Abstract
Dendritic cells (DC) at mucosal surfaces mature when exposed to “danger” signals such as LPS. Bacterial vaginosis (BV) is a prevalent alteration of the vaginal bacterial flora associated with preterm childbirth and increased risk for HIV acquisition. We examined the effect of mucosal fluid from women with BV or healthy flora on DC function. IL-12, IL-23 and p40 production by monocyte-derived dendritic cells (MDDC) were all induced by BV samples. Activation/maturation markers HLA-DR, CD40, and CD83 on MDDC incubated with BV CVL were also induced. BV CVL also decreased the endocytic ability of MDDC and increased proliferation of T-cells in allogeneic MLR. Plasmacytoid dendritic cell (pDC) CD86 expression was induced by BV CVL. Healthy flora CVL had little effect in any of the tests. This study suggests that BV, but not healthy flora, affects local dendritic cell function in vivo suggesting a mechanism through which BV affects mucosal immunity.
Keywords: Myeloid dendritic cells, plasmacytoid dendritic cells, Bacterial Vaginosis, Bacterial flora, Antigen presentation, Activation, Maturation, Endocytosis, IL-12, Mucosal immunity
INTRODUCTION
Immature dendritic cells (DC) play a key role in the initiation of adaptive immune responses by capturing antigen in tissues, including mucosal tissues [1]. When immature DC sense danger signals in the environment via pathogen recognition receptors such as toll-like receptors (TLR), they are induced to mature into efficient antigen-presenting cells [2]. Maturation is accompanied by changes in DC important for stimulating T cells such as increased expression of co-stimulatory molecules, increased expression of MHC class II, and secretion of cytokines that induce T cell differentiation including IL-12 and IL-23 [1, 3]. Secretion of IL-12 by DC is critical for induction of T cell responses to pathogens while IL-23 secretion by DC can skew responses towards a T-cell subset that secretes IL-17, a subset that is implicated in autoimmune disease [4]. DC can also secrete free p40, a subunit of both heterodimeric IL-12 and IL-23, which can inhibit IL-12 activity [5]. In this regard, the inclusion of TLR ligands, such as lipopolysaccharide (LPS) and other bacterial products, during immunization dramatically enhances immune responsiveness to vaccines [6]. Naturally occurring commensal bacterial flora or infections at mucosal sites such as the lower genital tract, intestine or mouth may alter DC phenotype and function thereby affecting downstream adaptive immune responses although this has not been studied in detail [7, 8].
Bacterial Vaginosis (BV) is a common alteration of the vaginal flora in women that involves a change from predominantly lactobacilli in healthy women to a polymicrobial overgrowth of both gram-positive and gram-negative bacteria including Gardnerella vaginalis, anaerobic gram-negative rods and numerous types of other bacteria [9]. Episodes of BV commonly span several days to weeks [10] and the incidence of BV ranges from 5–30% in women of child bearing age in North America to over 50% in some populations [11-13]. BV has several severe consequences including increasing the risk of preterm birth, miscarriage and pelvic inflammatory disease [14-16]. BV also increases the risk of infection with human immunodeficiency virus (HIV) and herpes simplex virus type 2 [13, 17, 18].
Since BV provides an environment in the lower genital tract that has a relatively high concentration of bacterial products, we hypothesized that BV would be highly stimulatory to DC and induce their maturation into efficient antigen presenting cells. We also postulated that healthy genital tract flora, because it is also comprised of bacteria, would be stimulatory to DC. These hypotheses were tested by obtaining cervicovaginal lavage (CVL) samples from women with either BV or healthy genital tract flora and testing their effects on DC activation and maturation. The effect of genital fluids on plasmacytoid dendritic cells (pDC) were also tested since these cells are also found in the lower genital tract, express pathogen pattern recognition receptors, and can impact adaptive immune responses after stimulation [19-21].
MATERIAL AND METHODS
CVL Samples
CVL samples were obtained from a cohort of women, with informed consent, attending a fertility clinic (UNICAMP, Sao Paulo, Brazil). CVL samples were collected by irrigation of the cervix with 10 ml of nonbacteriostatic sterile saline followed by aspiration from the posterior fornix. CVL samples were centrifuged at 2200 RPM for 20 min to remove cell debris. The supernatants were then frozen at −80°C. Gram stains of vaginal swabs were evaluated using the Nugent criteria [22]. Studies were performed only on samples with scores 8–10 (positive for BV) or with scores 0–3 (negative for BV, healthy flora). The samples used in this study were also negative for yeast, Chlamydia, Neisseria gonorrhoeae and cervical dysplasia.
DC generation and stimulation
Monocyte derived dendritic cells (MDDCs) were generated from peripheral blood mononuclear cells (PBMC) isolated from the blood of healthy donors by centrifugation on Lymphocyte Separation Medium (BioWhittaker, Walkerville, MD). The blood donors provided informed consent and the study was approved by the Rush institutional review board. CD14+ cells were isolated with CD14+ microbeads and the autoMACS cell separation system yielding greater than 95% purity (Miltenyi Biotec, Auburn, CA). Isolated monocytes were cultured in RPMI 1640 medium supplemented with 10% heat inactivated Fetal Bovine Serum (FBS) (BioWhittaker, Walkersville, MD), 2mM L-Glutamine (BioWhittaker, Walkersville, MD), 1000U/ml GM-CSF (Leukine, Berlex, Richmond CA) and 2900U/ml IL-4 (R&D Systems, Minneapolis, MN) at 2x106 cells/ml for 6 days at 37°C in 5% CO2. Cytokines were replenished on days 2 and 4. On day 6 the MDDCs were harvested and cultured for a further 24 or 48 hrs with either medium alone, 1μg/ml LPS (Sigma Aldrich, St. Louis MO), 4μg/ml Class A CpG-ODN (2216) (Coley Pharmaceutical Group, Wellesley, MA) or CVL samples at 10% of total culture volume. For pDC stimulation experiments, PBMC were isolated and cultured with stimuli for 24h. pDCs were then identified based on their cell surface markers by flow cytometry as described below. The number of pDC from each donor varied between 0.2% and 0.5% of total PBMCs.
