Abstract
Hox transcription factors are essential for shaping body morphology in development and evolution. The control of Hox protein activity in part arises from interaction with the PBC class of partners, pre-B cell transcription factor (Pbx) proteins in vertebrates and Extradenticle (Exd) in Drosophila. Characterized interactions occur through a single mode, involving a short hexapeptide motif in the Hox protein. This apparent uniqueness in Hox–PBC interaction provides little mechanistic insight in how the same cofactors endow Hox proteins with specific and diverse activities. Here, we identify in the Drosophila Ultrabithorax (Ubx) protein a short motif responsible for an alternative mode of Exd recruitment. Together with previous reports, this finding highlights that the Hox protein Ubx has multiple ways to interact with the Exd cofactor and suggests that flexibility in Hox–PBC contacts contributes to specify and diversify Hox protein function.
Keywords: development, transcription, hexapeptide, UbdA
Hox genes play fundamental roles in shaping animal body plans in development and evolution (1), yet the mechanisms underlying Hox protein function remain poorly understood. Questions to be solved include how the same Hox protein directs distinct biological responses and how different Hox proteins bearing similar or identical biochemical properties promote specific developmental programs. These questions highlight diversity and specificity as intrinsic characteristics of Hox protein activity, which are likely to be mechanistically linked, as relying on Hox transcriptional properties. Although other proteins are presumably involved (2), our current knowledge of Hox protein mode of action mainly stands from the interaction with the PBC class of cofactors that specifies target site recognition (3, 4).
Our current view of Hox–PBC interactions is that they all occur through a single mode, involving a short hexapeptide (HX) motif. The importance of the Hox HX motif in mediating interaction with PBC proteins is extensively supported by its requirement in in vitro interaction assays (for examples, see ref. 5 and 6) and by crystallographic studies that showed that the HX provides most if not all major contacts (7–9). In contrast, in vivo functional support for a role of the HX in mediating interaction with PBC proteins is still limited, mainly because effects of HX mutations during development have only been assessed for two vertebrate Hox proteins, Hoxa-1 (10) and Hoxb-8 (11), and for three Drosophila proteins, Labial (Lab) (12), Ubx (13), and Abdominal-A (AbdA) (14).
Mutation of the HX in Hoxb-8 results in dominant phenotypes, which are at present difficult to interpret with regard to Pbx recruitment. Hoxa-1 HX mutation mimics Hoxa-1 loss of function, including defects in the hindbrain that could relate to loss of Pbx recruitment because inactivation of Pbx2 and Pbx4 in the zebrafish affects hindbrain patterning (15). However, addressing in vertebrates whether phenotypes resulting from HX mutations are consequences of defects in Pbx interaction will require examination of combinations of Hox-1 paralogous and Pbx gene mutations. In Drosophila, mutation of the HX in Lab, the only representative of Hox-1 class genes, results in an hyperactive protein when assayed for its potential to activate transcription through an evolutionarily conserved Hoxb-1 autoregulatory element (12, 16). This hyperactivity results from the loss of an inhibitory action of the HX on Lab DNA binding. In this context, it was proposed that HX-mediated recruitment of Extradenticle (Exd) acts to mask the DNA-binding inhibitory activity of the HX motif (12).
Although the Hoxa-1 and Lab studies support, yet not exclusively, a role of the HX in mediating recruitment of PBC class proteins during development, work on Drosophila Ubx and AbdA has provided evidences for HX-independent mode of Exd recruitment. Regarding Ubx, a truncated protein lacking N-terminal sequences (including the HX) was shown in vitro to retain Exd recruitment potential (17) and to interact weakly with Exd in yeast two-hybrid assays (5). More specifically, mutation of the HX does not affect the capacity to recruit Exd on a Hox/Exd consensus target sequence in vitro and to repress in an Exd-dependent manner the limb-promoting gene Distalless [Dll (13, 18)], which has served as a paradigm to study Hox–Exd interactions (2, 19–21). Concerning AbdA, the HX-deficient protein was shown to recruit Exd on the Dll regulatory element that mediates Dll repression (14), consistent with its retained ability to repress Dll.
Thus, Hox–PBC interactions are not limited to HX-mediated interactions, highlighting that another Hox protein motif, yet to be identified, may also assume this function. The Ubx C terminus [sequences downstream of the homeodomain (HD), UC], important for Ubx segment identity functions (22) and shown to increase the ability of the Ubx HD to associate with Exd in yeast two-hybrid assays (5), harbors an 8-aa peptide previously termed UbdA (22) as well as a QA repression domain responsible for changes in Ubx activity (23, 24). Although evolutionarily conserved, the precise function of the UbdA motif, only present in Ubx and AbdA proteins but absent from any other Drosophila Hox protein (ref. 22 and Fig. 1A), is not known. In this work, we report on the function of the UbdA motif in the context of the Ubx protein. Because this motif is specific to Ubx and AbdA, which share the HX-independent mode of Exd recruitment, we focused our analysis on the possible implication of this motif in mediating Exd recruitment.
