Abstract
Visual selection is influenced by items in working memory (WM) and priming from implicit memory when a stimulus is repeated across time. WM effects are typically held to be top-down in nature [Soto D, Heinke D, Humphreys GW, Blanco MJ (2005) J Exp Psychol Hum Percept Perform 31:248–261], whereas implicit priming may operate in a bottom-up manner [Theeuwes J, Reimann B, Mortier K (2006) Vis Cogn 14: 466–489]. How WM and implicit priming affects influence visual selection remains poorly understood, however. Here, we report functional MRI evidence that dissociates the neural mechanisms involved in these memory-based effects on selection. The reappearance of a stimulus held in WM enhanced activity in superior frontal gyrus, midtemporal, and occipital areas that are known to encode the prior occurrence of stimuli. In contrast, mere stimulus repetition elicited a suppressive response in the same regions. An additional finding was that a frontothalamic network was sensitive to the behavioral relevance of a match between the contents of WM and the visual search array, enhancing activity when the contents of WM matched the critical target of selection. Items held in WM influence selection by using neural coding distinct to effects of mere repetition.
Keywords: repetition priming, working memory, fMRI, anterior prefrontal cortex: BA10, thalamus
Imagine you are in love and the memory of your beloved is constantly active in your thoughts. Your task requires that you focus attention on your computer screen; despite it being detrimental to the task, you cannot avoid attending to the picture of your loved one placed on the desk nearby. This example shows how the contents of working memory (WM) can capture visual attention. The aim of the current study is to investigate the neural mechanisms that give rise to such phenomena.
Over the last 20 years, there has been active study of the mechanisms involved in memory-based guidance of visual attention. This work has led to the development of the influentially biased competition model of visual selection (1), where it is hypothesized that memory acts to bias the competition for selection between different objects in the visual scene. However, at least two forms of memory may influence visual selection: an active WM for a stimulus (2–14) and a more passive form of implicit memory generated by the mere reappearance of a stimulus across time (15–20). Conceptually, there is an important difference between WM processes and other forms of implicit memory, which are based on the mere repetition of a stimulus. The maintenance of information in WM entails an internal representation that is kept active online through a period by a network of relatively anterior neural regions (14). In contrast, repetition priming does not require the active maintenance of any internal representation and may reflect automatic, experience-based changes. Because these changes may operate through the processing pathways leading to a response, they may generate bottom-up changes in signal strength without necessarily invoking the need for top-down feedback from an active memory representation. The effects of both forms of memory on visual selection have proved difficult to distinguish by using typical measures of performance (i.e., response latencies) because at a behavioral level both WM and repetition priming can facilitate selection of matching stimuli in visual search tasks. For example, it has been demonstrated that the contents of WM can automatically draw our attention to matching objects in the visual field, and this process occurs even when the information in WM is detrimental to performance (3–6). In contrast, visual selection can be modulated automatically by repetition priming from stimuli of which we are not aware. For instance, a search for a given target is facilitated if the target is repeated across successive trials (15, 16), and this benefit can arise even when a stimulus is not consciously detected on the earlier trial (17). To assess whether the underlying mechanisms of WM and priming from implicit memory can be distinguished, we examined the neural correlates of their effects on selection.
Previously, researchers have used delayed match to sample tasks to study memory-based influences on neural responses. In these tasks, the presentation of a sample item (A) is followed after a time delay by one or more test stimuli (i.e., A-B-B-C-A). The observer has to indicate whether the test (i.e., the final A in the sequence) matched the initial sample. Single-cell recording studies demonstrate that a match between a stimulus and a WM representation is associated with increased responses in the inferior and medial temporal cortex (7–9) and prefrontal cortex (PFC) (10–14). In contrast, the mere repetition of a stimulus has mostly been shown to reduce neuronal responses in similar regions (7, 19–20). Intriguingly, most neuroimaging studies using similar paradigms with human participants suggest that different neural regions are influenced by mere repetition of an item and active memory processes (21–23). Thus, another aim of the current study was to test whether dissociation between WM and stimulus repetition could be observed within the same brain regions, as suggested by electrophysiological recording, or just across regions, as suggested by human functional MRI (fMRI).
Furthermore, we extended the delayed-match-to-sample studies in an important way. In such studies, the neural responses to the reappearance of the WM item typically coincide with neural responses based on the relevance of the item for the task because the WM item also is the target of selection. Thus, it is unclear whether any differential response to the WM item, relative to a novel stimulus, reflects the influence of WM on selection or preparation of a response to the task-relevant stimulus. This same problem exists in studies contrasting explicit and implicit memory in humans because a response is typically required on the basis of the stimulus matching the explicit memory, but not on the basis of whether there is a match to an implicit memory (22). Consequently, it could be that the enhancement of neuronal responses found in these tasks for the memorized item may not be specifically related to memory, but instead due to the stimulus representing the critical target of selection or to it being a signal for response preparation. Here, we used an experimental protocol where memory and attention are independently manipulated within a single paradigm (i.e., the memory stimulus is different from the critical target of selection). Observers were presented with an object cue (to either hold in WM or merely identify) and subsequently had to search for a target that could appear surrounded by either a cued or noncued object. In contrast to previous studies, our experimental design allowed us to specify the relations between these memory-based effects at a neural level and their role in visual selection. We first aimed to delineate those brain regions sensitive to matches between memory and search displays (regions where activity is modulated as a function of whether the memory information is represented in the array). In addition, we assessed responses in regions involved in monitoring the behavioral relevance of matches between memory and search display (i.e., when the reappearance of the cue coincides with the relevant target of selection, compared with when it coincides with an irrelevant distracter). Because the memory content was unrelated to the critical target of selection, we were able to assess the influence of memory on attention while avoiding any strategic effects that could arise if the cue were the target of selection, as is usual in delayed-match-to-sample tasks (9, 12) and explicit memory tests (22, 23).