Detection of Cytokines
The supernatants from 48 hr incubations of MDDC with stimuli were collected and stored at −20°C until the ELISAs were performed. Cytokines levels in undiluted CVL samples were also assessed. The Human p40 Cytoset ELISA kit was from Biosource (Camarillo, CA), the IL-12p70 kit was from BioLegend, (San Diego CA) and the IL-23 Elisa kit was from eBioscience (San Diego, CA). The IL-12 and IL-23 kits are both specific for their targets and do not cross-react with the other cytokines while the IL-12+p40 kit detects IL-12 p40 monomer, homodimer and IL-12 p70.
Flow Cytometric Analysis
Cell surface markers were evaluated using APC-conjugated monoclonal antibodies (mAb) to CD40, CD83, CD86 (BD Pharmingen, San Jose, CA) and PerCP-conjugated Ab to HLA-DR (BD Bioscience, San Jose, CA). In addition, PE-conjugated mAbs to CD1a (Immunotech, Beckman Coulter, Marseille France) CD123, CD11c and Fluorescein isothiocyanate (FITC)-conjugated to CD14 and Lineage cocktail (Lin1) consisting of FITC-conjugated CD3, CD14, CD16, CD19, CD20, and CD56 (BD Biosciences) were also used as markers to define the populations. The CVL samples were cultured with MDDCs at 10% of culture volume for 24 or 48 hrs and the level of surface markers was measured by flow cytometry, first by identifying the MDDC as CD14−, CD1a+, HLA-DR+ and then further defining their level of CD40, CD83 and HLA-DR. pDCs were also cultured for 24 hours in 10% CVL. They were then analyzed for activation marker CD86 by identifying the cells as Lin1 negative, CD123+, HLA-DR+. All gates were set based on staining of isotype controls for each fluorochrome.
Endocytosis
Endocytosis was measured by uptake of FITC–dextran with molecular weight of 40,000 (Sigma-Aldrich, St.Louis, MO) and was similar to the methods used by Spisek et al [23]. MDDC (200,000 cells/ml) were incubated in medium containing 0.5mg/ml FITC-dextran for 120min at 37°C or at 4°C (control). Cells were then washed in ice-cold PBS and fluorescence was determined by flow cytometry.
Allogeneic antigen stimulation
Allogeneic mixed lymphocyte reactions (MLR) were performed similar to the methods of Hartmann et al [24]. Briefly, PBMCs were depleted of adherent cells by adherence to plastic for 2hr at 37°C. The non-adherent cells were then labeled with Carboxyfluorescein diacetate succinimidyl ester (CFSE) (Molecular Probes, Carlsbad, CA) by incubating 20 × 106 cells in 1ml 0.1% bovine serum albumin in PBS with 5μM CFSE for 10 minutes at 37°C. Stimulated MDDCs from a different donor were washed and mixed with 106 CFSE-labeled PBMCs at a ratio of 1:300 (stimulator MDDCs to responder cells) in cell culture medium and cultured for 7 days before analysis of proliferation by 3 color flow cytometry using CD4 PE and CD8 PerCP. For each experiment, PBMCs incubated in media alone with no MDDC was used as a negative control.
Statistical Analysis
The Mann Whitney test was used to determine statistically significant differences between groups. The analysis was performed using GraphPad Prism 4 software (San Diego, CA).
RESULTS
CVL samples from women with BV induce IL-12, IL-23 and p40 production by MDDC
To determine if vaginal flora from women with bacterial vaginosis (BV) induce IL-12, IL-23 or p40 production by MDDC, CVL samples from 23 women with BV flora (BV CVL) and from 17 women with normal vaginal flora (normal CVL) were tested. Incubation of BV CVL with MDDC induced significantly higher levels of IL-12 p70 (mean of 720 pg/ml, S.D/S.E=1378/227), IL-23 (mean of 3120 pg/ml, S.D/S.E=2557/522) and p40 (mean of 12100 pg/ml, S.D/S.E=7925/1652) in culture supernatants when compared to control-treated MDDC (means of 19 pg/ml (p=0.0139) S.D/S.E=4.8/2.4, 92pg/ml (p=0.0109) S.D/S.E=61.6/35.6 and 63 pg/ml (p<0.01) S.D/S.E=41.8/17.1 respectively) (Fig. 1). The cytokines detected in culture supernatants were produced by the DC since they were undetectable in CVL samples (data not shown). Incubating MDDC with CVL from women with healthy flora did not induce significant levels of IL-12 (mean of 40 pg/ml, S.D/S.E=64.4/13.4) or IL-23 (mean of 220 pg/ml S.D/S.E=254.6/61.7), although p40 levels (mean of 1200 pg/ml S.D/S.E=3149/764 were higher than control (Fig. 1). For all three cytokines, CVL from women with BV induced significantly higher levels than CVL from women without BV. A pool of all 23 BV CVL samples significantly increased secretion of all three cytokines when compared to a pool of the 17 normal CVL samples (BV pool means; p40=20000 pg/ml; IL-23=4799pg/ml; IL-12p70=1222 pg/ml vs. normal pool means of p40=950 pg/ml, IL-23=128 pg/ml, and IL-12p70=38 pg/ml).
Figure 1.
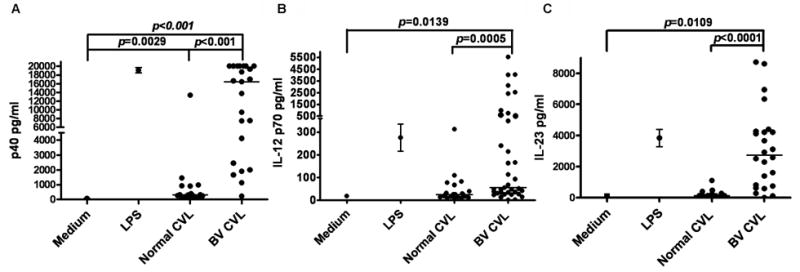
Cytokine production of MDDCs induced by CVL samples. (A) Individual CVL sample induction of IL-12 p40, (B) IL-12 p70 and (C) IL-23. CVL samples were incubated with MDDC for 24hr. Culture supernatants were harvested and cytokine levels measured by ELISA. Each point represents the average of three experiments, the line indicates the median value of each group. p values were determined by Mann Whitney.