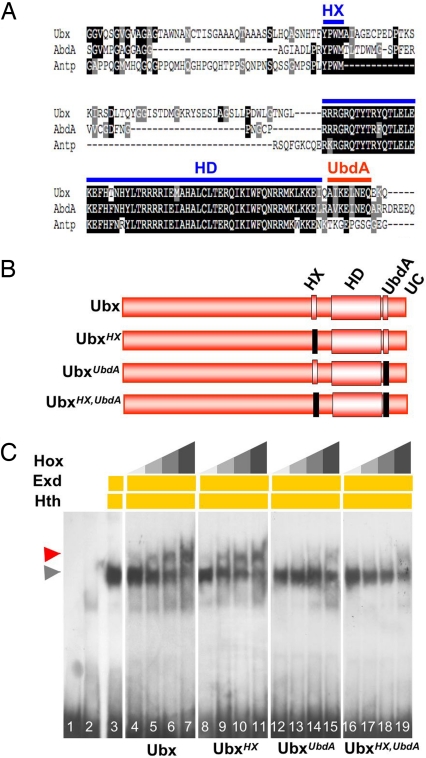
Fig. 1.
The UbdA motif mediates Exd/Hth recruitment. (A) Sequence alignment (ClustalW and Boxshade treatment) of AbdA, Ubx, and Antp protein sequences including and flanking the HD. Except for the HD and HX (blue bars), the UbdA motif (red bar) is the most conserved sequence between Ubx and AbdA. The UbdA motif is not found in any other Drosophila Hox protein, as illustrated here by its absence in Antp. (B) Structure of the Ubx variants used. Mutations, indicated as black boxes, are YPWM to YAAA for the HX and AIKELNEQ to AIVVLIVA for the UbdA motif. (C) EMSA shows that assembling the Ubx–Exd–Hth trimeric complex on DllR (lanes 4–7, red arrowhead) does not depend on HX (lanes 8–11) but on UbdA motif integrity (lanes 12–15). Mutation of both the HX and UbdA motifs (lanes 16–19) does not decrease Ubx–Exd–Hth complex formation compared with mutation of the UbdA motif alone. Six, 12, 25, and 50 ng of Ubx or its variant were used. Lanes 1–3 are, respectively, the DIIR probe alone, nonprogrammed, and Exd–Hth programmed lysate. The gray arrowhead indicates the position of Exd–Hth–DNA complexes.
Results
The UbdA Motif Is Required for Exd Recruitment on a Dll Repressor Element.
The abdominal repression of Dll by Ubx relies on assembling a ternary complex among the Hox protein, Exd, and Homothorax (Hth), a HD-containing protein of the Meis superfamily (25), on Dll regulatory sequences (DllR; see refs. 2, 19–21). The formation of the ternary complex, where Exd interacts directly with Ubx and Hth but the Hox protein does not contact Hth (14, 26), constitutes an in vitro readout of Hox–Exd interactions. EMSAs (Fig. 1C) with Ubx variants (UbxHX, UbxUbdA; Fig. 1B) showed that mutation of the UbdA motif strikingly reduces Exd recruitment, whereas that of the HX does not. Sequences C-terminal to UbdA, in particular the evolutionarily conserved QA motif (23), are not required for UbdA-mediated Exd recruitment (data not shown), further supporting that the UbdA motif, independently of more downstream sequences, constitutes an essential domain for in vitro Exd recruitment by Ubx. Because UbxUbdA retains some potential to recruit Exd, we tested a possible compensatory role of the HX by mutating both the HX and UbdA motifs. We found that UbxHX,UbdA recruits Exd with a low affinity similar to that of UbxUbdA (Fig. 1C), supporting that the HX is not responsible for the residual Exd recruitment potential of UbxUbdA. These observations support a major role for the UbdA motif in Exd recruitment and suggest that additional amino acids marginally contribute to Ubx–Exd interaction, consistent with previous results involving Ubx HD residues (17).
Exd-Dependent Dll Repression by Ubx Relies on the Integrity of the UbdA Motif.
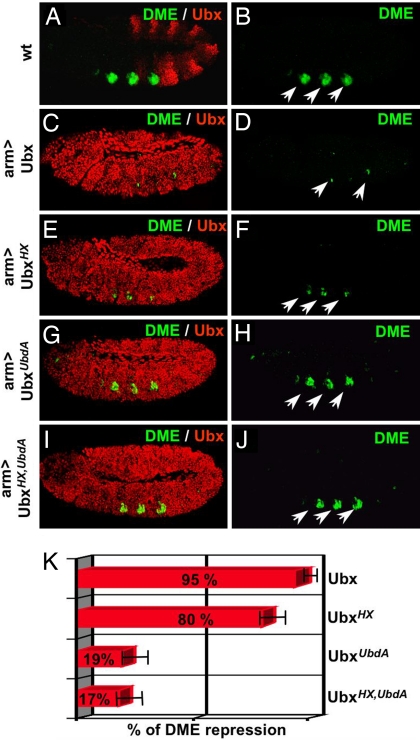
The transcriptional response of a DllR-containing minimal Hox response element (DME; ref. 21) to ubiquitous expression of similar physiological levels of wild-type (Fig. 2 C and D), HX- (Fig. 2 E and F), UbdA- (Fig. 2 G and H), or HX,UbdA- (Fig. 2 I and J) deficient forms of Ubx was taken as a measure of their in vivo repressive activities. Quantification (Fig. 2K) of DME-controlled reporter activity showed that Ubx and HX-deficient Ubx are strong DME repressors (95% and 80% of Ubx repressive activity, respectively), whereas UbxUbdA and UbxHX,UbdA have lost most of wild-type repression efficiency (19% and 17%, respectively). The similar levels of repression by UbxUbdA and UbxHX,UbdA indicate that the two Exd recruitment motifs in Ubx do not perform redundant functions in vivo. Together, the positive correlation between the ability to repress DME in vivo and to recruit Exd on DllR in vitro supports that the UbdA motif is required for proper Exd recruitment in the process of Dll repression.
