Abstract
With aging there is a decline in the number of newly generated neurons in the dentate gyrus of the hippocampus. In rodents and tree shrews, this age-related decrease in neurogenesis is evident long before the animals become aged. No previous studies have investigated whether primates exhibit a similar decline in hippocampal neurogenesis with aging. To investigate this possibility, young to middle aged adult common marmosets (Callithrix jacchus) were injected with BrdU and perfused 3 weeks later. The number of newly generated cells in the subgranular zone/granule cell layer of the dentate gyrus was significantly lower in older animals and decreased linearly with age. A similar age-related decline in new cells was observed in the subventricular zone but not in the hilar region of the dentate gyrus. These data demonstrate that a substantial decrease in neurogenesis occurs before the onset of old age in the adult marmoset brain, suggesting the possibility that similar alterations occur in the human brain.
Keywords: aging, BrdU, dentate gyrus, hippocampus, subventricular zone
New neurons are continually added to the dentate gyrus of the hippocampus throughout life in several species, from rodents to humans (1–8). Evidence of neurogenesis has been observed in the dentate gyrus even in aged animals, including rodents over 2 years of age (9–14), dogs as old as 15 years (15), and humans as old as 72 years (4). However, substantial reductions in adult neurogenesis occur by old age in rodents and dogs (7, 9, 15–17). In adult rats, mice, and tree shrews, rates of neurogenesis begin to slow by 1 year of age, well before the onset of senescence (9, 13, 14, 16, 18–22). Some age-associated cognitive deficits first appear in middle aged individuals, considerably before old age (23–25), raising the possibility that reduced neurogenesis may contribute to these problems.
There are data suggesting that non-human primates exhibit a similar decrease in neurogenesis with advancing age. We have observed reduced numbers of proliferating cells and immature granule neurons in the dentate gyrus of middle aged (7–16 years) and aged (23 years) macaque monkeys (6). However, the small sample size in that study precluded any systematic analysis of the relationship between age and neurogenesis. Thus, it remains unclear whether neurogenesis in the adult primate brain is sensitive to aging and, moreover, whether the decline in neurogenesis becomes evident before old age. Evidence for an age-related decrease in neurogenesis in the nonhuman primate brain would suggest that similar alterations may occur in the human brain, where it is more difficult to study (26).
The common marmoset (Callithrix jacchus) is a New World monkey that reaches sexual maturity at ≈1.5 years of age and has a lifespan that ranges from ≈8 to 16 years (27). Senescence in marmosets begins around the age of 8–10 years as measured by neuropathology related to Alzheimer's (28) and Parkinson's (29) diseases, hearing loss (30), and cartilage aging (31). Here, we examined whether there is a relationship between age and the number of new cells generated in the dentate gyrus and subventricular zone (SVZ) of adult marmosets 1.5–7 years of age.
Results
In all monkeys, BrdU-labeled cells were observed in the two brain regions examined in this study: the dentate gyrus [including the granule cell layer (GCL), subgranular zone (SGZ), and hilus] and the SVZ lining the wall of the lateral ventricle.
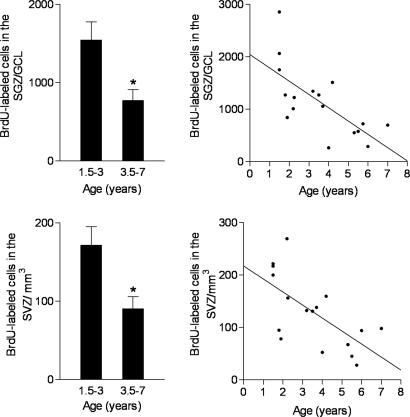
In the dentate gyrus, age was associated with changes in the number of BrdU-labeled cells in the SGZ/GCL by using two different methods of statistical analysis. First, when marmosets were divided into two groups by using median age as the criterion, the older age group (3.5–7 years) had significantly fewer BrdU-labeled cells than the younger age group (1.5–3 years) [t(15) = 2.9, P = 0.01] (Fig. 1). Second, regression analysis revealed a significant linear decrease with age of ≈253 ± 69 BrdU-labeled cells per day in the SGZ/GCL (r2 = 0.48, P = 0.002) (Fig. 1), resulting in a loss of at least 90,000 new cells over the course of an entire year. In contrast, there was no effect of age on the number of BrdU-labeled cells in the hilus [1.5–3 years: 1,542 ± 396; 3.5–7 years: 1,144 ± 162; t(15) = 0.97, P = 0.35] and no significant change with age in the number of BrdU-labeled cells in the hilus (r2 = 0.13, P = 0.16). The total volume of the SGZ/GCL was not affected by age [1.5–3 years: 6.32 ± 0.50 mm3; 3.5–7 years: 6.24 ± 0.59 mm3; t(15) = 0.10, P = 0.92; r2 = 0.05, P = 0.37].