BV CVL increase activation and maturation markers on MDDC
To determine the effect of BV CVL on MDDC activation and maturation, specific cell surface markers were measured by flow cytometry. The level of CD83 is correlated with maturation of MDDCs [23, 25] while an increase in CD40 or HLA-DR indicate activation of MDDC [25, 26]. The mean fluorescence intensity (MFI) of all surface markers (CD83, CD40 and HLA-DR) was significantly increased due to BV CVL (57%, 62% and 52% of LPS respectively) when compared to normal CVL (29%, 22% and 9% of LPS) (Fig. 2). Pooled BV CVL samples also induced significantly higher levels of CD83 when compared to normal CVL (85% vs. 13% of LPS, Fig 2D). Markers on MDDC treated with CVL samples from women (individual or pooled) with normal vaginal flora were not significantly higher than medium-treated MDDC, although a few individual samples from women with normal vaginal flora induced substantial expression of CD83, CD40 and HLA-DR. MDDC were also treated with medium alone as a negative control. The activation/maturation marker levels on BV CVL treated cells were consistently higher than on medium-treated cells. This relationship is reflected in Figure 2D since data are normalized for negative control (medium alone) and positive control (LPS treated).
Figure 2.
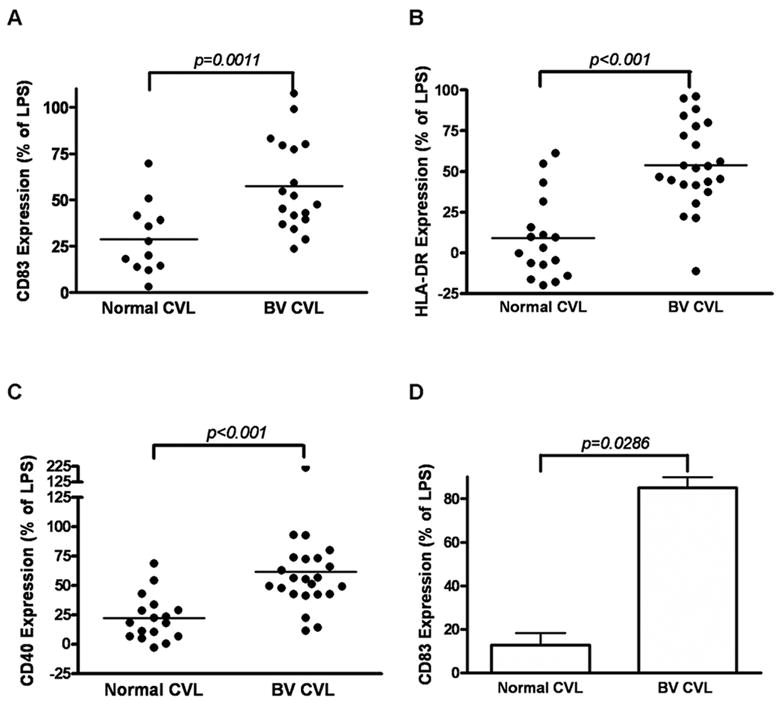
Effect of mucosal fluids on levels of MDDC cell-surface markers of activation and maturation. MDDCs were incubated with CVL samples from individual patient samples (A, B, C) or pooled CVL samples (pools of all 17 normal CVL samples from and all 23 BV CVL samples) (D). Expression (Mean Fluorescence Intensity) of CD83 (A, D) HLA-DR (B) and CD40 (C). Data were adjusted to % of the LPS positive control (100%) and medium treatment (0%) and each point is the average of two independent experiments. The line in figs A,B,C represents the median value of each group, and D is Mean +SEM. p values were determined by Mann Whitney..
BV CVL-treated MDDC display reduced endocytic activity
To characterize functional changes in MDDC following incubation with CVL samples, endocytosis of FITC-dextran was measured. The MFI of MDDC following 48-hour stimulation with pooled BV CVL samples was 83% lower than MDDC incubated with normal CVL samples (Fig. 3). There was an approximately 6 fold difference in MFI between MDDC incubated in medium alone and BV CVL while there was a 25% decrease in MFI between MDDC incubated in medium and normal CVL. Lower endocytic ability after exposure to BV CVL was observed when MDDC were incubated with FITC-dextran for 30, 60, or 90 minutes (data not shown), although the most pronounced differences were at 120 minutes (Fig. 3).
Figure 3.
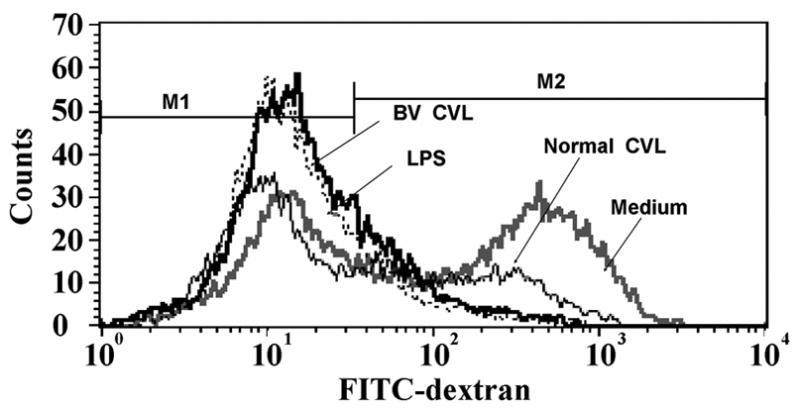
FITC-dextran uptake by MDDCs following incubation with CVL samples. MDDCs were pretreated for 48hr with either medium alone, LPS, or CVL from women with healthy flora or BV flora. MDDCs were then pulsed with FITC-dextran and analyzed by flow cytometry 2 hrs later. Median values from three independent experiments are as follows: 44.2 for medium alone, 32.5 for normal CVL, 7.9 for BV CVL, and 5.9 for LPS. One representative experiment of three independent experiments is shown.
BV CVL increase allo-antigen–driven T-cell proliferation
Previous studies show that activation of DC results in increased proliferation of allogeneic T cells [27]. Therefore, BV CVL- and normal CVL- treated MDDCs were tested for their capacity to stimulate allogeneic CD4+ and CD8+ T-cell proliferation. Relatively low levels of T-cell proliferation were observed in 48hr cultures containing unstimulated MDDC at a 1:300 stimulator to responder ratio (8% proliferation for CD4 and 7% for CD8 Fig. 4). LPS-treated MDDC, a positive control for MDDC maturation, resulted in 23% and 28% proliferation for CD4+ and CD8+ cells respectively. MDDC stimulated with BV CVL, increased CD4+ (42%) and CD8+ (54%) T-cell proliferative responses whereas normal CVL-stimulated MDDC induced levels of T-cell proliferation that were similar to unstimulated MDDC (9% proliferation for CD4+ and 9% for CD8+).