Fig. 2.
The UbdA motif is required for Exd-dependent Dll repression. (A and B) DME activity, detected by an anti-β-galactosidase staining (green and highlighted by arrows), is in wild-type embryos restricted to thoracic segments where Ubx (detected by an anti-Ubx staining, red) is not expressed. (C–J) Ubiquitous expression of wild-type and Ubx variants was driven by arm-Gal4. Ubx (C and D) and UbxHX(E and F) repress DME activity, whereas UbxUbdA (G and H) and UbxHX,UbdA (I and J) do so only poorly. Quantification of the repressive activity of Ubx and its variants on DME reporter expression (K, see Materials and Methods). Embryos, anterior to the left and ventral side down, are at stage 11.
To ascertain that the loss of Exd recruitment on DllR and the loss of Dll repression do not result from a general loss of UbxUbdA activity, we analyzed its potential to repress wing promoting genes, including spalt (sal), Blistered/dSrf and vestigial (vg), a function known to be Exd-independent (13). Using the MS1096-Gal4 driver, preferentially active in the dorsal wing pouch compartment, we found that both wild-type Ubx and UbxUbdA repress dSrf expression as well as Vg-Q, sal-328, and sal-1.1 enhancer activities in a dorsal compartment-specific manner [supporting information (SI) Fig. 7]. EMSAs performed using Ubx-responsive cis-regulatory sequences of sal (13) showed that the UbdA mutation does not alter the capacity of Ubx to associate as a monomer to sal enhancers (SI Fig. 8). Taken together, these data indicate that the UbdA mutation does not result in a globally nonfunctional protein and that the UbdA motif appears nonessential for Exd-independent repression of wing fate by Ubx. We thus propose that loss of Dll repression by mutation of the UbdA motif results from the lack of Exd recruitment.
The UbdA Motif Confers Exd Recruitment Potential to an HX-Deficient Antennapedia Protein.
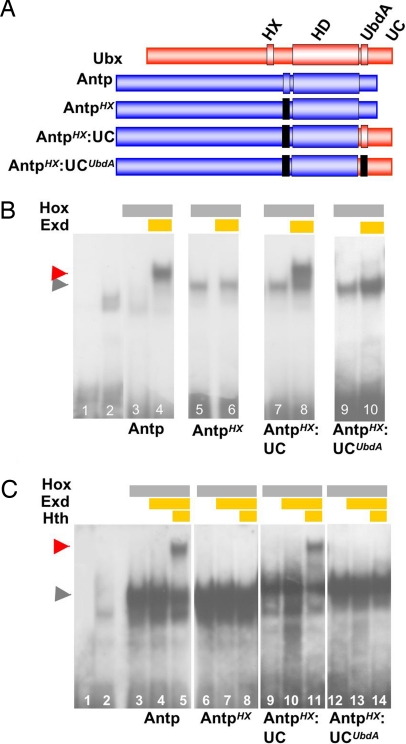
If the UbdA motif acts as a bona fide Exd recruitment domain, then it should be able to confer de novo Exd-binding activity to a Hox protein deficient for Exd binding. To test this possibility, we first used a Hox/Exd consensus sequence derived from DllR, DllRcon, that was in particular shown to assemble an Antennapedia (Antp)–Exd complex (21). Interestingly, on this specific target sequence, the Hox–Exd complex forms in the absence of Hth (Fig. 3B). Mutation of the HX motif in Antp (Fig. 3A) results in the loss of Exd recruitment (Fig. 3B), which provides the appropriate context to ask whether the UbdA motif has the potential to restore Exd-binding activity. We found that Exd recruitment is restored by swapping the Antp C-terminal region with that of Ubx (Fig. 3 A and B). This de novo Exd-binding activity is abolished by point mutations within UbdA (Fig. 3 A and B), demonstrating that the UbdA motif confers Exd recruitment potential in vitro on this Hox/Exd consensus sequence.
Fig. 3.
