doi: 10.1016/j.resmic.2005.10.002. (Electronic publication 2005 Nov 2009) [DOI] [PubMed] [Google Scholar]
- 8.Fevrier B., Vilette D., Laude H., Raposo G. Exosomes: a bubble ride for prions? Traffic. 2005;6:10–17. doi: 10.1111/j.1600-0854.2004.00247.x. [DOI] [PubMed] [Google Scholar]
- 9.Porto-Carreiro I., Fevrier B., Paquet S., Vilette D., Raposo G. Prions and exosomes: from PrPc trafficking to PrPsc propagation. Blood Cells Mol. Dis. 2005;35:143–148. doi: 10.1016/j.bcmd.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 10.Wagner E. Application of membrane-active peptides for nonviral gene delivery. Adv. Drug Deliv. Rev. 1999;38:279–289. doi: 10.1016/s0169-409x(99)00033-2. [DOI] [PubMed] [Google Scholar]
- 11.Lukacs G.L., Haggie P., Seksek O., Lechardeur D., Freedman N., Verkman A.S. Size-dependent DNA mobility in cytoplasm and nucleus. J. Biol. Chem. 2000;275:1625–1629. doi: 10.1074/jbc.275.3.1625. [DOI] [PubMed] [Google Scholar]
- 12.Pollard H., Toumaniantz G., Amos J.L., Avet-Loiseau H., Guihard G., Behr J.P., Escande D. Ca2+-sensitive cytosolic nucleases prevent efficient delivery to the nucleus of injected plasmids. J. Gene Med. 2001;3:153–164. doi: 10.1002/jgm.160. [DOI] [PubMed] [Google Scholar]
- 13.Lord J.M., Roberts L.M. Toxin entry: retrograde transport through the secretory pathway. J. Cell. Biol. 1998;140:733–736. doi: 10.1083/jcb.140.4.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sandvig K., van Deurs B. Entry of ricin and Shiga toxin into cells: molecular mechanisms and medical perspectives. EMBO J. 2000;19:5943–5950. doi: 10.1093/emboj/19.22.5943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Endo Y., Tsurugi K. RNA N-glycosidase activity of ricin A-chain. Mechanism of action of the toxic lectin ricin on eukaryotic ribosomes. J. Biol. Chem. 1987;262:8128–8130. [PubMed] [Google Scholar]
- 16.Endo Y., Tsurugi K., Yutsudo T., Takeda Y., Ogasawara T., Igarashi K. Site of action of a Vero toxin (VT2) from Escherichia coli O157:H7 and of Shiga toxin on eukaryotic ribosomes. RNA N-glycosidase activity of the toxins. Eur. J. Biochem. 1988;171:45–50. doi: 10.1111/j.1432-1033.1988.tb13756.x. [DOI] [PubMed] [Google Scholar]
- 17.Moss J., Vaughan M. Mechanism of action of choleragen. Evidence for ADP-ribosyltransferase activity with arginine as an acceptor. J. Biol. Chem. 1977;252:2455–2457. [PubMed] [Google Scholar]
- 18.Kaper J.B., Morris J.G., Jr., Levine M.M. Cholera. Clin. Microbiol. Rev. 1995;8:48–86. doi: 10.1128/cmr.8.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iglewski B.H., Kabat D. NAD-dependent inhibition of protein synthesis by Pseudomonas aeruginosa toxin. Proc. Natl. Acad. Sci. U. S. A. 1975;72:2284–2288. doi: 10.1073/pnas.72.6.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Waddell T., Cohen A., Lingwood C.A. Induction of verotoxin sensitivity in receptor-deficient cell lines using the receptor glycolipid globotriosylceramide. Proc. Natl. Acad. Sci. U. S. A. 1990;87:7898–7901. doi: 10.1073/pnas.87.20.7898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jacewicz M., Clausen H., Nudelman E., Donohue-Rolfe A., Keusch G.T. Pathogenesis of shigella diarrhea. XI. Isolation of a shigella toxin-binding glycolipid from rabbit jejunum and HeLa cells and its identification as globotriaosylceramide. J. Exp. Med. 1986;163:1391–1404. doi: 10.1084/jem.163.6.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lindberg A.A., Brown J.E., Stromberg N., Westling-Ryd M., Schultz J.E., Karlsson K.A. Identification of the carbohydrate receptor for Shiga toxin produced by Shigella dysenteriae type 1. J. Biol. Chem. 1987;262:1779–1785. [PubMed] [Google Scholar]
- 23.Holmgren J., Lonnroth I., Svennerholm L. Tissue receptor for cholera exotoxin: postulated structure from studies with GM1 ganglioside and related glycolipids. Infect. Immun. 1973;8:208–214. doi: 10.1128/iai.8.2.208-214.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holmgren J., Lonnroth I., Svennerholm L. Fixation and inactivation of cholera toxin by GM1 ganglioside. Scand. J. Infect. Dis. 1973;5:77–78. doi: 10.3109/inf.1973.5.issue-1.15. [DOI] [PubMed] [Google Scholar]
- 25.Kounnas M.Z., Morris R.E., Thompson M.R., FitzGerald D.J., Strickland D.K., Saelinger C.B. The alpha 2-macroglobulin receptor/low density lipoprotein receptor-related protein binds and internalizes Pseudomonas exotoxin A. J. Biol. Chem. 1992;267:12420–12423. [PubMed] [Google Scholar]
- 26.Olsnes S., Pihl A. Toxic lectins and related proteins. In: van Heyningen S., editor. Molecular Action of Toxins and Viruses. Elsevier; Amsterdam: 1982. pp. 51–105. [Google Scholar]