Figure 4.
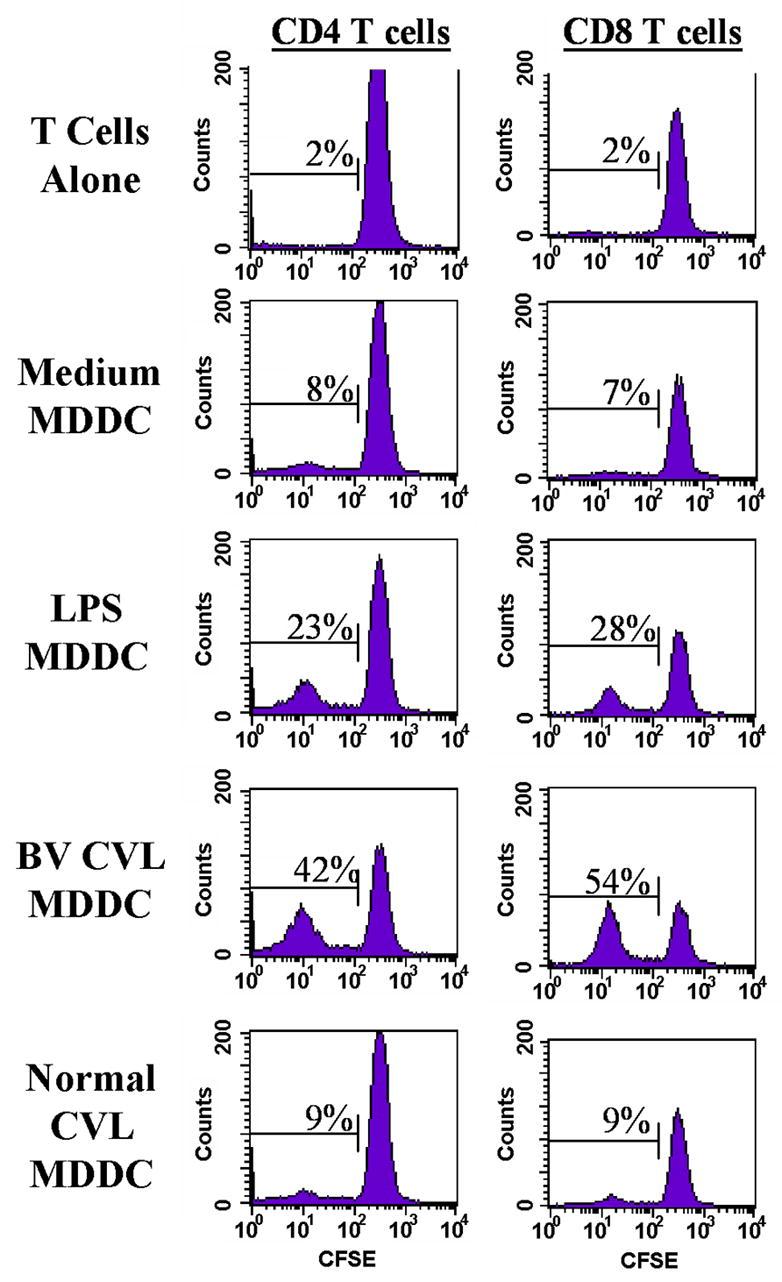
Effect of CVL on allo-antigen-driven T-cell proliferation. MDDC were incubated with either LPS at 1 ng/ml, a pool of 23 BV CVL or a pool of 17 normal CVL for 48hr; CFSE-labeled allogeneic lymphocytes (monocyte depleted) were incubated with treated MDDCs for 7 days. Proliferation of CD4+ and CD8+ T-cells was determined by decreased levels of CFSE. Data show one representative experiment of four independent experiments, each performed with duplicate cultures and with a stimulator (MDDC) to responder ratio of 1:300.
BV CVL increase activation markers on pDC
Activation of the plasmacytoid dendritic cells (pDC) was assessed by changes in CD86 after incubation for 24h with CVL samples [21, 28, 29]. As a positive control, 4μg/ml of CpG2216 class A induced substantial CD86 expression (MFI of 176) compared to medium (MFI of 76). There was a significant difference between the increase of the MFI of CD86 on the surface of pDC incubated overnight with BV CVL vs. Normal CVL (156% vs. 17% of CPG p<0.001 Fig 5.). These results show that pDC are also affected by BV CVL.
Figure 5.

Expression of CD86 on plasmacytoid dendritic cells following incubation with CVL samples. PBMCs were incubated overnight with either medium alone, CPG, healthy flora CVL or BV flora CVL. The pDC population was identified using pDC specific mAbs (lineage-/CD123+/HLA-DR+) and the level of CD86 (MFI) was determined using flow cytometry. Each individual sample was adjusted to % of CPG positive control (100%) and is the mean of three independent experiments, p<0.001 as determined by Mann Whitney test. The lines show the median values.
DISCUSSION
Dendritic cells are potent antigen presenting cells and are present in the vaginal mucosa. Although BV is highly prevalent in women of childbearing age throughout the world, adversely affecting pregnancy and increasing acquisition of HIV, the effect of BV flora and healthy flora on dendritic cell function was not previously assessed. Recent studies show that mucosal DC interact with genital tract luminal substances, highlighting the need to study the effect of BV on antigen presentation and DC function [30]. The experiments in this study show that mucosal fluids from women with BV stimulate and mature DC in vitro. The level of DC stimulation in many of the assays was higher than the control of LPS at 1μg/ml. These results suggest that within the genital tract of women with BV, DC become stimulated. Since in many cases BV is chronic [31] the stimulation of DC by BV may eventually result in reduced DC function due to over-stimulation or exhaustion as has been observed in chronic infections by other microorganisms. For example, chronic infection by hepatitis B virus or hepatitis C virus (HCV) reduces the function of peripheral blood myeloid DC [32-34]. pDC function may also be altered by HCV [35]. Infection with HIV also reduces pDC function, both in chronic and primary HIV infection although the decrease in function may be mostly due to reduced pDC numbers [36, 37].