The UbdA motif confers Exd recruitment potential to Antp. (A) Structure of the Ubx/Antp variants. A color code identifies Ubx (red) and Antp (blue) sequences. Mutations are indicated as black boxes and are YPWM to YAAA for the HX and AIKELNEQ to AIVVLIVA for the UbdA motif. Antp and Ubx C termini are, respectively, from amino acids 360–378 and 358–389. (B) EMSA of the DllRcon probe, on which Antp recruits Exd in the absence of Hth (lanes 3 and 4, red arrowhead). Mutation of the HX (AntpHX) impairs Exd recruitment (lanes 5 and 6). Note that the HX mutation increases Antp monomer binding, as observed previously for the HX mutation in the Lab protein (12). Recruitment is restored upon swapping Antp and Ubx C termini (AntpHX:UC; lanes 7 and 8). Altering the integrity of the UbdA motif (AntpHX:UCUbdA) alleviates Exd recruitment (lanes 9 and 10). Lanes 1 and 2 are, respectively, the Dllcons probe alone and nonprogrammed lysate. The gray arrowhead indicates the position of the Hox–DNA complexes. Twenty-five nanograms of Antp or its variants was used. (C) A Hox–Exd–Hth trimeric complex forms in vitro on tsh cis-regulatory DNA (box2, ref. 28) using Antp (lanes 3–5, red arrowhead) or AntpHX:UC (lanes 9–11) but not AntpHX (lanes 6–8) or AntpHX:UCUbdA (lanes 12–14). The two first lanes are, respectively, the probe alone and nonprogrammed lysate. The gray arrowhead indicates the position of the Hox–DNA complexes. Twenty-five nanograms of Antp or its variants was used.
The UbdA Motif Confers Exd-Dependent Activity to an HX-Deficient Antp Protein.
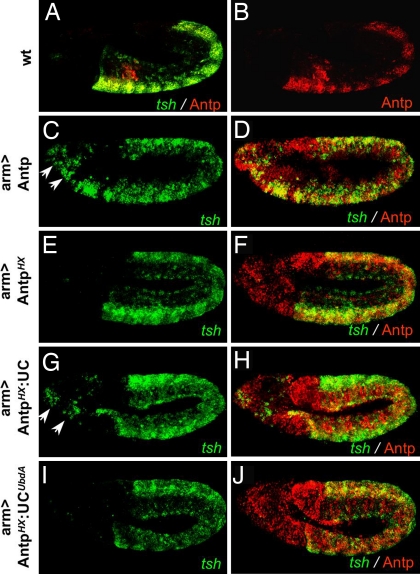
To assess the in vivo properties of the Antp variants, we analyzed their ability to control the expression of teashirt (tsh). tsh is a target of Antp/Exd activity in the embryonic epidermis (27, 28), and ubiquitous expression of Antp induces ectopic tsh transcription in the head region (27) (Fig. 4 C and D). This activity is abolished in the HX-deficient Antp protein (Fig. 4 E and F). Grafting the UC restores tsh inducibility (Fig. 4 G and H) in a manner that strictly depends on the integrity of the UbdA motif (Fig. 4 I and J). To establish firmly that these in vivo properties parallel the capacity of the protein to recruit Exd, we scanned for Antp—Exd-binding sites in the tsh epidermal enhancer (27). We found that trimeric Antp–Exd–Hth complexes assemble on tsh regulatory sequences. Mutation of the HX results in the loss of Exd–Hth recruitment (Fig. 3C). This loss is reversed upon grafting wild-type but not the UbdA-mutated UC (Fig. 3C). Together with the requirement of the motif for Dll repression, these data provide strong support for the direct implication of the UbdA motif in mediating Exd recruitment.
Fig. 4.
The UbdA motif confers Exd-dependent activity to Antp. (A and B) tsh transcripts (detected by in situ hybridization, green) and Antp protein (detected by an anti-Antp staining, red) are absent from wild-type head segments. (C–J) Ubiquitous expression driven by arm-Gal4 of Antp (C and D) or of AntpHX:UC (G and H) induces ectopic tsh in the head (arrows in C and G), whereas that of AntpHX (E and F) or of AntpHX:UCUbdA (I and J) does not. Embryos, anterior to the left and ventral side down, are at stage 11.
The UbdA Motif Is Dispensable for Activation of the Ubx/Exd Target Gene decapentaplegic.
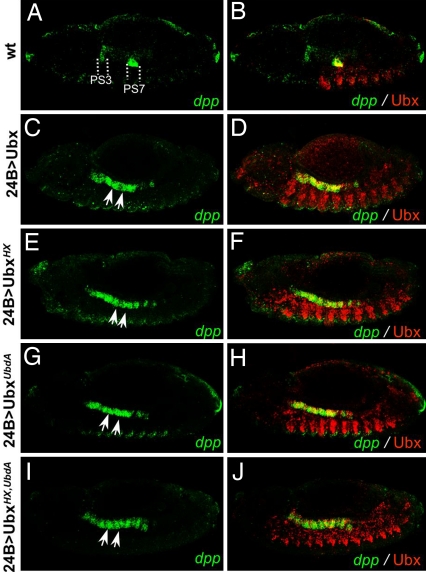
To investigate further the function of the UbdA motif, we analyzed its requirement for the activation of the decapentaplegic (dpp) gene in the embryonic visceral mesoderm (Fig. 5 A and B). dpp was previously shown to be activated in the central part of the midgut [parasegment (PS) 7] in response to Ubx and Exd activity (17, 29, 30). Functional binding sites for both proteins were identified, establishing dpp as one of the few molecularly characterized Ubx/Exd direct target genes. Mesodermal ubiquitous expression of Ubx induces anterior ectopic expression of dpp in PS4–5 (Fig. 5 C and D). A previous report showed that a Ubx protein bearing a short deletion covering the HX retained dpp-inducing capabilities (18). Consistent with this report, we also found that UbxHX still activates dpp (Fig. 5 E and F), therefore raising the possibility that the UbdA motif may be required for dpp activation. However, we found that UbxUbdA retains its dpp-activating potential (Fig. 5 G and H), which is not caused by functional redundancy of the HX and UbdA motifs because UbxHX,UbdA still activates dpp (Fig. 5 I and J). Thus, unlike for Dll regulation, the UbdA motif is dispensable for Exd-dependent regulation of dpp.