One possibility is that WM and repetition priming use common neural mechanisms to guide and bias selection. In line with this idea, the WM effect on selection may simply amplify any process associated with repetition priming. Here, we would expect that similar regions are engaged in both cases, but merely showing quantitative differences in the level of activity. Alternatively, it may be that WM affects selection by using qualitative differences in the neural signal relative to the effects of repetition priming. According to this later hypothesis, we should find evidence for dissociable neural mechanisms within the same or across different brain regions, supporting, on the one hand, memory-based guidance of visual selection from WM and, on the other hand, implicit priming.
Results
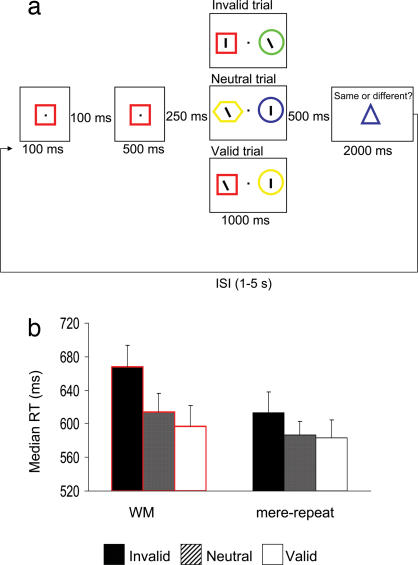
We had observers identify a target line presented within one of two colored shapes. Before the target display, participants saw two exposures of a colored shape cue, and this cue could later be re-presented in the target display where it surrounded either the target (on valid trials) or a distracter (on invalid trials). On neutral trials, the cue was absent from the search display (Fig. 1a). Observers had to either hold the cue in memory (the WM condition) or just identify it (the mere repetition condition). In the mere repetition condition, observers were instructed to compare the two cue exposures and withhold their response to the target when the second cue differed in color or shape from the first (there were 20% catch trials with two different cues). If both instances of the cue matched, then participants had to carry out the search task. In the WM condition, both instances of the cue were always the same, and participants had to hold the cue in memory through the trial (memory was tested on 20% of the trials). The 20% catch trials of WM and mere repetition were not included in the analysis. Hence, the visual displays and trial sequence used in the WM and mere repetition conditions were identical in the visual sequence of events, differing only in how participants were instructed to represent the cue (hold it in WM or merely identify it).
Fig. 1.
Illustration of the experimental stimuli and behavioral data. (a) An example of the display sequences used. (b) Median RTs across the different validity conditions when the cue was held in WM and when it was merely identified (mean ± SEM).
Behavioral Data.
Accuracy in the search task was good (94% correct), and memory performance on catch trials was high (89% correct). Search responses on catch trials in the mere repetition condition also were withheld adequately (98% correct). Catch trials and incorrect search responses were removed from all of the following analyses. We carried out a 2 (task: WM vs. mere repetition) × 3(validity: invalid, neutral, valid) ANOVA over the median reaction times (RTs) and used Greenhouse–Geisser correction to account for nonsphericity effects in the data (Fig. 1b). Search RTs were slower in the WM condition than in the mere repetition condition [F(1, 9) = 19.83, P = 0.002]. There also was a reliable validity effect [F(2, 18) = 9.17, P = 0.008]. Relative to the neutral baseline, performance was slower on invalid trials (when the cue reappeared and contained a distracter) (P = 0.001). RTs on valid trials (when the cue reappeared and contained the target) did not significantly differ from the neutral baseline (P = 0.41). Crucially, the effect of cue validity was modulated by the memory requirements of the task [F(2, 18) = 4.91, P = 0.025]. Follow-up analyses showed a significant cue validity effect in the WM condition [F(2, 18) = 14.15, P = 0.002], whereas there was only a nonreliable trend for a validity effect in the mere repetition case [F(2, 18) = 2.66, P = 0.12]. The costs from an invalid cue (i.e., the RT difference between invalid and neutral trials) were reliable in both tasks (WM, 54 ms; mere repetition, 27 ms) (both P < 0.035), but the costs were significantly larger in the WM condition [interaction between task and validity cost (invalid vs. neutral); F(1, 9) = 5.97, P = 0.037]. The data indicate that the cue validity effect was stronger when the cue was maintained in WM, compared with when it was merely repeated. There was no evidence of a speed–accuracy tradeoff [see supporting information (SI) Materials and Methods].
fMRI Data.