Another microorganism that affects DC is the mucosal pathogen Helicobacter pylori which stimulates MDDC, but prolonged stimulation reduces DC antigen-presenting function, possibly due to DC exhaustion after prolonged exposure [38]. Interestingly, H. pylori organisms, when added to MDDC, do not directly stimulate IL-12 production [38] but instead induce IL-23. The lack of IL-12 secretion was suggested to contribute to the lack of adequate T cell responses and subsequent lack of clearance of H. pylori. In our studies, stimulation of MDDC with mucosal fluid from women with BV efficiently induced both IL-12 and IL-23.
Our studies show that mucosal fluids collected from women with BV flora had an activating and maturing effect on MDDC while fluids from healthy vaginal flora had much less of an effect. Multiple bacteria species are present in BV flora [9, 39-41], while normal flora species consist predominantly of lactobacilli [42, 43]. Therefore, the results suggest that DC recognize products released by bacteria responsible for BV but not those released by lactobacilli. However, many of the bacteria present in BV are also present at low levels in healthy vaginal flora, such as Lactobacillus inners, G. vaginalis, and Mycoplasma hominis [39, 42-44]. Therefore, both the type and number of DC-stimulating bacteria may be limiting factors for activation of DC by normal flora. Lactobacillus crispatus is found frequently in normal flora but at much lower levels in subjects with BV, whereas L. inners is found in subjects with and without BV [45]. In terms of the number of bacteria present in the genital tract, there can be greater than 108 lactobacilli/ml in CVL samples in women with healthy vaginal flora [40, 44, 46]. The number of bacteria present in the genital tract of women with BV can be equal to or much greater than 108 bacterial counts/ml and composed of a variety of bacterial species including Prevotella species, Atopobium species, and Megasphaera species [39, 42]. Some unculturable bacteria are also present [46].
The flora responsible for BV consists of gram positive, gram-negative and gram variable organisms (G. vaginalis). Gram negative bacteria contain LPS, whereas gram positive bacteria have no LPS but do contain peptidoglycans (PGN). LPS and PGN can be released from live or dying bacteria at stimulatory levels and have been shown in numerous studies to activate DC through toll like receptors (TLR) [25, 26, 47]. The two TLR most likely responsible for MDDC activation are TLR2 (activated by PGN) and TLR4 (activated by LPS). Our studies of NF-kB activation, induced by CVL from women with BV, show strong induction through TLR2 suggesting that stimulatory levels of gram-positive bacterial products are present during BV (GTS, unpublished observations). It has been reported that lipoprotein (TLR2 ligand) stimulation of MDDC induces high levels of IL-12 [48]. Thus both TLR2 and TLR4 ligands are efficient stimulators of DC and are likely present in BV CVL. While the maturing effect of BV on MDDC is likely due to bacterial products in the CVL samples as discussed above, a role for cytokines or other host factors stimulating MDDC in the CVL cannot be ruled out. For example, IL-6, IL-8, and MCP-1 can be present in healthy vaginal secretions, [49, 50] and the levels are elevated in vaginal secretions during infection with M. hominis or Ureaplasma urealyticum or during intra-amniotic inflammation [51]. IL-1β is consistently higher in genital fluids from women with BV [49, 52, 53]. However, a recent study by Nakahara et al. [54] concluded that IL-1β alone did not induce the phenotypic maturation of MDDC, but maturation required the presence of IFN-γ to induce differentiation. Hence, IL-1β is likely not to be the sole inducer of MDDC maturation.
Stimulation of DC can be mediated by microbes that cross the epithelial barrier via transport mechanisms [55]. Alternatively, intraepithelial DC can directly interact with luminal pathogens via dendrites that penetrate epithelium and sample the bacteria in the lumen [55, 56]. The ability of both DEC 205+ DC and pDC to extend dendrites into the vaginal lumen was recently demonstrated by LeBlanc et al. in a murine model of vaginal Candida albicans infection [57]. This model also revealed a high concentration of DC in the T-cell zone of the draining vaginal lymph nodes throughout the duration of Candida infection, suggesting that following sampling of the bacteria in the vaginal lumen, DC migrate to lymph nodes for presentation of antigen to T-cells and induce a cell-mediated immune response. Similar DC sampling of the vaginal lumen and migration may occur in humans with BV although this has not been reported.
DC are abundant in the vaginal sub mucosa where they are potentially affected by BV flora as discussed above. In turn, the susceptibility of individuals to other infections may be altered. For example, epidemiological studies suggest that BV increases susceptibility to HIV-1 infection in women [13, 18, 58, 59]. Increased susceptibility due to BV may occur through few mechanisms. First, mature dendritic induce more efficient T-cell activation leading to increased HIV infection of T cells[60] and second, direct DC infection and DC-mediated trans-infection of HIV can also be affected by the maturation state of DC [61, 62]. Langerhans Cells (LC), which are a subtype of DC found in tissues, have increased HIV uptake after stimulation[63]. This could parallel stimulation of DC by BV CVL samples leading to an increase in the macropinocytosis of virions [64].
In conclusion, we observed that a change in vaginal flora from normal to BV causes DC to reach a matured and activated state. The BV CVL treated MDDC produced high amounts of IL-12p40, IL-12p70 and IL-23. BV CVL also induced expression of CD40/HLA-DR/CD83 and reduced MDDC endocytic activity. Finally, BV CVL also increased MDDC stimulation of allogeneic T-cell proliferation suggesting that BV CVL can induce an increase of in-vivo MDDC antigen presentation to T-cells Furthermore, BV CVL increased CD86 on pDC. The change in maturation and activation status of the DC induced by BV indicates the dramatic effects that changes in vaginal flora may have on local dendritic cell function and points to a mechanism whereby BV may impact local host immunity.