Fig. 5.
The HX and UbdA motifs are dispensable for Exd-dependent activation of dpp. Distribution of dpp transcripts (detected by in situ hybridization, green) and Ubx proteins (detected by an anti-Ubx staining, red) is shown. Dashed lines delineate ps3 and ps7 in the midgut of a wild-type embryo (A and B), and arrows point toward dpp ectopic transcription similarly induced by mesodermal ubiquitous expression (24B-Gal4) of Ubx (C and D), UbxHX (E and F), UbxUbdA (G and H), or of UbxHX,UbdA (I and J). Embryos, anterior to the left and ventral side down, are at stage 13.
Discussion
A Unique Mode of Hox–PBC Interaction.
Our results strongly support that the UbdA motif mediates Exd recruitment by the Ubx protein. This finding is first established by the requirement of the motif for Exd recruitment in the process of Dll regulation: mutation of the motif impairs the capacity of Ubx to mediate interaction with Exd on Dll regulatory sequences in vitro, which correlates with the reduced ability of UbxUbdA to perform Exd-dependent repression of Dll in vivo. We also provide evidence that mutation of the UbdA motif does not result in a globally defective protein: the UbdA mutated protein still binds DNA with appropriate affinities as a monomer, still represses the wing promoting genes dSRF, sal, and vg and still activates the dpp target gene in the visceral mesoderm. Thus, mutating the UbdA motif selectively affects a subset of Ubx functions. Importantly, the conclusion that the UbdA motif mediates Exd recruitment is also supported by the demonstration that the motif provides de novo Exd recruitment potential to a Hox protein that has been rendered deficient for this function. This finding is shown both in vitro by the potential of the motif to confer Exd recruitment to Antp on a Hox/Exd consensus sequence and on a cis-regulatory sequence of the Antp/Exd target gene tsh, and in vivo by its potential to restore Exd-dependent activation of tsh.
Complexity in Hox–Exd Interactions.
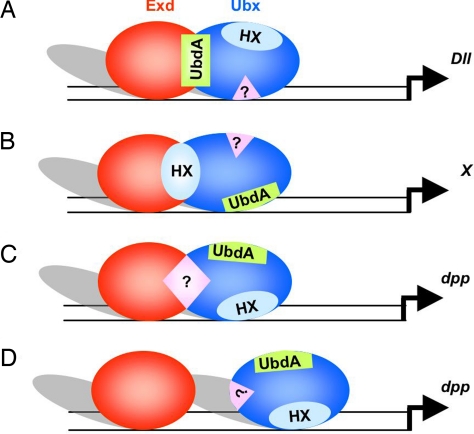
The Ubx protein provides a so far unique situation wherein two identified protein motifs within the same Hox protein have the potential to perform the recruitment of the Exd cofactor, which raises the question of whether these two motifs are effectively used for Exd recruitment by Ubx. Previous work has shown that an HX-deficient Ubx protein was altered in its segment identity specification: whereas Ubx specifies A1 segment identity (31, 32), the mutated form specifies A2-like identity (13). Interestingly, in a context deficient for zygotic Exd contribution (33), Ubx also specifies A2-like identity, suggesting that the HX motif is required for Exd-dependent A1 specification. These observations support that within this context, the HX is the motif used to perform Exd recruitment (Fig. 6B), although definitive support awaits characterization of Ubx-Exd interaction on a Ubx downstream target gene involved in segment identity specification (gene X in Fig. 6B). Considering our finding that the UbdA motif mediates Exd recruitment in the process of Dll regulation (Fig. 6A), we propose that depending on the developmental context, i.e., on the target gene regulated, Ubx uses different protein motifs for Exd recruitment. The contextual (gene-specific) use of the HX and UbdA protein motifs introduce a first level of complexity in Ubx–Exd interactions.
Fig. 6.
Complexity in Ubx–Exd functional interactions. The scheme highlights the diversity of interactions underlying Ubx–Exd functional interactions. The motif mediating Exd recruitment varies according to the target gene regulated: UbdA for Dll down-regulation (A), HX for regulation of genes (gene X) involved in segment identity specification (B), or a third unidentified motif in the process of dpp activation (C). In the last case, functional interplay may also not rely on direct interaction between Exd and Ubx (D).