We report findings for the main effect of task in SI Materials and Methods. In brief, similar to previous fMRI studies, larger neuronal responses were observed in the WM condition, compared with the mere repetition condition in a network of areas: bilateral prefrontal cortices, bilateral intraparietal sulcus, lateral occipital cortices, and calcarine sulcus (14). There also was a larger response in the mere repetition condition relative to the WM condition in the midanterior cingulate gyrus. This later finding is consistent with previous evidence that this area increases activity during the less cognitive demanding tasks (24) (see SI Fig. 4, SI Table 3, and SI Materials and Methods). Next we describe results relating to the main focus of the current study, which deals with the way in which WM and priming through implicit memory affect visual selection.
Cue Repetition Effects on Neural Responses.
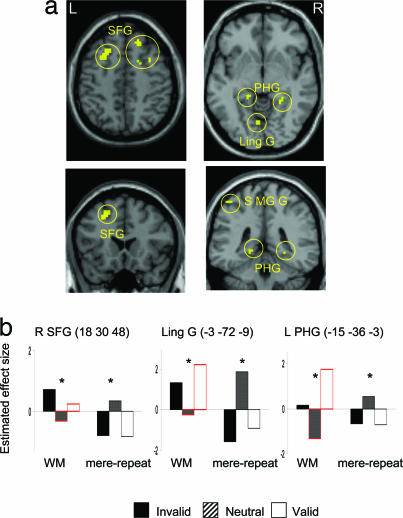
To delineate the mechanisms subserving the effects of different memory types on visual selection, we compared the brain response when the cue reappeared in the search display (on valid and invalid trials) with responses in the neutral baseline (where the cue did not reappear) for the WM versus the mere repetition condition. Note that this comparison is independent of the relevance of the cue item to the search task. Interestingly, the superior frontal gyrus [(SFG) in the vicinity of the frontal eye fields], the lingual gyrus (Ling G), and the bilateral parahippocampal gyrus (PHG) (Table 1 and Fig. 2) were sensitive to the reappearance of the cue, and, more important, their responses depended on the memory requirements of the task. When the task required active maintenance in WM, the reappearance of the memory cue (across invalid and valid trials) increased activity (compared with neutral trials). In contrast, in the mere repetition condition, these same regions showed decreased responses when the cue reappeared in the search array, compared with when it was absent.
Table 1.
Cue repetition effects
| Anatomical label | BA | H | Z | MNI, mm |
|---|---|---|---|---|
| Interaction task × cue repetition: mere repetition: cue repeat [Valid + Invalid] < no repeat [neutral trials] and WM: cue repeat [Valid + Invalid] > no repeat [neutral trials] | ||||
| Frontal | ||||
| SFG | 6, 8 | R | 4.15 | 18, 30, 48 |
| SFG | 6 | L | 4.09 | −24, 18, 45 |
| Parietal | ||||
| SMG | 40 | L | 3.36 | −42, −36, 60* |
| Temporal | ||||
| PHG | 30 | R | 3.54 | 24, −42, −6* |
| PHG | 30 | L | 3.62 | −15, −36, −3* |
| Occipital | ||||
| Ling G | 18 | L | 3.47 | −3, −72, −9 |
| Subcortical | ||||
| Cerebellum | — | R | 3.85 | 3, −87, −36 |
| Cerebellum | — | L | 3.67 | −15, −57, −15, |
| Effects of cue repetition common to both tasks: repeat [Valid + Invalid] < nonrepeat [neutral] | ||||
| Frontal | ||||
| aCG | 32 | R | 4.06 | 15, 51, 15 |
| Temporal | ||||
| ITG | 20 | L | 3.81 | −27, −36, −24 |
Anatomical label based on the Duvernoy Human Brain Atlas: BA, Brodmann's area. Anatomical labels based on the Wake Forest University Pickatlas: H, hemisphere; SMG, supramarginal gyrus; Ling G, lingual gyrus; aCG, anterior cingulate gyrus; ITG, inferior temporal gyrus. All clusters of activation reported were threshold at P < 0.001 uncorrected, with cluster size >45 mm3, but the ones shown with an asterisk have a cluster size >15 mm3.
Fig. 2.
The interaction between cue repetition and task in the fMRI data. (a) In yellow, regions showing an increased response for the reappearance of the cue in the WM condition and a decreased response in the mere repetition condition, compared with when the cue did not reappear. The SPM thresholds were set at P < 0.001, uncorrected, with clusters >15 mm3 overlaid on axial and coronal views of a T1 single-subject template image. (b) The graphs depict the responses (estimated effect size) of three regions across the different conditions. The coordinates in MNI space and the anatomical labels for the regions are written above the graphs. The asterisks indicate significant simple effects (invalid + valid vs. neutral) (P < 0.05). L, left; R, right; SFG/FEF, superior frontal gyrus; SMG, supramarginal gyrus; PHG, parahippocampal gyrus.
In addition to these regions showing qualitatively different patterns of response, a common pattern of response to the reappearance of the cue in the search array was observed in anterior cingulate and left inferior temporal gyrus (Table 1). During both WM and mere repetition, these regions decreased their response when the cue reappeared in the search array, compared with when it did not reappear. There was no above-threshold response showing increased response to the reappearance of the cue in the mere repetition condition.
Cue Validity Effects on Neural Responses.