Acknowledgments
We would like to thank all of the women who participated in this study. This work was supported by National Institutes of Health Grant P01 HD40539.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Steinman RM, Inaba K. Myeloid dendritic cells. J Leukoc Biol. 1999;66:205–208. doi: 10.1002/jlb.66.2.205. [DOI] [PubMed] [Google Scholar]
- 2.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5:987–995. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 3.Napolitani G, Rinaldi A, Bertoni F, Sallusto F, Lanzavecchia A. Selected toll-like receptor agonist combinations synergistically trigger a t helper type 1-polarizing program in dendritic cells. Nat Immunol. 2005;6:769–776. doi: 10.1038/ni1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bowman EP, Chackerian AA, Cua DJ. Rationale and safety of anti-interleukin-23 and anti-interieukin-17a therapy. Current Opinion in Infectious Diseases. 2006;19:245–252. doi: 10.1097/01.qco.0000224818.42729.67. [DOI] [PubMed] [Google Scholar]
- 5.Mattner F, Fischer S, Guckes S, Jin SC, Kaulen H, Schmitt E, Rude E, Germann T. The interleukin-12 subunit p40 specifically inhibits effects of the interleukin-12 heterodimer. European Journal of Immunology. 1993;23:2202–2208. doi: 10.1002/eji.1830230923. [DOI] [PubMed] [Google Scholar]
- 6.Kaisho T, Akira S. Toll-like receptors as adjuvant receptors. Biochim Biophys Acta. 2002;1589:1–13. doi: 10.1016/s0167-4889(01)00182-3. [DOI] [PubMed] [Google Scholar]
- 7.Sallusto F, Lanzavecchia A. Mobilizing dendritic cells for tolerance, priming, and chronic inflammation. J Exp Med. 1999;189:611–614. doi: 10.1084/jem.189.4.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Veckman V, Miettinen M, Pirhonen J, Siren J, Matikainen S, Julkunen I. Streptococcus pyogenes and lactobacillus rhamnosus differentially induce maturation and production of th1-type cytokines and chemokines in human monocyte-derived dendritic cells. J Leukoc Biol. 2004;75:764–771. doi: 10.1189/jlb.1003461. [DOI] [PubMed] [Google Scholar]
- 9.Hillier SL. Diagnostic microbiology of bacterial vaginosis. Am J Obstet Gynecol. 1993;169:455–459. doi: 10.1016/0002-9378(93)90340-o. [DOI] [PubMed] [Google Scholar]
- 10.Hay PE, Ugwumadu A, Chowns J. Sex, thrush and bacterial vaginosis. International Journal of Std & Aids. 1997;8:603–608. doi: 10.1258/0956462971918850. [DOI] [PubMed] [Google Scholar]
- 11.Greenblatt RM, Bacchetti P, Barkan S, Augenbraun M, Silver S, Delapenha R, Garcia P, Mathur U, Miotti P, Burns D. Lower genital tract infections among hiv-infected and high-risk uninfected women: Findings of the women’s interagency hiv study (wihs) Sex Transm Dis. 1999;26:143–151. doi: 10.1097/00007435-199903000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Schwebke JR. Gynecologic consequences of bacterial vaginosis. Obstet Gynecol Clin North Am. 2003;30:685–694. doi: 10.1016/s0889-8545(03)00086-x. [DOI] [PubMed] [Google Scholar]
- 13.Sewankambo N, Gray RH, Wawer MJ, Paxton L, McNaim D, Wabwire-Mangen F, Serwadda D, Li C, Kiwanuka N, Hillier SL, Rabe L, Gaydos CA, Quinn TC, Konde-Lule J. Hiv-1 infection associated with abnormal vaginal flora morphology and bacterial vaginosis. Lancet. 1997;350:546–550. doi: 10.1016/s0140-6736(97)01063-5. [DOI] [PubMed] [Google Scholar]
- 14.Hillier SL, Kiviat NB, Hawes SE, Hasselquist MB, Hanssen PW, Eschenbach DA, Holmes KK. Role of bacterial vaginosis-associated microorganisms in endometritis. Am J Obstet Gynecol. 1996;175:435–441. doi: 10.1016/s0002-9378(96)70158-8. [DOI] [PubMed] [Google Scholar]
- 15.Hillier SL, Nugent RP, Eschenbach DA, Krohn MA, Gibbs RS, Martin DH, Cotch MF, Edelman R, Pastorek JG, 2nd, Rao AV, et al. Association between bacterial vaginosis and preterm delivery of a low-birth-weight infant. The vaginal infections and prematurity study group. N Engl J Med. 1995;333:1737–1742. doi: 10.1056/NEJM199512283332604. [DOI] [PubMed] [Google Scholar]
- 16.Ralph SG, Rutherford AJ, Wilson JD. Influence of bacterial vaginosis on conception and miscarriage in the first trimester: Cohort study. Bmj. 1999;319:220–223. doi: 10.1136/bmj.319.7204.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cherpes TL, Melan MA, Kant JA, Cosentino LA, Meyn LA, Hillier SL. Genital tract shedding of herpes simplex virus type 2 in women: Effects of hormonal contraception, bacterial vaginosis, and vaginal group b streptococcus colonization. Clin Infect Dis. 2005;40:1422–1428. doi: 10.1086/429622. [DOI] [PubMed] [Google Scholar]
- 18.Martin HL, Richardson BA, Nyange PM, Lavreys L, Hillier SL, Chohan B, Mandaliya K, Ndinya-Achola JO, Bwayo J, Kreiss J. Vaginal lactobacilli, microbial flora, and risk of human immunodeficiency virus type 1 and sexually transmitted disease acquisition. J Infect Dis. 1999;180:1863–1868. doi: 10.1086/315127. [DOI] [PubMed] [Google Scholar]
- 19.Barchet W, Cella M, Colonna M. Plasmacytoid dendritic cells--virus experts of innate immunity. Semin Immunol. 2005;17:253–261. doi: 10.1016/j.smim.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 20.Fonteneau JF, Gilliet M, Larsson M, Dasilva I, Munz C, Liu YJ, Bhardwaj N. Activation of influenza virus-specific cd4+ and cd8+ t cells: A new role for plasmacytoid dendritic cells in adaptive immunity. Blood. 2003;101:3520–3526. doi: 10.1182/blood-2002-10-3063. [DOI] [PubMed] [Google Scholar]