- 27.Smith D.C., Sillence D.J., Falguieres T., Jarvis R.M., Johannes L., Lord J.M., Platt F.M., Roberts L.M. The association of Shiga-like toxin with detergent-resistant membranes is modulated by glucosylceramide and is an essential requirement in the endoplasmic reticulum for a cytotoxic effect. Mol. Biol. Cell. 2006;17:1375–1387. doi: 10.1091/mbc.E05-11-1035. (Electronic publication 2005 Dec 1328) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith D.C., Spooner R.A., Watson P.D., Murray J.L., Hodge T.W., Amessou M., Johannes L., Lord J.M., Roberts L.M. Internalized Pseudomonas exotoxin A can exploit multiple pathways to reach the endoplasmic reticulum. Traffic. 2006;7:379–393. doi: 10.1111/j.1600-0854.2006.00391.x. [DOI] [PubMed] [Google Scholar]
- 29.Lombardi D., Soldati T., Riederer M.A., Goda Y., Zerial M., Pfeffer S.R. Rab9 functions in transport between late endosomes and the trans Golgi network. EMBO J. 1993;12:677–682. doi: 10.1002/j.1460-2075.1993.tb05701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sandvig K., Grimmer S., Lauvrak S.U., Torgersen M.L., Skretting G., van Deurs B., Iversen T.G. Pathways followed by ricin and Shiga toxin into cells. Histochem. Cell Biol. 2002;117:131–141. doi: 10.1007/s00418-001-0346-2. (Electronic publication 2001 Nov 2020) [DOI] [PubMed] [Google Scholar]
- 31.Bonifacino J.S., Rojas R. Retrograde transport from endosomes to the trans-Golgi network. Nat. Rev., Mol. Cell Biol. 2006;7:568–579. doi: 10.1038/nrm1985. [DOI] [PubMed] [Google Scholar]
- 32.Lewis M.J., Nichols B.J., Prescianotto-Baschong C., Riezman H., Pelham H.R. Specific retrieval of the exocytic SNARE Snc1p from early yeast endosomes. Mol. Biol. Cell. 2000;11:23–38. doi: 10.1091/mbc.11.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miesenbock G., Rothman J.E. The capacity to retrieve escaped ER proteins extends to the trans-most cisterna of the Golgi stack. J. Cell Biol. 1995;129:309–319. doi: 10.1083/jcb.129.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cosson P., Letourneur F. Coatomer (COPI)-coated vesicles: role in intracellular transport and protein sorting. Curr. Opin. Cell Biol. 1997;9:484–487. doi: 10.1016/s0955-0674(97)80023-3. [DOI] [PubMed] [Google Scholar]
- 35.Letourneur F., Gaynor E.C., Hennecke S., Demolliere C., Duden R., Emr S.D., Riezman H., Cosson P. Coatomer is essential for retrieval of dilysine-tagged proteins to the endoplasmic reticulum. Cell. 1994;79:1199–1207. doi: 10.1016/0092-8674(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 36.Chaudhary V.K., Jinno Y., FitzGerald D., Pastan I. Pseudomonas exotoxin contains a specific sequence at the carboxyl terminus that is required for cytotoxicity. Proc. Natl. Acad. Sci. U. S. A. 1990;87:308–312. doi: 10.1073/pnas.87.1.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jackson M.E., Simpson J.C., Girod A., Pepperkok R., Roberts L.M., Lord J.M. The KDEL retrieval system is exploited by Pseudomonas exotoxin A, but not by Shiga-like toxin-1, during retrograde transport from the Golgi complex to the endoplasmic reticulum. J. Cell Sci. 1999;112:467–475. doi: 10.1242/jcs.112.4.467. [DOI] [PubMed] [Google Scholar]
- 38.Kreitman R.J., Pastan I. Importance of the glutamate residue of KDEL in increasing the cytotoxicity of Pseudomonas exotoxin derivatives and for increased binding to the KDEL receptor. Biochem. J. 1995;307:29–37. doi: 10.1042/bj3070029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seetharam S., Chaudhary V.K., FitzGerald D., Pastan I. Increased cytotoxic activity of Pseudomonas exotoxin and two chimeric toxins ending in KDEL. J. Biol. Chem. 1991;266:17376–17381. [PubMed] [Google Scholar]
- 40.Mallard F., Tang B.L., Galli T., Tenza D., Saint-Pol A., Yue X., Antony C., Hong W., Goud B., Johannes L. Early/recycling endosomes-to-TGN transport involves two SNARE complexes and a Rab6 isoform. J. Cell Biol. 2002;156:653–664. doi: 10.1083/jcb.200110081. (Electronic publication 2002 Feb 2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Girod A., Storrie B., Simpson J.C., Johannes L., Goud B., Roberts L.M., Lord J.M., Nilsson T., Pepperkok R. Evidence for a COP-I-independent transport route from the Golgi complex to the endoplasmic reticulum. Nat. Cell Biol. 1999;1:423–430. doi: 10.1038/15658. [DOI] [PubMed] [Google Scholar]
- 42.White J., Johannes L., Mallard F., Girod A., Grill S., Reinsch S., Keller P., Tzschaschel B., Echard A., Goud B., Stelzer E.H. Rab6 coordinates a novel Golgi to ER retrograde transport pathway in live cells. J. Cell Biol. 1999;147:743–760. doi: 10.1083/jcb.147.4.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Monier S., Jollivet F., Janoueix-Lerosey I., Johannes L., Goud B. Characterization of novel Rab6-interacting proteins involved in endosome-to-TGN transport. Traffic. 2002;3:289–297. doi: 10.1034/j.1600-0854.2002.030406.x. [DOI] [PubMed] [Google Scholar]