Here, we delineated the brain regions sensitive to the relevance of the cue for the search task, contrasting activity when the memory item contained the search target (on valid trials) versus when it contained a distracter (on invalid trials). The left amygdala [Montreal Neurological Institute (MNI) coordinates (−18, 9, −21)] showed an increased response to valid compared with neutral and invalid trials for both the WM and mere repetition tasks [Z = 4.64; F(2, 18) = 17.5; P < 0.001; Cluster = 120 mm3]. However, cue validity had a strong effect in the WM condition [Z = 2.9; F(1, 9) = 8.8; P < 0.05], and there was only a nonsignificant trend in the mere repetition condition [Z = 1.23; F(1, 9) = 2.47; P = 0.11].
More interestingly, we found that the validity effect on brain responses also was modulated by the memory type. Clusters within bilateral anterior PFC [peaking in the right Brodmann's area (BA)10] and bilateral thalamic nuclei (including the pulvinar) (Table 2 and Fig. 3) responded differentially only during the WM condition. In the WM condition, these regions showed larger responses when the cue contained the target (on valid trials) and suppressed responses when it contained a distracter (on invalid trials) relative to the neutral trials. There was no above-threshold response showing a validity effect during the mere repetition condition.
Table 2.
Effects of WM on search WM
| Anatomical label | BA | H | Z | MNI, mm |
|---|---|---|---|---|
| Frontal | ||||
| aPFC | 10 | R | 4.12 | 21, 63, 6 |
| aPFC | 10 | L | 3.59 | −24, 63, 0 |
| DLPFC | 46 | R | 3.51 | 42, 45, 9 |
| SFG | 6 | R | 4.75 | 18, 3, 54 |
| medOFC | 11 | L | 4.40 | −18, 36, −12 |
| CG | 24 | L | 4.64 | −3, 6, 30 |
| Temporal | ||||
| STG | 22 | L | 3.71 | −51, −15, −3 |
| Subcortical | ||||
| MD Thalamus/Pul | — | R | 3.47 | 9, −21, 9 |
| VL Thalamus | — | R | 4.10 | 24, −18, 3 |
| Putamen | — | R | 3.99 | 30, 6, 0 |
| Pulvinar | — | L | 3.24 | −15, −33, 3 |
| VL Thalamus/Pul | — | L | 3.87 | −15, −15, 6 |
| Putamen | — | L | 3.96 | −30, −3, 6 |
| Amygdala | — | L | 4.76 | −18, 9, −18 |
Valid > neutral > invalid (masked by the interaction between task and validity) (P < 0.05). Anatomical label based on the Duvernoy Human Brain Atlas: BA, Brodmann's area. Anatomical labels based on the Wake Forest University Pickatlas: H, hemisphere; aPFC, anterior prefrontal cortex; DLPFC, dorsolateral prefrontal cortex; CG, cingulated gyrus; IPS, intraparietal sulcus; MTG, middle temporal gyrus; MD, middle dorsal; Pul, pulvinar; VL, ventral lateral; CM, centromedial. All clusters of activation reported were threshold at P < 0.001 uncorrected, with cortical cluster size >45 mm3 and subcortical cluster size >15 mm3.
Fig. 3.
Validity effects in the WM condition. (a) In yellow, regions showing a reliably increased response to the reappearance of the cue on valid trials and a decreased response on invalid trials, relative to the neutral baseline. The SPM threshold was set at P < 0.001, uncorrected, with cluster sizes >15 mm3, with clusters overlaid on a T1 single-subject template image. (b) The yellow rectangle in a depicts the thalamic nuclei involved. The fMRI clusters are overlaid on diffusion tensor imaging probabilistic maps describing connections between various regions of the thalamus and cortex. The diffusion tensor imaging maps were generated and reported by Johansen-Berg et al. (25). The different colors within the different thalamic nuclei illustrate the cortical regions with which they are interconnected. For example, the blue regions are likely to be connected with PFC, whereas the cyan regions are likely to be connected with occipital cortices. (c) The graphs depict the responses (estimated effect size) of three regions across the different conditions. The coordinates in MNI space and the anatomical labels for the regions are written above the graphs. The asterisks indicate significant simple effects (invalid + valid vs. neutral) (P < 0.001). L, left; R, right; aPFC, anterior prefrontal cortex; MD, middle.
To explore the role of the thalamic clusters, we overlapped our functional clusters with probabilistic maps based on diffusion tensor imaging (25). Based on these maps, our thalamic clusters can be identified as having reciprocal connections with the prefrontal, premotor, parietal, temporal, and occipital cortices (Fig. 3b). A functional connectivity analysis [based on a psychophysiological interaction (PPI)] (26) showed that these structural anatomical connections also were functionally connected in our experiment during the WM condition. We observed increased coupling between right BA10 (MNI: 21, 63, 6) and clusters within bilateral thalamic nuclei (left ventral lateral: −15, −3, 0; Z = 2.86, P = 0.002; right lateral dorsal: 9, −6, 6; Z = 2.99, P = 0.001; right ventral lateral: 15, −6, −9; Z = 2.91, P = 0.002) when the WM item reappeared in the search array (on valid and invalid trials) relative to the neutral condition. Activation in right BA10 also correlated with the response of bilateral dorsolateral PFC (BA46, left: −36, 45, 30; Z = 3.57; P < 0.001; right: 36, 36, 33; Z = 2.85, P = 0.002) on trials where the cue reappeared in the search array relative to the neutral condition.