Fig. 1.
The number of BrdU-labeled cells in the dentate gyrus (Upper) and SVZ (Lower) of marmoset monkeys ranging in age from 1.5 to 7 years (median age of 3.5 years) was examined 3 weeks after BrdU injection. According to whether their age fell above or below the median, animals were assigned to one of two age groups: 1.5–3 years or 3.5–7 years. Animals in the older age group had significantly fewer BrdU-labeled cells in the dentate gyrus and SVZ (Left). Bars represent mean ± SEM; ∗, P < 0.05. Moreover, regression analysis showed that the number of BrdU-labeled cells in the dentate gyrus and SVZ declined linearly with age (Right).
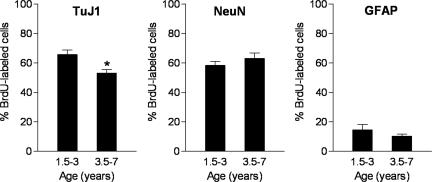
In the SGZ/GCL, the percentage of BrdU-labeled cells expressing class III β-tubulin (TuJ1), a marker of immature and mature neurons, was significantly reduced in the older age group [3.5–7 years; t(15) = 3.2, P = 0.006] (Figs. 2 and 3). The neuronal phenotype of BrdU-labeled cells was confirmed by using neuronal nuclei (NeuN), a marker of mature neurons. The majority of BrdU-labeled cells expressed NeuN but no difference in the percentage of such cells was observed in the older versus younger marmosets [t(15) = 0.96, P = 0.35] (Figs. 2 and 3). A considerably smaller proportion of BrdU-labeled cells expressed glial fibrillary acidic protein (GFAP), an astroglial marker. There was no difference in the percentage of BrdU-labeled cells that expressed GFAP between the age groups [t(14) = 1.19, P = 0.25] (Fig. 2).
Fig. 2.
The majority of BrdU-labeled cells expressed the immature and mature neuronal marker TuJ1 or the mature neuronal marker NeuN. A considerably smaller proportion of BrdU-labeled cells expressed GFAP, an astroglial marker. In the older age group, the percentage of BrdU-labeled cells expressing TuJ1 was reduced. There was no difference in the percentage of BrdU-labeled cells that expressed NeuN or GFAP between the age groups. Bars represent mean ± SEM; ∗, P < 0.05.
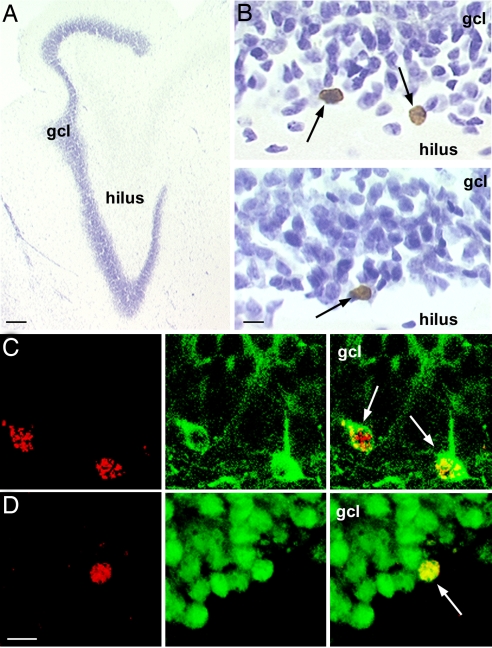
Fig. 3.
Age-related decline in adult hippocampal neurogenesis in the marmoset. (A) Photomicrograph of the adult marmoset dentate gyrus. (Scale bar: 200 μm.) (B) Younger marmosets 1.5–3 years of age (Upper) had a greater number of BrdU-labeled cells (arrows) in the dentate gyrus compared with older animals 3.5–7 years of age (Lower). (Scale bar: 10 μm.) The majority of BrdU-labeled cells expressed the immature and mature neuronal marker TuJ1 (C) or the mature neuronal marker NeuN (D). (C Left and D Left) BrdU-labeled cells. (C Center and D Center) TuJ1 (C) or NeuN (D). (C Right and D Right) Merged images with BrdU-labeled cells indicated by arrows. (Scale bar for C and D: 10 μm.) gcl, granule cell layer.
In the SVZ, the number of BrdU-labeled cells was also affected by age [t(15) = 2.9, P = 0.01]. Animals in the 1.5–3 years age group had significantly more BrdU-labeled cells relative to the 3.5–7 years age group (Fig. 1). As in the SGZ/GCL, regression analysis showed a significant linear decrease in the number of BrdU cells labeled in the SVZ (r2 = 0.43, P = 0.005) (Fig. 1). A comparison of the linear regression slopes revealed that the rate of the decline with age was similar for the SGZ/GCL and SVZ.