A second level of complexity in the Ubx–Exd relationship is illustrated by the regulation of the dpp target gene. In this case, we found that neither the HX nor the UbdA motif was required for Exd-dependent activation by Ubx. The possibility that these two motifs were acting in a redundant way was excluded by the observation that a Ubx protein mutant for both motifs still activates dpp. Thus, other protein motifs, yet to be identified, could confer an additional mode of Exd interaction, further increasing the diversity by which Ubx could contact the Exd cofactor (Fig. 6C). Alternatively, the dispensability of the HX and UbdA motifs for dpp activation may also suggest that Ubx/Exd contacts are not required (Fig. 6D). The latter hypothesis is supported by the existence in dpp regulatory regions of Exd-binding sites that are not closely associated to Hox-binding sequences (29, 30) and by the previous observation that Exd can improve Ubx monomer binding to dpp regulatory sequences in a manner that does not require the formation of a Ubx–Exd–DNA tripartite complex (17). In any case, the regulation of dpp suggests further complexity in the Ubx–Exd relationship, which, by extension, highlights that the functional interplay of Hox–PBC proteins is likely to be more diverse than our current view.
Implications for Hox Protein Diversity and Specificity.
Although previous studies showed that HX-deficient Hox proteins retain the capacity to interact with Exd and to mediate Exd-dependent functions (13, 14), motifs responsible for alternative modes of interaction were not identified. This work identifies a so far unique HX-alternative mode of PBC recruitment, introducing the notion of flexibility in Hox–PBC contacts. This interaction mode was not anticipated from previous crystallographic studies because the truncated Ubx protein used was lacking the UbdA motif (7). Given the divergence of the primary sequences of the HX and UbdA motifs, their distinct location in the protein, and the absence of functional redundancy, the UbdA- and HX-mediated interaction modes are likely to be structurally distinct.
Our findings have also implications with regard to Hox protein diversity and specificity. Flexibility in Hox–PBC interactions allows us to address the issue of diversity from a mechanistic point of view: depending on the motif involved in the interaction, which likely relies on the target sequence (Fig. 6), the Hox–PBC complex may adopt different conformations, which in turn set structural bases for distinct activities. This process therefore provides cues to explain how diversity can be generated through qualitatively distinct interaction modes involving the same protein partners. Furthermore, because the UbdA motif is only found in Ubx and AbdA, it likely endows these two proteins with a specific Exd interaction mode. This mode may serve to distinguish Ubx and AbdA from other Drosophila Hox proteins, therefore providing basis for Hox protein specificity. Finally, our study questions whether additional HX-independent modes of PBC interaction exist. It was reported previously that the HX-deficient Lab protein retains Exd interaction potential and in vivo Exd-dependent activity (12). As Lab does not bear a UbdA motif, it supports further flexibility in Hox-PBC interaction. Addressing the issue of diversity in Hox-PBC interaction thus appears as a necessary step to understand the mechanisms underlying Hox protein activity in development and evolution.
Materials and Methods
Flies, Egg Collections, Cuticle Preparations, in Situ Hybridization, and Immunostaining.
24B-Gal4 and armadillo(arm)-Gal4 were used as embryonic mesodermal and ubiquitous drivers, respectively, and MS1096-Gal4 for preferential expression in the dorsal compartment of the wing pouch. UAS-Antp and UAS-Ubx were provided by M. Akam (Cambridge University, Cambridge, U.K.) and R. Mann (Columbia University, New York, NY). sal328-lacZ, sal1.1-lacZ, and VgQ-lacZ reporter lines were from S. Carroll (University of Wisconsin, Madison, WI), and the DME-lacZ line from R. Mann. Embryo collections, cuticle preparations, in situ hybridizations, and immunodetections were performed according to standard procedures. Digoxigenin RNA-labeled probes were generated according to the manufacturer's protocol (Boehringer Mannheim, Gaithersburg, MD) from dpp and tsh cDNAs cloned in Bluescript. dSRF antibody was used at a 1/50 dilution. Monoclonal antibodies to Ubx (FP3.38, dilution 1/100), Antp (4C3, dilution 1/100) and Wg (4D4, dilution 1/10) are from R. White (Cambridge University, Cambridge, U.K.) and Developmental Studies Hybridoma Bank (DSHB) (Iowa City, IA) The rabbit (MP Biomedicals, Solon, OH) or mouse (Promega, Madison, WI) anti-β-galactosidase antibodies were used at a 1/500 dilution.
Constructs, Transgenic Lines, and Gain-of-Function Experiments.