Discussion
In this study, we investigated the neural mechanisms that underlie the effects of WM and priming from implicit memory on visual selection. We delineated two different networks. The first network is involved in both WM and implicit memory effects, but shows an opposite pattern of response depending on whether there is a match to WM or merely repetition priming. This network includes the PHG, Ling G, and SFG, and it was sensitive to the reappearance of visual stimuli independent of their relevance to the search task. The reappearance of the cue in the search array caused an increased response when the cue was actively held in WM, whereas there was a decreased response when the cue reappeared in the mere repetition condition. A second frontothalamic network also was found. This network was linked to the relevance of the contents of WM, and it was not responsive to the reappearance and relevance of the cue in the mere repetition condition. The frontothalamic network enhanced activity when there was a relevant match (on valid trials, when the memory item contained the relevant target), whereas activity was suppressed when the memory information was detrimental for target selection (on invalid trials).
The first network, including the PHG, Lin G, and SFG, has been implicated previously in memory processes. In accordance with our findings, these regions typically exhibit either repetition suppression or enhancement depending on whether active memory is involved (7, 10–12, 18). In contrast with prior studies, the increases in activity linked to the reappearance of the WM item here occurred even though the item in WM was not the relevant target of attention. In previous studies, any neuronal enhancement may not reflect WM per se, but rather the WM item being the target for selection (7, 9–14) or response (21). Our data demonstrate that the increases of activity in SFG, Ling G, and PHG (see Table 1) do not reflect the relevance of the object for visual selection, but rather reflect matches between the contents of WM and external stimuli. Moreover, although the WM and mere repetition effects were mediated through the same brain regions (see Table 1), the neuronal mechanisms were qualitatively distinct. We found that, relative to the neutral baseline (where there was no matching between the memory cue and the visual search array), the reappearance of the cue led to an enhanced response when the cue was held in WM, but it led to a suppressed response when the cue was merely repeated. This finding is in line with data from studies using single-cell recording, and it suggests that neurons within the same brain regions can respond differently according to the context of a prior experience (7, 8, 18). The context of merely being repeated or matching an item in WM effects a change from response suppression to enhancement. We propose that response suppression due to mere stimulus repetition reflects neuronal changes related to increased efficiency of processing when items match across successive presentations (27). In contrast, we suggest that enhancement of the neuronal responses based on visual stimuli matching the contents of WM is responsible for the capture of attention by the matching object (3, 4). This capture of attention by response enhancement in the WM condition produces larger biases on visual selection than mere repetition (4–6). This result also was confirmed by our behavioral data here, where the effects of the cue on visual search were more pronounced in the WM condition than in the mere repetition case.
In addition to the differential responses in the WM and mere repetition conditions, we also observed common mechanisms for the two conditions. For example, the anterior cingulate gyrus and the left inferior temporal gyrus both showed reduced responses when the memory item reappeared in the search display, and this finding was independent of whether the cue was maintained in WM or merely identified. This result may relate to increased efficiency in the processing of repeated visual input and indicates that some degree of perceptual priming occurred in the WM condition and not only in the mere repetition condition. Our data provide strong evidence to suggest that guidance of attention by the contents of WM is mediated mostly by neuronal responses that dissociate from the effects caused by mere repetition of the stimulus.
The second network we identified, involving frontal and thalamic regions, was only evident during the WM condition (Table 2). This network showed enhanced responses on valid trials and suppressed responses on invalid trials, compared with the neutral conditions. We propose that when the memory cue is re-presented participants monitor the validity of the cue in relation to the target (the oriented line in our study) to allow the task-relevant response to take place in search. This monitoring was linked to an increased coupling among BA10, the thalamus, and the right dorsolateral PFC when the cue coincided with the target relative to when it did not (valid relative to neutral trials) and when the cue and the target fell at different locations (invalid relative to neutral trials). This network was involved only in the WM condition, suggesting that it was sensitive to the congruence between internal (item held in WM) and external (visual information in the search array) signals based on their relevance for current task goals. For example, enhanced activity on valid trials reflects the case where internal and external signals fully matched both observers' goals (holding an item in WM and identifying the search target). Decreased activity reflects the competition between the internal and external signals. Through its sensitivity to the congruence between stimuli and task goals, this frontothalamic network may complement the well known frontoparietal system controlling spatial attention and awareness (28–30). The existence of a frontothalamic attention system (that bypasses parietal cortex) also can explain how patients with visual extinction following damage to posterior parietal cortex show enhanced awareness for contralesional targets that match the contents of WM (3). The thalamus, through its reciprocal connections with most of the cortex, may have a privileged role in modulating responses in other cortical regions involved in selection. Our findings support the role of thalamic nuclei (i.e., pulvinar and ventrolateral parts) in controlling visual attention (31–33), particularly in guiding attention based on goal-relevant information held in prefrontal areas linked to WM.