- 44.Feng Y., Jadhav A.P., Rodighiero C., Fujinaga Y., Kirchhausen T., Lencer W.I. Retrograde transport of cholera toxin from the plasma membrane to the endoplasmic reticulum requires the trans-Golgi network but not the Golgi apparatus in Exo2-treated cells. EMBO Rep. 2004;5:596–601. doi: 10.1038/sj.embor.7400152. (Electronic publication 2004 May 2021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Day P.J., Owens S.R., Wesche J., Olsnes S., Roberts L.M., Lord J.M. An interaction between ricin and calreticulin that may have implications for toxin trafficking. J. Biol. Chem. 2001;276:7202–7208. doi: 10.1074/jbc.M009499200. (Electronic publication 2000 Dec 7211) [DOI] [PubMed] [Google Scholar]
- 46.Chen A., AbuJarour R.J., Draper R.K. Evidence that the transport of ricin to the cytoplasm is independent of both Rab6A and COPI. J. Cell Sci. 2003;116:3503–3510. doi: 10.1242/jcs.00641. (Electronic publication 2003 Jul 3515) [DOI] [PubMed] [Google Scholar]
- 47.Clemons W.M., Jr., Menetret J.F., Akey C.W., Rapoport T.A. Structural insight into the protein translocation channel. Curr. Opin. Struct. Biol. 2004;14:390–396. doi: 10.1016/j.sbi.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 48.Van den Berg B., Clemons W.M., Jr., Collinson I., Modis Y., Hartmann E., Harrison S.C., Rapoport T.A. X-ray structure of a protein-conducting channel. Nature. 2004;427:36–44. doi: 10.1038/nature02218. (Electronic publication 2003 Dec 2003) [DOI] [PubMed] [Google Scholar]
- 49.Hazes B., Read R.J. Accumulating evidence suggests that several AB-toxins subvert the endoplasmic reticulum-associated protein degradation pathway to enter target cells. Biochemistry. 1997;36:11051–11054. doi: 10.1021/bi971383p. [DOI] [PubMed] [Google Scholar]
- 50.Fisher K.J., Wilson J.M. The transmembrane domain of diphtheria toxin improves molecular conjugate gene transfer. Biochem. J. 1997;321:49–58. doi: 10.1042/bj3210049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Madshus I.H., Olsnes S., Stenmark H. Membrane translocation of diphtheria toxin carrying passenger protein domains. Infect. Immun. 1992;60:3296–3302. doi: 10.1128/iai.60.8.3296-3302.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stenmark H., Moskaug J.O., Madshus I.H., Sandvig K., Olsnes S. Peptides fused to the amino-terminal end of diphtheria toxin are translocated to the cytosol. J. Cell Biol. 1991;113:1025–1032. doi: 10.1083/jcb.113.5.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fominaya J., Uherek C., Wels W. A chimeric fusion protein containing transforming growth factor-α mediates gene transfer via binding to the EGF receptor. Gene Ther. 1998;5:521–530. doi: 10.1038/sj.gt.3300614. [DOI] [PubMed] [Google Scholar]
- 54.Fominaya J., Wels W. Target cell-specific DNA transfer mediated by a chimeric multidomain protein. Novel non-viral gene delivery system. J. Biol. Chem. 1996;271:10560–10568. doi: 10.1074/jbc.271.18.10560. [DOI] [PubMed] [Google Scholar]
- 55.Barrett L.B., Berry M., Ying W.B., Hodgkin M.N., Seymour L.W., Gonzalez A.M., Read M.L., Baird A., Logan A. CTb targeted non-viral cDNA delivery enhances transgene expression in neurons. J. Gene Med. 2004;6:429–438. doi: 10.1002/jgm.524. [DOI] [PubMed] [Google Scholar]
- 56.Barrett L.B., Logan A., Berry M., Ying W., Gonzalez A.M., Baird A., Seymour L.W. Targeted transfection of neuronal cells using a poly(d-lysine)-cholera-toxin b chain conjugate. Biochem. Soc. Trans. 1999;27:851–857. doi: 10.1042/bst0270851. [DOI] [PubMed] [Google Scholar]
- 57.Gaur R., Gupta P.K., Goyal A., Wels W., Singh Y. Delivery of nucleic acid into mammalian cells by anthrax toxin. Biochem. Biophys. Res. Commun. 2002;297:1121–1127. doi: 10.1016/s0006-291x(02)02299-4. [DOI] [PubMed] [Google Scholar]
- 58.Facchini L.M., Lingwood C.A. A verotoxin 1 B subunit-lambda CRO chimeric protein specifically binds both DNA and globotriaosylceramide (Gb(3)) to effect nuclear targeting of exogenous DNA in Gb(3) positive cells. Exp. Cell Res. 2001;269:117–129. doi: 10.1006/excr.2001.5297. [DOI] [PubMed] [Google Scholar]
- 59.Smith D.C., Lord J.M., Roberts L.M., Tartour E., Johannes L. 1st class ticket to class I: protein toxins as pathfinders for antigen presentation. Traffic. 2002;3:697–704. doi: 10.1034/j.1600-0854.2002.31001.x. [DOI] [PubMed] [Google Scholar]
- 60.Cabiaux V. pH-sensitive toxins: interactions with membrane bilayers and application to drug delivery. Adv. Drug Deliv. Rev. 2004;56:987–997. doi: 10.1016/j.addr.2003.10.044. [DOI] [PubMed] [Google Scholar]