Hox variant constructs (see precise modifications in SI Fig. 9 and figure legends) were generated by PCR from full-length Ubx and Antp cDNAs. Primers used are available upon request. All constructs were cloned in the pcDNA3 vector and were sequenced-verified. Constructs for fly transformation were cloned in the pUAST vector, and transgenic lines were established by P element-mediated germ line transformation. P insertions were genetically mapped. For each variant, two lines were crossed with the arm-Gal4 driver at 22, 25, or 29°C. Collected embryos were stained with anti-Ubx or anti-Antp to select the conditions (line and temperature) that result in expression levels similar (±15%) to Ubx and Antp wild-type levels in A1 and T2, respectively. Levels of Ubx and Antp in wild-type embryos were assessed in a sized region in the middle of A1 and T2, respectively. The mean luminosity values for these regions were established by using the AxioVision LE4.5 measurement tool. The same-sized region was then used in T1 to establish the mean luminosity value in arm>Ubx and arm>Antp embryos. Quantification of DME repression was achieved by using the same DME-lacZ insertion. The levels of DME enhancer repression were estimated by quantifying the surface reduction in T2 of the DME-positive cell cluster by using the AxioVision LE4.5 measurement tool. Quantification was done on three individual experiments for each genotype. The error bars in Fig. 1C represent the deviation from the average value established from the three sets of quantification. For experiments with the MS1096-Gal4 and the 24B-Gal4 drivers, we used line and temperature conditions that were chosen for the arm-Gal4 driven experiments, ensuring that similar levels of wild-type and UbdA-deficient proteins were produced.
Protein Expression and EMSAs.
Exd and Hth were full-length, and Hox proteins and their variants were as schematized in Figs. 1B and 3A. Proteins were produced with the TNT (T7)-coupled in vitro transcription/translation system (Promega). Production yields of Hox proteins and variants were estimated by [35S]methionine labeling. The amounts of Hox proteins used in the band-shift assays are indicated in the respective figures. EMSA were performed as described previously (14) with 5 μl of a doubly programmed Exd/Hth lysate. Probes used were double-stranded DllR [5′-TATTTGGGAAATTAAATCATTCCCGCGGACAGTT (21)], DllRcon [5′-TATTTGGGCCATAAATCATTCCCGCGGACAGTT (21)], box2′ from the tsh epidermal enhancer [5′-TCATGGACTGAAAACCATAAATTTGATAATTGACTTTCCAC (27)], and Ubx-binding site 4 (5′-TGGGGTTGGTAATTAAAGGGG) and 7 (5′-TGGGGTTTTTTAATAAGTTGGGG) from the sal enhancers (13). Ten micrograms of FP3.38 was used for the “supershift” experiments. EMSAs on the different probes were performed at least once with proteins produced from the same in vitro transcription/translation reactions.
Supplementary Material
Acknowledgments
We thank R. Mann, S. Carroll, M. Akam, D. Coiffier, S. Kerridge (Institut de Biologie du Développement de Marseille Luminy, Marseille, France), and the Bloomington Stock Center (Bloomington, IN) for providing fly stocks and cDNAs; R. White and the DSHB for antibodies. We also acknowledge S. Kerridge, M. Semeriva, L. Perrin, B. Monier, and A. Saurin for discussions and comments on the manuscript. This work was supported by the Centre National de la Recherche Scientifique, grants from l'Association pour la Recherche contre le Cancer, la Ligue Nationale Contre le Cancer, l'Agence Nationale pour la Recherche, and the Indo French Center for the Promotion of Advanced Research.
Abbreviations
- AbdA
Abdominal-A
- Antp
Antennapedia
- arm
armadillo
- DllR
Dll regulatory sequence
- DME
DllR-containing minimal Hox response element
- Exd
Extradenticle
- HD
homeodomain
- Hth
Homothorax
- HX
hexapeptide
- Lab
Labial
- Pbx
pre-B cell transcription factor
- Ubx
Ultrabithorax
- UC
Ubx C terminus.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0705832104/DC1.
References
- 1.Pearson JC, Lemons D, McGinnis W. Nat Rev Genet. 2005;6:893–904. doi: 10.1038/nrg1726. [DOI] [PubMed] [Google Scholar]
- 2.Gebelein B, McKay DJ, Mann RS. Nature. 2004;431:653–659. doi: 10.1038/nature02946. [DOI] [PubMed] [Google Scholar]
- 3.Mann RS, Chan S-K. Trends Genet. 1996;12:258–262. doi: 10.1016/0168-9525(96)10026-3. [DOI] [PubMed] [Google Scholar]