Additionally, the current findings suggest the existence of functional dissociations within the PFC for memory and selection processes. PFC regions subserving the maintenance of information in WM (left dorsolateral PFC) and the encoding of memory traces regardless of their behavioral significance (SFG) are distinct from processes that monitor the relevance of memory information for current task goals (anterior PFC). The results have specific implications for the role of anterior PFC (BA10) in cognitive processing. Previous studies have implicated BA10 in problem-solving tasks that require an interplay between WM and attention. For example, activity in anterior PFC is found when the observer has to keep a task goal in WM while performing other task goals (34) or when the observer switches from performing a task based on incoming stimulation to another based on internal representations or vice versa (35). Such cognitive operations were likely present across the different trials here regardless of whether the memory item matched a target or a distracter, and therefore cannot fully explain our pattern of results. We instead propose that anterior PFC plays a role within the frontothalamic network by comparing internal and external representations and prioritizing them in accordance with the behavioral goals of the task.
The current study supports the argument that WM effects on selection reflect top-down influences from anterior neural circuits that modulate visual selection, whereas mere repetition leads to bottom-up changes in search efficiency. The influences of WM and mere repetition on visual selection reflect qualitatively different functional operations within and across different brain regions.
Methods
Participants.
Participants included 10 students from the University of Birmingham's School of Psychology who were all unaware of the purpose of the experiment. The students were between the ages of 18 and 23 years, were all right handed, did not have a history of any neurological or psychiatric disorders, and had normal vision. Course credits or cash were given for their participation. The study was approved by the local ethics committee.
Stimuli, Task, and Procedure.
Each trial began with a fixation display for 500 ms, followed by a cue displayed two times (100 and 500 ms, respectively) with a 100-ms blank interval in between. After a 250-ms blank interval, the search display appeared. The cue could be a circle, diamond, square, triangle, or hexagon. The color of the objects could be red, green, blue, yellow, or pink, and the background was gray. There were two lines (0.88° length) drawn in black ink at the center of each colored shape. The distracter line was vertical, and the target was tilted 26° to either the left or right. Each of the objects surrounding the lines was unique in color and shape (Fig. 1a). The objects were placed to the left and right of the fixation point with a center-to-center distance of 18°. Observers had to discriminate the orientation of the target line during a time window of 1,000 ms by pressing one of two different buttons, for left and right orientations. In the WM condition, observers were required to hold in memory the color and shape of the cue through the trial. In the mere repetition condition, observers were instructed to perceptually compare the two instances of the cue and withhold their response to the search display whenever the second presentation of the cue differed (either in color or in shape) from the first presentation. We introduced two types of catch trials that were not included in the analysis and that appeared with 20% likelihood throughout the experiment. During the WM condition, a memory test followed 500 ms after completion of the search task. Here, an item was displayed and the observers had to indicate whether it was the same color and shape as the cue or whether it was different (either in color or shape) within a 2-s limited time window. During the mere repetition condition, 20% of the trials featured two instances of the cue not matching, and participants then had to withhold their response to the search target. The visual display and events sequence used during the WM and mere repetition conditions was identical for all trials included in the analyses (i.e., the noncatch trials).
There were three different conditions determined by the validity of the cue for the search task. In the valid condition, the target line appeared within an object that matched both the color and shape of the cue. In the invalid condition, the precued object reappeared contained a distracter. A neutral condition also was included where the cue did not reappear in the search display. The different validity conditions occurred with the same probability and were selected randomly within each trial.
Participants were instructed about the probability of the different validity conditions and were encouraged to perform accurately in all tasks. Speeded responses also were required in the search task. The task factor was manipulated across blocks. Instructions about the task were presented for 3 s at the beginning of each block and were followed by two catch trials to minimize task-switching effects. Observers were instructed to maintain eye fixation at a dot placed at the center of the display throughout the whole experiment. Before scanning, the observers were familiarized with the task and performed several practice blocks where they were explicitly trained to maintain fixation during the task. Each participant took part in three fMRI sessions. Each session contained four randomly selected blocks (two WM and two mere repetition conditions) of 30 trials each for a grand total of 360 trials per participant (3 × 4 × 30). Stimuli were presented by using E-prime (36).
fMRI Data Acquisition.
We used a Phillips 3T Achieva system to acquire blood oxygenated level-dependent, contrast-weighted echoplanar images during the functional scans. Then 39 oblique slices, 2 mm thick with a 1-mm gap, were acquired, resulting in an in-plane resolution of 3 × 3 × 3 mm, with an 80° flip angle, 30-ms echo time, and 2,110-ms slice repetition time. Images were acquired by using an eight-channel phase array coil with a sense factor of 2. To minimize susceptibility artifacts, slices were tilted 30° along the frontal-temporal cortex (37).
Data Analysis.
The data were analyzed by using SPM5 (Wellcome Department of Imaging Neuroscience, London; www.fil.ion.ucl.ac.uk/spm). Echoplanar image volumes were spatially realigned to correct for movement artifacts, transformed to the MNI standard space (38), and smoothed by using a 9-mm Gaussian kernel to account for residual intersubject differences.
Voxel-Based Analysis.