- 61.Donnelly J.J., Ulmer J.B., Hawe L.A., Friedman A., Shi X.P., Leander K.R., Shiver J.W., Oliff A.I., Martinez D., Montgomery D., Liu M.A. Targeted delivery of peptide epitopes to class I major histocompatibility molecules by a modified Pseudomonas exotoxin. Proc. Natl. Acad. Sci. U. S. A. 1993;90:3530–3534. doi: 10.1073/pnas.90.8.3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Haicheur N., Bismuth E., Bosset S., Adotevi O., Warnier G., Lacabanne V., Regnault A., Desaymard C., Amigorena S., Ricciardi-Castagnoli P., Goud B., Fridman W.H., Johannes L., Tartour E. The B subunit of Shiga toxin fused to a tumor antigen elicits CTL and targets dendritic cells to allow MHC class I-restricted presentation of peptides derived from exogenous antigens. J. Immunol. 2000;165:3301–3308. doi: 10.4049/jimmunol.165.6.3301. [DOI] [PubMed] [Google Scholar]
- 63.Lasmezas C.I. Putative functions of PrP(C) Br. Med. Bull. 2003;66:61–70. doi: 10.1093/bmb/66.1.61. [DOI] [PubMed] [Google Scholar]
- 64.Martins V.R., Linden R., Prado M.A., Walz R., Sakamoto A.C., Izquierdo I., Brentani R.R. Cellular prion protein: on the road for functions. FEBS Lett. 2002;512:25–28. doi: 10.1016/s0014-5793(02)02291-3. [DOI] [PubMed] [Google Scholar]
- 65.Prusiner S.B. Prions. Proc. Natl. Acad. Sci. U. S. A. 1998;95:13363–13383. doi: 10.1073/pnas.95.23.13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Collinge J. Prion diseases of humans and animals: their causes and molecular basis. Annu. Rev. Neurosci. 2001;24:519–550. doi: 10.1146/annurev.neuro.24.1.519. [DOI] [PubMed] [Google Scholar]
- 67.Tuite M.F., Koloteva-Levin N. Propagating prions in fungi and mammals. Mol. Cell. 2004;14:541–552. doi: 10.1016/j.molcel.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 68.Aguzzi A. Prions and the immune system: a journey through gut, spleen, and nerves. Adv. Immunol. 2003;81:123–171. doi: 10.1016/s0065-2776(03)81004-0. [DOI] [PubMed] [Google Scholar]
- 69.Haik S., Faucheux B.A., Hauw J.J. Brain targeting through the autonomous nervous system: lessons from prion diseases. Trends Mol. Med. 2004;10:107–112. doi: 10.1016/j.molmed.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 70.Mabbott N.A., Bruce M.E. The immunobiology of TSE diseases. J. Gen. Virol. 2001;82:2307–2318. doi: 10.1099/0022-1317-82-10-2307. [DOI] [PubMed] [Google Scholar]
- 71.Aucouturier P., Carnaud C. The immune system and prion diseases: a relationship of complicity and blindness. J. Leukoc. Biol. 2002;72:1075–1083. [PubMed] [Google Scholar]
- 72.Fevrier B., Vilette D., Archer F., Loew D., Faigle W., Vidal M., Laude H., Raposo G. Cells release prions in association with exosomes. Proc. Natl. Acad. Sci. U. S. A. 2004;101:9683–9688. doi: 10.1073/pnas.0308413101. (Electronic publication 2004 Jun 9621) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Campana V., Sarnataro D., Zurzolo C. The highways and byways of prion protein trafficking. Trends Cell Biol. 2005;15:102–111. doi: 10.1016/j.tcb.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 74.Harris D.A. Trafficking, turnover and membrane topology of PrP. Br. Med. Bull. 2003;66:71–85. doi: 10.1093/bmb/66.1.71. [DOI] [PubMed] [Google Scholar]
- 75.Simons K., Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 76.Peters P.J., Mironov A., Jr., Peretz D., van Donselaar E., Leclerc E., Erpel S., DeArmond S.J., Burton D.R., Williamson R.A., Vey M., Prusiner S.B. Trafficking of prion proteins through a caveolae-mediated endosomal pathway. J. Cell Biol. 2003;162:703–717. doi: 10.1083/jcb.200304140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shyng S.L., Heuser J.E., Harris D.A. A glycolipid-anchored prion protein is endocytosed via clathrin-coated pits. J. Cell Biol. 1994;125:1239–1250. doi: 10.1083/jcb.125.6.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mironov A., Jr., Latawiec D., Wille H., Bouzamondo-Bernstein E., Legname G., Williamson R.A., Burton D., DeArmond S.J., Prusiner S.B., Peters P.J. Cytosolic prion protein in neurons. J. Neurosci. 2003;23:7183–7193. doi: 10.1523/JNEUROSCI.23-18-07183.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Trowbridge I.S., Collawn J.F., Hopkins C.R. Signal-dependent membrane protein trafficking in the endocytic pathway. Annu. Rev. Cell Biol. 1993;9:129–161. doi: 10.1146/annurev.cb.09.110193.001021. [DOI] [PubMed] [Google Scholar]
- 80.van Deurs B., Holm P.K., Kayser L., Sandvig K., Hansen S.H. Multivesicular bodies in HEp-2 cells are maturing endosomes. Eur. J. Cell Biol. 1993;61:208–224. [PubMed] [Google Scholar]
- 81.Babst M. A protein's final ESCRT. Traffic. 2005;6:2–9. doi: 10.1111/j.1600-0854.2004.00246.x. [DOI] [PubMed] [Google Scholar]
- 82.van Niel G., Porto-Carreiro I., Simoes S., Raposo G. Exosomes: a common pathway for a specialized function. J. Biochem. (Tokyo) 2006;140:13–21. doi: 10.1093/jb/mvj128. [DOI] [PubMed] [Google Scholar]