- 4.Chan SK, Ryoo HD, Gould A, Krumlauf R, Mann RS. Development (Cambridge, UK) 1997;124:2007–2014. doi: 10.1242/dev.124.10.2007. [DOI] [PubMed] [Google Scholar]
- 5.Johnson FB, Parker E, Krasnow MA. Proc Natl Acad Sci USA. 1995;92:739–743. doi: 10.1073/pnas.92.3.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knoepfler PS, Kamps MP. Mol Cell Biol. 1995;15:5811–5819. doi: 10.1128/mcb.15.10.5811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Passner JM, Ryoo HD, Shen L, Mann RS, Aggarwal AK. Nature. 1999;397:714–719. doi: 10.1038/17833. [DOI] [PubMed] [Google Scholar]
- 8.Piper DE, Batchelor AH, Chang CP, Cleary ML, Wolberger C. Cell. 1999;96:587–597. doi: 10.1016/s0092-8674(00)80662-5. [DOI] [PubMed] [Google Scholar]
- 9.LaRonde-LeBlanc NA, Wolberger C. Genes Dev. 2003;17:2060–2072. doi: 10.1101/gad.1103303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Remacle S, Abbas L, De Backer O, Pacico N, Gavalas A, Gofflot F, Picard JJ, Rezsohazy R. Mol Cell Biol. 2004;24:8567–8575. doi: 10.1128/MCB.24.19.8567-8575.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Medina-Martinez O, Ramirez-Solis R. Dev Biol. 2003;264:77–90. doi: 10.1016/j.ydbio.2003.07.020. [DOI] [PubMed] [Google Scholar]
- 12.Chan S-K, Pöpperl H, Krumlauf R, Mann RS. EMBO J. 1996;15:2476–2487. [PMC free article] [PubMed] [Google Scholar]
- 13.Galant R, Walsh CM, Carroll SB. Development (Cambridge, UK) 2002;129:3115–3126. doi: 10.1242/dev.129.13.3115. [DOI] [PubMed] [Google Scholar]
- 14.Merabet S, Kambris Z, Capovilla M, Berenger H, Pradel J, Graba Y. Dev Cell. 2003;4:761–768. doi: 10.1016/s1534-5807(03)00126-6. [DOI] [PubMed] [Google Scholar]
- 15.Moens CB, Selleri L. Dev Biol. 2006;291:193–206. doi: 10.1016/j.ydbio.2005.10.032. [DOI] [PubMed] [Google Scholar]
- 16.Pöpperl H, Bienz M, Studer M, Chan S-K, Aparicio S, Brenner S, Mann RS, Krumlauf R. Cell. 1995;81:1031–1042. doi: 10.1016/s0092-8674(05)80008-x. [DOI] [PubMed] [Google Scholar]
- 17.Chan SK, Jaffe L, Capovilla M, Botas J, Mann RS. Cell. 1994;78:603–615. doi: 10.1016/0092-8674(94)90525-8. [DOI] [PubMed] [Google Scholar]
- 18.Tour E, Hittinger CT, McGinnis W. Development (Cambridge, UK) 2005;132:5271–5281. doi: 10.1242/dev.02138. [DOI] [PubMed] [Google Scholar]
- 19.Vachon G, Cohen B, Pfeifle C, McGuffin ME, Botas J, Cohen SM. Cell. 1992;71:437–450. doi: 10.1016/0092-8674(92)90513-c. [DOI] [PubMed] [Google Scholar]
- 20.White RA, Aspland SE, Brookman JJ, Clayton L, Sproat G. Mech Dev. 2000;91:217–226. doi: 10.1016/s0925-4773(99)00306-8. [DOI] [PubMed] [Google Scholar]
- 21.Gebelein B, Culi J, Ryoo HD, Zhang W, Mann RS. Dev Cell. 2002;3:487–498. doi: 10.1016/s1534-5807(02)00257-5. [DOI] [PubMed] [Google Scholar]
- 22.Chan S-K, Mann RS. Genes Dev. 1993;7:796–811. doi: 10.1101/gad.7.5.796. [DOI] [PubMed] [Google Scholar]
- 23.Galant R, Carroll SB. Nature. 2002;415:910–913. doi: 10.1038/nature717. [DOI] [PubMed] [Google Scholar]
- 24.Ronshaugen M, McGinnis N, McGinnis W. Nature. 2002;415:914–917. doi: 10.1038/nature716. [DOI] [PubMed] [Google Scholar]
- 25.Rieckhof GE, Casares F, Ryoo HD, Abu-Shaar M, Mann RS. Cell. 1997;91:171–183. doi: 10.1016/s0092-8674(00)80400-6. [DOI] [PubMed] [Google Scholar]
- 26.Ryoo HD, Marty T, Casares F, Affolter M, Mann RS. Development (Cambridge, UK) 1999;126:5137–5148. doi: 10.1242/dev.126.22.5137. [DOI] [PubMed] [Google Scholar]
- 27.McCormick A, Core N, Kerridge S, Scott MP. Development (Cambridge, UK) 1995;121:2799–2812. doi: 10.1242/dev.121.9.2799. [DOI] [PubMed] [Google Scholar]
- 28.Rauskolb C, Wieschaus E. EMBO J. 1994;13:3561–3569. doi: 10.1002/j.1460-2075.1994.tb06663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun B, Hursh DA, Jackson D, Beachy PA. EMBO J. 1995;14:520–535. doi: 10.1002/j.1460-2075.1995.tb07028.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Manak JR, Mathies LC, Scott MP. Development (Cambridge, UK) 1994;120:3605–3619. doi: 10.1242/dev.120.12.3605. [DOI] [PubMed] [Google Scholar]
- 31.Gonzalez-Reyes A, Morata G. Cell. 1990;61:515–522. doi: 10.1016/0092-8674(90)90533-k. [DOI] [PubMed] [Google Scholar]
- 32.Mann RS, Hogness DS. Cell. 1990;60:597–610. doi: 10.1016/0092-8674(90)90663-y. [DOI] [PubMed] [Google Scholar]
- 33.Peifer M, Wieschaus E. Genes Dev. 1990;4:1209–1223. doi: 10.1101/gad.4.7.1209. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.