This analysis was performed by using a random effects model. For each subject, we first estimated the effect size on each condition averaged across the three sessions. We used a first-level model that included a regressor of the onsets of the stimuli on each trial across the six different experimental conditions, following our 2 (task) × 3 (validity) design, which also were split in two regressors depending on the target location (left, right). In addition, we modeled two regressors for the onset of catch trials, error trials, and instructions separately for the WM and mere repetition conditions. Further, for each of the previous regressors, we included the RT of each event as a covariate to control for RT differences. These regressors were convolved with the canonical hemodynamic response function. To correct for signal changes because of head movement, the six realignment parameters were included in the design matrix. An additional set of harmonic regressors was used to account for any low-pass frequency variance within the data, along with a cutoff of 1/128 Hz. There were no above-threshold responses to target location or any interaction of target location with any of our effects of interest. Therefore, we report and discuss effects that collapsed across the target location factor. For each subject, we computed the averaged estimated response across the three sessions on each of the different conditions. Consistent effects across subjects (random effects second-level analysis) were then tested by using ANOVA with 2 (WM and mere repetition) × 3 (valid, neutral, invalid) within-subject factors. In the model, we did not assume independency, nor was equal variance between the conditions assumed. For cortical structures, we report >45-mm3 clusters at P < 0.001 uncorrected unless specified otherwise. Simple effects were tested on the estimated effect size of the maxima from the voxel-based analysis by using SPSS13 with repeated-measures ANOVA.
PPI Analyses.
These analyses were performed in SPM5 to test the changes in the coupling strength between different brain regions that responded to the experimental manipulations. The aim of the PPI analyses was to provide confirmatory evidence for the pattern of connectivity suggested by diffusion tensor imaging analysis between the thalamus and prefrontal regions. Specifically, we aimed to show that BA10 and thalamic nuclei were functionally connected. For each subject, we extracted an Eigen vector that represents the response within a 3-mm sphere centered around our region of interest, right BA10 (MNI: 21, 63, 6). Using deconvolution (26), the underlying neuronal responses were estimated for this region. The PPI was then computed for the WM condition with increased coupling during reappearance of the WM item (valid + invalid trials), compared with the neutral baseline. Then a new model was estimated for each subject. This model included regressors of the comparison made [psychological regressors: (valid + invalid) − neutral], a regressor for the time course of the region of interest (physiological regressor), and a regressor representing the PPI (psychological × physiological). In addition, we included regressors for trials during the mere repetition condition, two for catch trials, error responses, and instructions separately for both the WM and mere repetition conditions. All regressors (apart from the physiological regressor) were convolved with the HRF responses. The six realignment parameters and harmonics were also included in this model to account for low-pass frequencies (1/128 Hz). Again, for each subject, we computed the effect size of each PPI averaged across the three sessions. Consistent effects across subjects were then tested by using two sampled t tests in a model that assumed neither independency nor equal variance between the conditions. The increase in positive coupling was tested by using t tests.
Supplementary Material
Acknowledgments
We thank Joy J. Geng for comments. This work was supported by British Academy, Biotechnology and Biological Sciences Research Council, Economic and Social Research Council, and Medical Research Council grants.
Abbreviations
- fMRI
functional MRI
- BA
Brodmann's area
- PPI
psychophysiological interaction
- RT
reaction time
- PFC
prefrontal cortex
- PHG
parahippocampal gyrus
- SFG
superior frontal gyrus
- WM
working memory.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0703706104/DC1.
References
- 1.Desimone R, Duncan J. Annu Rev Neurosci. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- 2.Downing PE. Psychol Sci. 2000;11:467–473. doi: 10.1111/1467-9280.00290. [DOI] [PubMed] [Google Scholar]
- 3.Soto D, Humphreys GW, Heinke D. Vision Res. 2006;46:1010–1018. doi: 10.1016/j.visres.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 4.Soto D, Humphreys GW. Proc Natl Acad Sci USA. 2006;103:4789–4792. doi: 10.1073/pnas.0510718103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soto D, Humphreys GW. J Exp Psychol Hum Percept Perform. 2007;33:730–737. doi: 10.1037/0096-1523.33.3.730. [DOI] [PubMed] [Google Scholar]