- 83.Baron G.S., Wehrly K., Dorward D.W., Chesebro B., Caughey B. Conversion of raft associated prion protein to the protease-resistant state requires insertion of PrP-res (PrP(Sc)) into contiguous membranes. EMBO J. 2002;21:1031–1040. doi: 10.1093/emboj/21.5.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Borchelt D.R., Taraboulos A., Prusiner S.B. Evidence for synthesis of scrapie prion proteins in the endocytic pathway. J. Biol. Chem. 1992;267:16188–16199. [PubMed] [Google Scholar]
- 85.Caughey B., Raymond G.J. The scrapie-associated form of PrP is made from a cell surface precursor that is both protease- and phospholipase-sensitive. J. Biol. Chem. 1991;266:18217–18223. [PubMed] [Google Scholar]
- 86.van Dillen I.J., Mulder N.H., Vaalburg W., de Vries E.F., Hospers G.A. Influence of the bystander effect on HSV-tk/GCV gene therapy. A review. Curr. Gene Ther. 2002;2:307–322. doi: 10.2174/1566523023347733. [DOI] [PubMed] [Google Scholar]
- 87.Liu C.S., Kong B., Xia H.H., Ellem K.A., Wei M.Q. VP22 enhanced intercellular trafficking of HSV thymidine kinase reduced the level of ganciclovir needed to cause suicide cell death. J. Gene Med. 2001;3:145–152. doi: 10.1002/jgm.164. [DOI] [PubMed] [Google Scholar]
- 88.Aints A., Guven H., Gahrton G., Smith C.I., Dilber M.S. Mapping of herpes simplex virus-1 VP22 functional domains for inter- and subcellular protein targeting. Gene Ther. 2001;8:1051–1056. doi: 10.1038/sj.gt.3301493. [DOI] [PubMed] [Google Scholar]
- 89.Elliott G., O'Hare P. Intercellular trafficking and protein delivery by a herpesvirus structural protein. Cell. 1997;88:223–233. doi: 10.1016/s0092-8674(00)81843-7. [DOI] [PubMed] [Google Scholar]
- 90.Yamada E. The fine structure of the gall bladder epithelium of the mouse. J. Biophys. Biochem. Cytol. 1955;1:445–458. doi: 10.1083/jcb.1.5.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rothberg K.G., Heuser J.E., Donzell W.C., Ying Y.S., Glenney J.R., Anderson R.G. Caveolin, a protein component of caveolae membrane coats. Cell. 1992;68:673–682. doi: 10.1016/0092-8674(92)90143-z. [DOI] [PubMed] [Google Scholar]
- 92.Kurzchalia T.V., Dupree P., Parton R.G., Kellner R., Virta H., Lehnert M., Simons K. VIP21, a 21-kD membrane protein is an integral component of trans-Golgi-network-derived transport vesicles. J. Cell Biol. 1992;118:1003–1014. doi: 10.1083/jcb.118.5.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dietzen D.J., Hastings W.R., Lublin D.M. Caveolin is palmitoylated on multiple cysteine residues. Palmitoylation is not necessary for localization of caveolin to caveolae. J. Biol. Chem. 1995;270:6838–6842. doi: 10.1074/jbc.270.12.6838. [DOI] [PubMed] [Google Scholar]
- 94.Murata M., Peranen J., Schreiner R., Wieland F., Kurzchalia T.V., Simons K. VIP21/caveolin is a cholesterol-binding protein. Proc. Natl. Acad. Sci. U. S. A. 1995;92:10339–10343. doi: 10.1073/pnas.92.22.10339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cohen A.W., Hnasko R., Schubert W., Lisanti M.P. Role of caveolae and caveolins in health and disease. Physiol. Rev. 2004;84:1341–1379. doi: 10.1152/physrev.00046.2003. [DOI] [PubMed] [Google Scholar]
- 96.Richterova Z., Liebl D., Horak M., Palkova Z., Stokrova J., Hozak P., Korb J., Forstova J. Caveolae are involved in the trafficking of mouse polyomavirus virions and artificial VP1 pseudocapsids toward cell nuclei. J. Virol. 2001;75:10880–10891. doi: 10.1128/JVI.75.22.10880-10891.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pietiainen V., Marjomaki V., Upla P., Pelkmans L., Helenius A., Hyypia T. Echovirus 1 endocytosis into caveosomes requires lipid rafts, dynamin II, and signaling events. Mol. Biol. Cell. 2004;15:4911–4925. doi: 10.1091/mbc.E04-01-0070. (Electronic publication 2004 Sep 4918) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Marjomaki V., Pietiainen V., Matilainen H., Upla P., Ivaska J., Nissinen L., Reunanen H., Huttunen P., Hyypia T., Heino J. Internalization of echovirus 1 in caveolae. J. Virol. 2002;76:1856–1865. doi: 10.1128/JVI.76.4.1856-1865.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nunes-Correia I., Eulalio A., Nir S., Pedroso de Lima M.C. Caveolae as an additional route for influenza virus endocytosis in MDCK cells. Cell Mol. Biol. Lett. 2004;9:47–60. [PubMed] [Google Scholar]
- 100.Nomura R., Kiyota A., Suzaki E., Kataoka K., Ohe Y., Miyamoto K., Senda T., Fujimoto T. Human coronavirus 229E binds to CD13 in rafts and enters the cell through caveolae. J. Virol. 2004;78:8701–8708. doi: 10.1128/JVI.78.16.8701-8708.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pelkmans L., Kartenbeck J., Helenius A. Caveolar endocytosis of simian virus 40 reveals a new two-step vesicular-transport pathway to the ER. Nat. Cell Biol. 2001;3:473–483. doi: 10.1038/35074539. [DOI] [PubMed] [Google Scholar]