Discussion
The results of this study indicate that adult neurogenesis in the dentate gyrus of the primate hippocampus undergoes a substantial decline with advancing age. The numbers of newly generated cells were significantly lower in older animals and declined linearly with age. In addition, the percentage of BrdU-labeled cells in the dentate gyrus expressing a marker of immature and mature neurons (TuJ1) also decreased with age, although the percentage of newly born cells expressing a marker of mature neurons (NeuN) or of astrocytes (GFAP) was unaffected. Collectively, these data suggest that there is an age-associated reduction in newly generated neurons in the hippocampus of marmosets. The older monkeys used in this study were between 3.5 and 7 years of age, a time considered to be before the onset of senescence, which begins at ≈8 years of age (27). These findings are consistent with previous reports in rats (9, 13, 14, 16, 18–20), mice (22), and tree shrews (21) suggesting that a decline in adult neurogenesis during midlife is a common feature of mammalian species, including primates.
Similar to the dentate gyrus, the number of BrdU-labeled cells in the SVZ was significantly less in older animals and showed a linear decrease with age. Although some studies have reported reduced neurogenesis in the SVZ of aged animals (17, 32, 33), others have not (9). The reason for this discrepancy is not known but may involve species differences, number, and timing of BrdU injections and/or survival time after BrdU administration. Regardless, the similar decrease in the number of newly generated cells in both the dentate gyrus and SVZ raises the possibility that aging may alter BrdU availability. However, we found that the number of BrdU-labeled cells in the hilus was unaffected by age, suggesting that aging does not compromise the availability of BrdU but instead affects the production of newly born cells in specific brain regions.
Factors Regulating Aging-Induced Decline in Adult Neurogenesis.
The factors that control the decrease in adult neurogenesis in the aging primate brain remain unknown. Because aging is associated with elevated basal levels of circulating corticosterone (34, 35), it has been suggested that glucocorticoids may be one candidate for the negative regulation of new neuron formation. Consistent with this idea are data showing that, under certain circumstances, glucocorticoids inhibit cell proliferation in the dentate gyrus (36). Moreover, removal of adrenal steroids has been shown to restore high levels of neurogenesis in the aged rat dentate gyrus (7, 10). NMDA receptor activation also modulates adult neurogenesis in the hippocampus (37–39). In the aged brain, reduced hippocampal neurogenesis can be reversed by NMDA receptor antagonist treatment (19). Some evidence suggests that adrenal steroids alter adult neurogenesis by acting through NMDA receptors (40), but the extent to which such an interaction exists in the aged brain has not been explored.
Another factor that may regulate the age-related decline in neurogenesis is serotonin, a neurotransmitter that promotes hippocampal neurogenesis through actions at the 5-HT1A receptor (41). Both serotonin levels and 5-HT1A receptor binding decrease with age, raising the possibility of a negative effect on neurogenesis (42, 43). In addition, numerous neurotrophic factors including IGF-1, VEGF, FGF-2, and BDNF decrease considerably by middle age and have been linked to reduced production of new cells in the dentate gyrus and SVZ with aging (11, 17, 44–46). In aged animals, central administration of several of these growth factors stimulates neurogenesis in both the dentate gyrus and SVZ. Lastly, the vascular environment, which has been shown to be an important regulator of new cell production (47), is altered during aging and thus may contribute to age-related decline in adult neurogenesis (48).
Enhanced production of new neurons in the dentate gyrus has also been shown to occur after physical activity (49), exposure to enriched environmental conditions (50), learning (51), and social dominance (52), possibly through their effects on the factors discussed above. In this regard, it should be noted that the considerable variability we observed in BrdU cell number within given age groups may be the result of individual differences in activity level, cognitive abilities, social interactions, and/or stress. Because neurogenesis may be modulated by numerous molecular, hormonal, and experiential factors, and because studies have demonstrated enhancements in adult neurogenesis in the aged rodent brain (3, 7, 11, 19, 53–55), the possibility exists that, under appropriate conditions, neurogenesis can be restored to young-adult levels in the aged primate brain as well.
Mechanism of Age-Related Decline in Adult Neurogenesis.