- 21.Kadowaki N, Antonenko S, Lau JY, Liu YJ. Natural interferon alpha/beta-producing cells link innate and adaptive immunity. J Exp Med. 2000;192:219–226. doi: 10.1084/jem.192.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol. 1991;29:297–301. doi: 10.1128/jcm.29.2.297-301.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spisek R, Brazova J, Rozkova D, Zapletalova K, Sediva A, Bartunkova J. Maturation of dendritic cells by bacterial immunomodulators. Vaccine. 2004;22:2761–2768. doi: 10.1016/j.vaccine.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 24.Hartmann G, Weiner GJ, Krieg AM. Cpg DNA: A potent signal for growth, activation, and maturation of human dendritic cells. Proc Natl Acad Sci U S A. 1999;96:9305–9310. doi: 10.1073/pnas.96.16.9305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 26.Langenkamp A, Messi M, Lanzavecchia A, Sallusto F. Kinetics of dendritic cell activation: Impact on priming of th1, th2 and nonpolarized t cells. Nat Immunol. 2000;1:311–316. doi: 10.1038/79758. [DOI] [PubMed] [Google Scholar]
- 27.Cella M, Scheidegger D, Palmer-Lehmann K, Lane P, Lanzavecchia A, Alber G. Ligation of cd40 on dendritic cells triggers production of high levels of interleukin-12 and enhances t cell stimulatory capacity: T-t help via apc activation. J Exp Med. 1996;184:747–752. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Colonna M, Trinchieri G, Liu YJ. Plasmacytoid dendritic cells in immunity. Nat Immunol. 2004;5:1219–1226. doi: 10.1038/ni1141. [DOI] [PubMed] [Google Scholar]
- 29.McKenna K, Beignon AS, Bhardwaj N. Plasmacytoid dendritic cells: Linking innate and adaptive immunity. J Virol. 2005;79:17–27. doi: 10.1128/JVI.79.1.17-27.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Foti M, Granucci F, Ricciardi-Castagnoli P. A central role for tissue-resident dendritic cells in innate responses. Trends Immunol. 2004;25:650–654. doi: 10.1016/j.it.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 31.Bradshaw CS, Morton AN, Hocking J, Garland SM, Morris MB, Moss LM, Horvath LB, Kuzevska I, Fairley CK. High recurrence rates of bacterial vaginosis over the course of 12 months after oral metronidazole therapy and factors associated with recurrence. J Infect Dis. 2006;193:1478–1486. doi: 10.1086/503780. [DOI] [PubMed] [Google Scholar]
- 32.Auffermann-Gretzinger S, Keeffe EB, Levy S. Impaired dendritic cell maturation in patients with chronic, but not resolved, hepatitis c virus infection. Blood. 2001;97:3171–3176. doi: 10.1182/blood.v97.10.3171. [DOI] [PubMed] [Google Scholar]
- 33.Kakumu S, Ito S, Ishikawa T, Mita Y, Tagaya T, Fukuzawa Y, Yoshioka K. Decreased function of peripheral blood dendritic cells in patients with hepatocellular carcinoma with hepatitis b and c virus infection. J Gastroenterol Hepatol. 2000;15:431–436. doi: 10.1046/j.1440-1746.2000.02161.x. [DOI] [PubMed] [Google Scholar]
- 34.Kanto T, Inoue M, Miyatake H, Sato A, Sakakibara M, Yakushijin T, Oki C, Itose I, Hiramatsu N, Takehara T, Kasahara A, Hayashi N. Reduced numbers and impaired ability of myeloid and plasmacytoid dendritic cells to polarize t helper cells in chronic hepatitis c virus infection. J Infect Dis. 2004;190:1919–1926. doi: 10.1086/425425. [DOI] [PubMed] [Google Scholar]
- 35.Dolganiuc A, Chang S, Kodys K, Mandrekar P, Bakis G, Cormier M, Szabo G. Hepatitis c virus (hcv) core protein-induced, monocyte-mediated mechanisms of reduced ifn-{alpha} and plasmacytoid dendritic cell loss in chronic hcv infection. J Immunol. 2006;177:6758–6768. doi: 10.4049/jimmunol.177.10.6758. [DOI] [PubMed] [Google Scholar]
- 36.Lopez C, Fitzgerald PA, Siegal FP. Severe acquired immune deficiency syndrome in male homosexuals: Diminished capacity to make interferon-alpha in vitro associated with severe opportunistic infections. J Infect Dis. 1983;148:962–966. doi: 10.1093/infdis/148.6.962. [DOI] [PubMed] [Google Scholar]
- 37.Kamga I, Kahi S, Develioglu L, Lichtner M, Maranon C, Deveau C, Meyer L, Goujard C, Lebon P, Sinet M, Hosmalin A. Type i interferon production is profoundly and transiently impaired in primary hiv-1 infection. J Infect Dis. 2005;192:303–310. doi: 10.1086/430931. [DOI] [PubMed] [Google Scholar]
- 38.Mitchell P, Germain C, Fiori PL, Khamri W, Foster GR, Ghosh S, Lechler RI, Bamford KB, Lombardi G. Chronic exposure to helicobacter pylori impairs dendritic cell function and inhibits th1 development. Infect Immun. 2007;75:810–819. doi: 10.1128/IAI.00228-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fredricks DN, Fiedler TL, Marrazzo JM. Molecular identification of bacteria associated with bacterial vaginosis. N Engl J Med. 2005;353:1899–1911. doi: 10.1056/NEJMoa043802. [DOI] [PubMed] [Google Scholar]
- 40.Hill GB. The microbiology of bacterial vaginosis. Am J Obstet Gynecol. 1993;169:450–454. doi: 10.1016/0002-9378(93)90339-k. [DOI] [PubMed] [Google Scholar]
- 41.Piot P, Van Dyck E, Godts P, Vanderheyden J. The vaginal microbial flora in non-specific vaginitis. Eur J Clin Microbiol. 1982;1:301–306. doi: 10.1007/BF02019976. [DOI] [PubMed] [Google Scholar]
- 42.Eschenbach DA, Davick PR, Williams BL, Klebanoff SJ, Young-Smith K, Critchlow CM, Holmes KK. Prevalence of hydrogen peroxide-producing lactobacillus species in normal women and women with bacterial vaginosis. J Clin Microbiol. 1989;27:251–256. doi: 10.1128/jcm.27.2.251-256.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Klebanoff SJ, Hillier SL, Eschenbach DA, Waltersdorph AM. Control of the microbial flora of the vagina by h2o2-generating lactobacilli. J Infect Dis. 1991;164:94–100. doi: 10.1093/infdis/164.1.94. [DOI] [PubMed] [Google Scholar]
- 44.Zariffard MR, Saifuddin M, Sha BE, Spear GT. Detection of bacterial vaginosis-related organisms by real-time pcr for lactobacilli, gardnerella vaginalis and mycoplasma hominis. FEMS Immunol Med Microbiol. 2002;34:277–281. doi: 10.1111/j.1574-695X.2002.tb00634.x. [DOI] [PubMed] [Google Scholar]