- 102.Damm E.M., Pelkmans L., Kartenbeck J., Mezzacasa A., Kurzchalia T., Helenius A. Clathrin- and caveolin-1-independent endocytosis: entry of simian virus 40 into cells devoid of caveolae. J. Cell Biol. 2005;168:477–488. doi: 10.1083/jcb.200407113. (Electronic publication 2005 Jan 2024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Anderson H.A., Chen Y., Norkin L.C. Bound simian virus 40 translocates to caveolin-enriched membrane domains, and its entry is inhibited by drugs that selectively disrupt caveolae. Mol. Biol. Cell. 1996;7:1825–1834. doi: 10.1091/mbc.7.11.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pelkmans L., Puntener D., Helenius A. Local actin polymerization and dynamin recruitment in SV40-induced internalization of caveolae. Science. 2002;296:535–539. doi: 10.1126/science.1069784. [DOI] [PubMed] [Google Scholar]
- 105.Tsai B., Gilbert J.M., Stehle T., Lencer W., Benjamin T.L., Rapoport T.A. Gangliosides are receptors for murine polyoma virus and SV40. EMBO J. 2003;22:4346–4355. doi: 10.1093/emboj/cdg439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bernacchi S., Mueller G., Langowski J., Waldeck W. Characterization of simian virus 40 on its infectious entry pathway in cells using fluorescence correlation spectroscopy. Biochem. Soc. Trans. 2004;32:746–749. doi: 10.1042/BST0320746. [DOI] [PubMed] [Google Scholar]
- 107.Anderson H.A., Chen Y., Norkin L.C. MHC class I molecules are enriched in caveolae but do not enter with simian virus 40. J. Gen. Virol. 1998;79:1469–1477. doi: 10.1099/0022-1317-79-6-1469. [DOI] [PubMed] [Google Scholar]
- 108.Stang E., Kartenbeck J., Parton R.G. Major histocompatibility complex class I molecules mediate association of SV40 with caveolae. Mol. Biol. Cell. 1997;8:47–57. doi: 10.1091/mbc.8.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dangoria N.S., Breau W.C., Anderson H.A., Cishek D.M., Norkin L.C. Extracellular simian virus 40 induces an ERK/MAP kinase-independent signalling pathway that activates primary response genes and promotes virus entry. J. Gen. Virol. 1996;77:2173–2182. doi: 10.1099/0022-1317-77-9-2173. [DOI] [PubMed] [Google Scholar]
- 110.Mundy D.I., Machleidt T., Ying Y.S., Anderson R.G., Bloom G.S. Dual control of caveolar membrane traffic by microtubules and the actin cytoskeleton. J. Cell Sci. 2002;115:4327–4339. doi: 10.1242/jcs.00117. [DOI] [PubMed] [Google Scholar]
- 111.Pelkmans L., Burli T., Zerial M., Helenius A. Caveolin-stabilized membrane domains as multifunctional transport and sorting devices in endocytic membrane traffic. Cell. 2004;118:767–780. doi: 10.1016/j.cell.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 112.Puri V., Watanabe R., Singh R.D., Dominguez M., Brown J.C., Wheatley C.L., Marks D.L., Pagano R.E. Clathrin-dependent and -independent internalization of plasma membrane sphingolipids initiates two Golgi targeting pathways. J. Cell Biol. 2001;154:535–547. doi: 10.1083/jcb.200102084. (Electronic publication 2001 Jul 2030) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nichols B.J. A distinct class of endosome mediates clathrin-independent endocytosis to the Golgi complex. Nat. Cell Biol. 2002;4:374–378. doi: 10.1038/ncb787. [DOI] [PubMed] [Google Scholar]
- 114.Di Guglielmo G.M., Le Roy C., Goodfellow A.F., Wrana J.L. Distinct endocytic pathways regulate TGF-beta receptor signalling and turnover. Nat. Cell Biol. 2003;5:410–421. doi: 10.1038/ncb975. [DOI] [PubMed] [Google Scholar]
- 115.Nabi I.R., Le P.U. Caveolae/raft-dependent endocytosis. J. Cell Biol. 2003;161:673–677. doi: 10.1083/jcb.200302028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Richards A.A., Stang E., Pepperkok R., Parton R.G. Inhibitors of COP-mediated transport and cholera toxin action inhibit simian virus 40 infection. Mol. Biol Cell. 2002;13:1750–1764. doi: 10.1091/mbc.01-12-0592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kasamatsu H., Nakanishi A. How do animal DNA viruses get to the nucleus? Annu. Rev. Microbiol. 1998;52:627–686. doi: 10.1146/annurev.micro.52.1.627. [DOI] [PubMed] [Google Scholar]