- 6.Olivers CNL, Meijer F, Theeuwes J. J Exp Psychol Hum Percept Perform. 2006;32:1243–1265. doi: 10.1037/0096-1523.32.5.1243. [DOI] [PubMed] [Google Scholar]
- 7.Desimone R. Proc Natl Acad Sci USA. 1996;93:13494–13499. doi: 10.1073/pnas.93.24.13494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown MW, Xiang JZ. Prog Neurobiol. 1998;55:149–189. doi: 10.1016/s0301-0082(98)00002-1. [DOI] [PubMed] [Google Scholar]
- 9.Chelazzi L, Miller EK, Duncan J, Desimone R. Nature. 1993;363:345–347. doi: 10.1038/363345a0. [DOI] [PubMed] [Google Scholar]
- 10.Miller EK, Erickson CA, Desimone R. J Neurosci. 1996;16:5154–5167. doi: 10.1523/JNEUROSCI.16-16-05154.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rao SC, Rainer G, Miller EK. Science. 1997;276:821–824. doi: 10.1126/science.276.5313.821. [DOI] [PubMed] [Google Scholar]
- 12.Wilson FA, O'Scalaidhe SP, Goldman-Rakic PS. Science. 1993;260:1955–1958. doi: 10.1126/science.8316836. [DOI] [PubMed] [Google Scholar]
- 13.Curtis CE, D'Esposito M. Trends Cognit Sci. 2003;7:415–423. doi: 10.1016/s1364-6613(03)00197-9. [DOI] [PubMed] [Google Scholar]
- 14.Courtney SM, Petit L, Haxby JV, Ungerleider LG. Philos Trans R Soc London Ser B. 1998;353:1819–1828. doi: 10.1098/rstb.1998.0334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maljkovic V, Nakayama K. Mem Cognit. 1994;226:657–672. doi: 10.3758/bf03209251. [DOI] [PubMed] [Google Scholar]
- 16.Kristjánsson Á, Vuilleumier P, Schwartz S, Macaluso E, Driver J. Cereb Cortex. 2007;17:1612–1624. doi: 10.1093/cercor/bhl072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kristjánsson Á, Vuilleumier P, Malhotra P, Husain M, Driver J. J Cognit Neurosci. 2005;17:859–873. doi: 10.1162/0898929054021148. [DOI] [PubMed] [Google Scholar]
- 18.Miller EK, Desimone R. Science. 1994;267:520–522. doi: 10.1126/science.8290960. [DOI] [PubMed] [Google Scholar]
- 19.Bunzeck N, Schutze H, Duzel H. Neuropsychologia. 2006;44:1765–1776. doi: 10.1016/j.neuropsychologia.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 20.Bichot NP, Schall JD. Nat Neurosci. 1999;2:549–555. doi: 10.1038/9205. [DOI] [PubMed] [Google Scholar]
- 21.Jiang Y, Haxby JV, Martin A, Ungerleider LG, Parasuraman R. Science. 2000;287:643–646. doi: 10.1126/science.287.5453.643. [DOI] [PubMed] [Google Scholar]
- 22.Henson RN, Shallice T, Gorno-Tempini M-L, Dolan RJ. Cereb Cortex. 2002;13:793–805. doi: 10.1093/cercor/12.2.178. [DOI] [PubMed] [Google Scholar]
- 23.Donaldson DI, Petersen SE, Buckner RL. Neuron. 2001;31:1047–1059. doi: 10.1016/s0896-6273(01)00429-9. [DOI] [PubMed] [Google Scholar]
- 24.Schwartz S, Vuilleumier P, Hutton C, Maravita A, Dolan RJ, Driver J. Cereb Cortex. 2005;15:770–786. doi: 10.1093/cercor/bhh178. [DOI] [PubMed] [Google Scholar]
- 25.Johansen-Berg H, Behrens TE, Sillery E, Ciccarelli O, Thompson AJ, Smith SM, Matthews PM. Cereb Cortex. 2005;15:31–39. doi: 10.1093/cercor/bhh105. [DOI] [PubMed] [Google Scholar]
- 26.Gitelman DR, Penny WD, Ashburner J, Friston KJ. NeuroImage. 2003;19:200–207. doi: 10.1016/s1053-8119(03)00058-2. [DOI] [PubMed] [Google Scholar]
- 27.Wiggs CL, Martin A. Curr Opin Neurobiol. 1998;8:227–233. doi: 10.1016/s0959-4388(98)80144-x. [DOI] [PubMed] [Google Scholar]
- 28.Thibaut de Schotten M, Urbanski M, Duffau H, Volle E, Levy R, Dubois B, Bartolomeo P. Science. 2005;309:2226–2228. doi: 10.1126/science.1116251. [DOI] [PubMed] [Google Scholar]
- 29.Beck DM, Rees G, Frith CD, Lavie N. Nat Neurosci. 2001;4:645–650. doi: 10.1038/88477. [DOI] [PubMed] [Google Scholar]
- 30.Corbetta M, Shulman GL. Nat Rev Neurosci. 2002;3:215–229. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- 31.Crick F. Proc Natl Acad Sci USA. 1984;81:4586–4590. doi: 10.1073/pnas.81.14.4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Danziger S, Ward R, Owen V, Rafal RD. Behav Neurol. 2001;13:95–104. doi: 10.1155/2002/917570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rafal RD, Posner MI. Proc Natl Acad Sci USA. 1987;84:7349–7353. doi: 10.1073/pnas.84.20.7349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koechlin E, Basso G, Pietrini P, Panzer S, Grafman J. Nature. 1999;399:148–151. doi: 10.1038/20178. [DOI] [PubMed] [Google Scholar]
- 35.Gilbert SJ, Frith CD, Burgess PW. Eur J Neurosci. 2005;21:1423–1431. doi: 10.1111/j.1460-9568.2005.03981.x. [DOI] [PubMed] [Google Scholar]
- 36.Psychology Software Tools. E-Prime. Pittsburgh, PA: Psychology Software; 2002. Version 1.0. [Google Scholar]
- 37.Deichmann R, Gottfried JA, Hutton C, Turner R. NeuroImage. 2003;19:430–441. doi: 10.1016/s1053-8119(03)00073-9. [DOI] [PubMed] [Google Scholar]
- 38.Ashburner J, Friston KJ. In: Human Brain Function. Frackowiak RS, Friston KJ, Frith C, Dolan RJ, Price C, Zeki S, Ashburner J, Penny W, editors. New York: Academic; 2003. pp. 635–654. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.