- 45.Antonio MA, Hawes SE, Hillier SL. The identification of vaginal lactobacillus species and the demographic and microbiologic characteristics of women colonized by these species. J Infect Dis. 1999;180:1950–1956. doi: 10.1086/315109. [DOI] [PubMed] [Google Scholar]
- 46.Masfari AN, Duerden BI, Kinghorn GR. Quantitative studies of vaginal bacteria. Genitourin Med. 1986;62:256–263. doi: 10.1136/sti.62.4.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Drakes M, Blanchard T, Czinn S. Bacterial probiotic modulation of dendritic cells. Infect Immun. 2004;72:3299–3309. doi: 10.1128/IAI.72.6.3299-3309.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thoma-Uszynski S, Kiertscher SM, Ochoa MT, Bouis DA, Norgard MV, Miyake K, Godowski PJ, Roth MD, Modlin RL. Activation of toll-like receptor 2 on human dendritic cells triggers induction of il-12, but not il-10. J Immunol. 2000;165:3804–3810. doi: 10.4049/jimmunol.165.7.3804. [DOI] [PubMed] [Google Scholar]
- 49.Hedges SR, Barrientes F, Desmond RA, Schwebke JR. Local and systemic cytokine levels in relation to changes in vaginal flora. J Infect Dis. 2006;193:556–562. doi: 10.1086/499824. [DOI] [PubMed] [Google Scholar]
- 50.Kayisli UA, Mahutte NG, Arici A. Uterine chemokines in reproductive physiology and pathology. Am J Reprod Immunol. 2002;47:213–221. doi: 10.1034/j.1600-0897.2002.01075.x. [DOI] [PubMed] [Google Scholar]
- 51.Jacobsson B, Holst RM, Wennerholm UB, Andersson B, Lilja H, Hagberg H. Monocyte chemotactic protein-1 in cervical and amniotic fluid: Relationship to microbial invasion of the amniotic cavity, intra-amniotic inflammation, and preterm delivery. Am J Obstet Gynecol. 2003;189:1161–1167. doi: 10.1067/s0002-9378(03)00594-5. [DOI] [PubMed] [Google Scholar]
- 52.Cauci S, Driussi S, Guaschino S, Isola M, Quadrifoglio F. Correlation of local interleukin-1beta levels with specific iga response against gardnerella vaginalis cytolysin in women with bacterial vaginosis. Am J Reprod Immunol. 2002;47:257–264. doi: 10.1034/j.1600-0897.2002.01096.x. [DOI] [PubMed] [Google Scholar]
- 53.Cauci S, Guaschino S, De Aloysio D, Driussi S, De Santo D, Penacchioni P, Quadrifoglio F. Interrelationships of interleukin-8 with interleukin-1beta and neutrophils in vaginal fluid of healthy and bacterial vaginosis positive women. Mol Hum Reprod. 2003;9:53–58. doi: 10.1093/molehr/gag003. [DOI] [PubMed] [Google Scholar]
- 54.Nakahara T, Urabe K, Fukagawa S, Uchi H, Inaba K, Furue M, Moroi Y. Engagement of human monocyte-derived dendritic cells into interleukin (il)-12 producers by il-1beta + interferon (ifn)-gamma. Clin Exp Immunol. 2005;139:476–482. doi: 10.1111/j.1365-2249.2004.02709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rescigno M, Urbano M, Valzasina B, Francolini M, Rotta G, Bonasio R, Granucci F, Kraehenbuhl JP, Ricciardi-Castagnoli P. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol. 2001;2:361–367. doi: 10.1038/86373. [DOI] [PubMed] [Google Scholar]
- 56.Niedergang F, Kweon MN. New trends in antigen uptake in the gut mucosa. Trends Microbiol. 2005;13:485–490. doi: 10.1016/j.tim.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 57.Leblanc DM, Barousse MM, Fidel PL., Jr Role for dendritic cells in immunoregulation during experimental vaginal candidiasis. Infect Immun. 2006;74:3213–3221. doi: 10.1128/IAI.01824-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cohn JA, Hashemi FB, Camarca M, Kong F, Xu J, Beckner SK, Kovacs AA, Reichelderfer PS, Spear GT. Hiv-inducing factor in cervicovaginal secretions is associated with bacterial vaginosis in hiv-1-infected women. J Acquir Immune Defic Syndr. 2005;39:340–346. doi: 10.1097/01.qai.0000146599.47925.e0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Taha TE, Hoover DR, Dallabetta GA, Kumwenda NI, Mtimavalye LA, Yang LP, Liomba GN, Broadhead RL, Chiphangwi JD, Miotti PG. Bacterial vaginosis and disturbances of vaginal flora: Association with increased acquisition of hiv. Aids. 1998;12:1699–1706. doi: 10.1097/00002030-199813000-00019. [DOI] [PubMed] [Google Scholar]
- 60.Weissman D, Li Y, Orenstein JM, Fauci AS. Both a precursor and a mature population of dendritic cells can bind hiv. However, only the mature population that expresses cd80 can pass infection to unstimulated cd4+ t cells. J Immunol. 1995;155:4111–4117. [PubMed] [Google Scholar]
- 61.Granelli-Piperno A, Moser B, Pope M, Chen D, Wei Y, Isdell F, O’Doherty U, Paxton W, Koup R, Mojsov S, Bhardwaj N, Clark-Lewis I, Baggiolini M, Steinman RM. Efficient interaction of hiv-1 with purified dendritic cells via multiple chemokine coreceptors. J Exp Med. 1996;184:2433–2438. doi: 10.1084/jem.184.6.2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pope M. Mucosal dendritic cells and immunodeficiency viruses. J Infect Dis. 1999;179(Suppl 3):S427–430. doi: 10.1086/314798. [DOI] [PubMed] [Google Scholar]
- 63.Hladik F, Lentz G, Akridge RE, Peterson G, Kelley H, McElroy A, McElrath MJ. Dendritic cell-t-cell interactions support coreceptor-independent human immunodeficiency virus type 1 transmission in the human genital tract. J Virol. 1999;73:5833–5842. doi: 10.1128/jvi.73.7.5833-5842.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Turville SG, Santos JJ, Frank I, Cameron PU, Wilkinson J, Miranda-Saksena M, Dable J, Stossel H, Romani N, Piatak M, Jr, Lifson JD, Pope M, Cunningham AL. Immunodeficiency virus uptake, turnover, and 2-phase transfer in human dendritic cells. Blood. 2004;103:2170–2179. doi: 10.1182/blood-2003-09-3129. [DOI] [PubMed] [Google Scholar]