- 118.Lipardi C., Mora R., Colomer V., Paladino S., Nitsch L., Rodriguez-Boulan E., Zurzolo C. Caveolin transfection results in caveolae formation but not apical sorting of glycosylphosphatidylinositol (GPI)-anchored proteins in epithelial cells. J. Cell Biol. 1998;140:617–626. doi: 10.1083/jcb.140.3.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Minshall R.D., Tiruppathi C., Vogel S.M., Niles W.D., Gilchrist A., Hamm H.E., Malik A.B. Endothelial cell-surface gp60 activates vesicle formation and trafficking via G(i)-coupled Src kinase signaling pathway. J. Cell Biol. 2000;150:1057–1070. doi: 10.1083/jcb.150.5.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Thomsen P., Roepstorff K., Stahlhut M., van Deurs B. Caveolae are highly immobile plasma membrane microdomains, which are not involved in constitutive endocytic trafficking. Mol Biol. Cell. 2002;13:238–250. doi: 10.1091/mbc.01-06-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Oh P., Borgstrom P., Witkiewicz H., Li Y., Borgstrom B.J., Chrastina A., Iwata K., Zinn K.R., Baldwin R., Testa J.E., Schnitzer J.E. Live dynamic imaging of caveolae pumping targeted antibody rapidly and specifically across endothelium in the lung. Nat. Biotechnol. 2007;25:327–337. doi: 10.1038/nbt1292. (Electronic publication 2007 Mar 2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rentsendorj A., Xie J., MacVeigh M., Agadjanian H., Bass S., Kim D.H., Rossi J., Hamm-Alvarez S.F., Medina-Kauwe L.K. Typical and atypical trafficking pathways of Ad5 penton base recombinant protein: implications for gene transfer. Gene Ther. 2006;13:821–836. doi: 10.1038/sj.gt.3302729. [DOI] [PubMed] [Google Scholar]
- 123.Huth S., Lausier J., Gersting S.W., Rudolph C., Plank C., Welsch U., Rosenecker J. Insights into the mechanism of magnetofection using PEI-based magnetofectins for gene transfer. J.Gene Med. 2004;6:923–936. doi: 10.1002/jgm.577. [DOI] [PubMed] [Google Scholar]
- 124.Rejman J., Oberle V., Zuhorn I.S., Hoekstra D. Size-dependent internalization of particles via the pathways of clathrin- and caveolae-mediated endocytosis. Biochem. J. 2004;377:159–169. doi: 10.1042/BJ20031253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.van der Aa M.A., Huth U.S., Hafele S.Y., Schubert R., Oosting R.S., Mastrobattista E., Hennink W.E., Peschka-Suss R., Koning G.A., Crommelin D.J. Cellular uptake of cationic polymer-DNA complexes via caveolae plays a pivotal role in gene transfection in COS-7 cells. Pharm. Res. 2007;24:24. doi: 10.1007/s11095-007-9287-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Goncalves C., Mennesson E., Fuchs R., Gorvel J.P., Midoux P., Pichon C. Macropinocytosis of polyplexes and recycling of plasmid via the clathrin-dependent pathway impair the transfection efficiency of human hepatocarcinoma cells. Mol. Ther. 2004;10:373–385. doi: 10.1016/j.ymthe.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 127.Pizarro-Cerda J., Moreno E., Desjardins M., Gorvel J.P. When intracellular pathogens invade the frontiers of cell biology and immunology. Histol. Histopathol. 1997;12:1027–1038. [PubMed] [Google Scholar]
- 128.Meresse S., Steele-Mortimer O., Moreno E., Desjardins M., Finlay B., Gorvel J.P. Controlling the maturation of pathogen-containing vacuoles: a matter of life and death. Nat. Cell Biol. 1999;1:E183–E188. doi: 10.1038/15620. [DOI] [PubMed] [Google Scholar]
- 129.Celli J., de Chastellier C., Franchini D.M., Pizarro-Cerda J., Moreno E., Gorvel J.P. Brucella evades macrophage killing via VirB-dependent sustained interactions with the endoplasmic reticulum. J. Exp. Med. 2003;198:545–556. doi: 10.1084/jem.20030088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gorvel J.P., Moreno E. Brucella intracellular life: from invasion to intracellular replication. Vet. Microbiol. 2002;90:281–297. doi: 10.1016/s0378-1135(02)00214-6. [DOI] [PubMed] [Google Scholar]
- 131.Al-Younes H.M., Rudel T., Meyer T.F. Characterization and intracellular trafficking pattern of vacuoles containing Chlamydia pneumoniae in human epithelial cells. Cell Microbiol. 1999;1:237–247. doi: 10.1046/j.1462-5822.1999.00024.x. [DOI] [PubMed] [Google Scholar]
- 132.Watarai M., Kim S., Erdenebaatar J., Makino S., Horiuchi M., Shirahata T., Sakaguchi S., Katamine S. Cellular prion protein promotes Brucella infection into macrophages. J. Exp. Med. 2003;198:5–17. doi: 10.1084/jem.20021980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sunyach C., Jen A., Deng J., Fitzgerald K.T., Frobert Y., Grassi J., McCaffrey M.W., Morris R. The mechanism of internalization of glycosylphosphatidylinositol-anchored prion protein. EMBO J. 2003;22:3591–3601. doi: 10.1093/emboj/cdg344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Naroeni A., Porte F. Role of cholesterol and the ganglioside GM(1) in entry and short-term survival of Brucella suis in murine macrophages. Infect. Immun. 2002;70:1640–1644. doi: 10.1128/IAI.70.3.1640-1644.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Pizarro-Cerda J., Meresse S., Parton R.G., van der Goot G., Sola-Landa A., Lopez-Goni I., Moreno E., Gorvel J.P. Brucella abortus transits through the autophagic pathway and replicates in the endoplasmic reticulum of nonprofessional phagocytes. Infect. Immun. 1998;66:5711–5724. doi: 10.1128/iai.66.12.5711-5724.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Pizarro-Cerda J., Moreno E., Sanguedolce V., Mege J.L., Gorvel J.P. Virulent Brucella abortus prevents lysosome fusion and is distributed within autophagosome-like compartments. Infect. Immun. 1998;66:2387–2392. doi: 10.1128/iai.66.5.2387-2392.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Celli J., Gorvel J.P. Organelle robbery: Brucella interactions with the endoplasmic reticulum. Curr. Opin. Microbiol. 2004;7:93–97. doi: 10.1016/j.mib.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 138.Porte F., Liautard J.P., Kohler S. Early acidification of phagosomes containing Brucella suis is essential for intracellular survival in murine macrophages. Infect. Immun. 1999;67:4041–4047. doi: 10.1128/iai.67.8.4041-4047.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kagan J.C., Roy C.R. Legionella phagosomes intercept vesicular traffic from endoplasmic reticulum exit sites. Nat. Cell Biol. 2002;4:945–954. doi: 10.1038/ncb883. [DOI] [PubMed] [Google Scholar]
- 140.Delrue R.M., Martinez-Lorenzo M., Lestrate P., Danese I., Bielarz V., Mertens P., De Bolle X., Tibor A., Gorvel J.P., Letesson J.J. Identification of Brucella spp. genes involved in intracellular trafficking. Cell Microbiol. 2001;3:487–497. doi: 10.1046/j.1462-5822.2001.00131.x. [DOI] [PubMed] [Google Scholar]
- 141.O'Callaghan D., Cazevieille C., Allardet-Servent A., Boschiroli M.L., Bourg G., Foulongne V., Frutos P., Kulakov Y., Ramuz M. A homologue of the Agrobacterium tumefaciens VirB and Bordetella pertussis Ptl type IV secretion systems is essential for intracellular survival of Brucella suis. Mol. Microbiol. 1999;33:1210–1220. doi: 10.1046/j.1365-2958.1999.01569.x. [DOI] [PubMed] [Google Scholar]
- 142.Rittig M.G., Alvarez-Martinez M.T., Porte F., Liautard J.P., Rouot B. Intracellular survival of Brucella spp. in human monocytes involves conventional uptake but special phagosomes. Infect. Immun. 2001;69:3995–4006. doi: 10.1128/IAI.69.6.3995-4006.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Gross A., Bouaboula M., Casellas P., Liautard J.P., Dornand J. Subversion and utilization of the host cell cyclic adenosine 5′-monophosphate/protein kinase A pathway by Brucella during macrophage infection. J. Immunol. 2003;170:5607–5614. doi: 10.4049/jimmunol.170.11.5607. [DOI] [PubMed] [Google Scholar]
- 144.Boschiroli M.L., Ouahrani-Bettache S., Foulongne V., Michaux-Charachon S., Bourg G., Allardet-Servent A., Cazevieille C., Liautard J.P., Ramuz M., O'Callaghan D. The Brucella suis virB operon is induced intracellularly in macrophages. Proc. Natl. Acad. Sci. U. S. A. 2002;99:1544–1549. doi: 10.1073/pnas.032514299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Check E. Gene therapy put on hold as third child develops cancer. Nature. 2005;433:561. doi: 10.1038/433561a. [DOI] [PubMed] [Google Scholar]
- 146.Hacein-Bey-Abina S., von Kalle C., Schmidt M., Le Deist F., Wulffraat N., McIntyre E., Radford I., Villeval J.L., Fraser C.C., Cavazzana-Calvo M., Fischer A. A serious adverse event after successful gene therapy for X-linked severe combined immunodeficiency. N. Engl. J. Med. 2003;348:255–256. doi: 10.1056/NEJM200301163480314. [DOI] [PubMed] [Google Scholar]
- 147.Hacein-Bey-Abina S., Von Kalle C., Schmidt M., McCormack M.P., Wulffraat N., Leboulch P., Lim A., Osborne C.S., Pawliuk R., Morillon E., Sorensen R., Forster A., Fraser P., Cohen J.I., de Saint Basile G., Alexander I., Wintergerst U., Frebourg T., Aurias A., Stoppa-Lyonnet D., Romana S., Radford-Weiss I., Gross F., Valensi F., Delabesse E., Macintyre E., Sigaux F., Soulier J., Leiva L.E., Wissler M., Prinz C., Rabbitts T.H., Le Deist F., Fischer A., Cavazzana-Calvo M. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science. 2003;302:415–419. doi: 10.1126/science.1088547. [DOI] [PubMed] [Google Scholar]
- 148.Calos M.P. The potential of extrachromosomal replicating vectors for gene therapy. Trends Genet. 1996;12:463–466. doi: 10.1016/0168-9525(96)40049-x. [DOI] [PubMed] [Google Scholar]
- 149.Ryan R.M., Green J., Lewis C.E. Use of bacteria in anti-cancer therapies. Bioessays. 2006;28:84–94. doi: 10.1002/bies.20336. [DOI] [PubMed] [Google Scholar]
- 150.Vassaux G., Nitcheu J., Jezzard S., Lemoine N.R. Bacterial gene therapy strategies. J. Pathol. 2006;208:290–298. doi: 10.1002/path.1865. [DOI] [PubMed] [Google Scholar]
- 151.Agorio C., Schreiber F., Sheppard M., Mastroeni P., Fernandez M., Martinez M.A., Chabalgoity J.A. Live attenuated Salmonella as a vector for oral cytokine gene therapy in melanoma. J. Gene Med. 2007;5:5. doi: 10.1002/jgm.1023. [DOI] [PubMed] [Google Scholar]