Although our study only examined one time point (3 weeks) after BrdU labeling, previous work in other species suggests that the age-associated decrease in adult neurogenesis is largely due to diminished production of new neurons, as opposed to a decrease in cell survival. Several studies have directly demonstrated diminished cell proliferation in the dentate gyrus of aged rodents, by using either short survival times after BrdU labeling or endogenous markers of cell proliferation (7, 9, 13, 14, 20, 55). A recent report has additionally demonstrated that the reduced cell proliferation arises from a decrease in the progenitor cell pool, as opposed to lengthening of the cell cycle (56). Likewise, some evidence suggests that decreased cell proliferation reduces adult neurogenesis in the rodent SVZ as a result of an age-related decrease in the total number of progenitors (57). Given the similarities between the regulation of adult neurogenesis in rodents and primates (see below), it is likely that the decline in new neurons in the marmoset dentate gyrus and SVZ we observed is also the result of a decrease in cell proliferation, possibly resulting from a diminished number of progenitors. However, there is also the possibility that decreased neurogenesis during aging results from alterations in the self-renewal capability and increased quiescence of progenitors over time (48).
A slight reduction in the number of new cells expressing the marker of immature and mature neurons, TuJ1, was also observed with age. However, the lack of a significant difference in the percentage of new cells that express NeuN suggests that effects on neuronal differentiation that occur with aging are not substantial at the time points we examined. The literature on aging rodents is mixed in this regard, with some studies reporting no difference in neuronal differentiation with advanced age (14, 20) and others reporting a reduced percentage of newborn cells becoming neurons in older animals (11, 13). Although a small percentage of the newborn cells developed into astrocytes, the fate of the remaining newborn cells—i.e., those that do not stain for GFAP or TuJ1 and/or NeuN—is unclear. The possibility exists that these cells remain undifferentiated or develop into other cell types such as microglia and/or oligodendrocytes.
Similarities Across Mammalian Species in Adult Neurogenesis.
In the context of the existing literature, our findings underscore the similarities in adult neurogenesis across mammalian species, including primates. A comparable decline in adult neurogenesis around mid-life in both the dentate gyrus and SVZ has been observed for rats (9, 13, 14, 16, 18, 20), mice (22), and tree shrews (21). This pattern of results resembles what we observed previously for macaques, albeit with a smaller set of animals (6). Although the current literature on adult neurogenesis is much more extensive for rodents than for primates, several studies suggest common regulatory factors. Stress has been shown to inhibit cell proliferation in the dentate gyrus across mammalian species, including mice, rats, tree shrews, and marmosets (5, 36, 58). On the other hand, antidepressant treatment stimulates adult neurogenesis in the dentate gyrus in mice, rats, tree shrews, and macaques (58–61). Taken together, these findings suggest that adult neurogenesis in humans may be under similar regulatory influences as those in rodents, tree shrews, and monkeys. Very little quantitative data exist on adult neurogenesis in humans. Indeed, the most definitive positive studies of adult neurogenesis in humans have examined BrdU labeling in cancer patients, all of whom were middle aged or older and all of whom were sick (4, 62). Because age and stress have negative effects on adult neurogenesis, these findings suggest that the reported rates of adult neurogenesis in humans do not accurately represent the young adult level of new neuron addition. In fact, given the similarities in the regulation of adult neurogenesis from rodents to primates, it is likely that adult neurogenesis in the young adult human is robust and subject to the same regulatory influences as has been observed in studies on experimental animals.
Functional Consequences of Age-Related Decline in Adult Neurogenesis.
New neurons in the dentate gyrus have been proposed to be involved in learning and memory (51, 63). Thus, reduced neurogenesis in the aged hippocampus may contribute to cognitive decline that often occurs with age in many mammalian species although data to support this idea are conflicting (12, 53–55, 64, 65). Nonetheless, it is interesting that some cognitive deficits begin to appear during middle age in rodents, monkeys, and humans (23–25, 66). These findings raise the possibility that the mid-life decline in adult neurogenesis may contribute to deficits in learning and memory that occur at this time. However, it is important to consider that aging is also accompanied by a loss of synaptic connectivity and altered synaptic plasticity (67, 68). It seems most likely that if the age-related decrease in adult neurogenesis contributes to a decline in cognitive function, it does so in combination with other processes that are compromised in the aged brain.
Materials and Methods
Animals.
Adult male (n = 5) and female (n = 12) marmoset monkeys (Callithrix jacchus) from the breeding colony at Princeton University were used. Marmosets weighed between 240 and 500 g and were 1.5–7 years old at the time of perfusion. Marmosets were housed in cages measuring a minimum of 29 × 30 × 32 inches. All of the males and eight of the females were housed with at least another member of the opposite sex or with their family group. The remaining females (n = 4) were individually housed for 3–4 months before being euthanized. No differences were observed between sex or housing condition so data along these variables were combined. All procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Animal Treatments.
To examine the relationship between age and adult neurogenesis, animals were injected once with the thymidine analog BrdU (200 mg/kg of body weight i.p., in saline plus 0.007 M NaOH; Sigma). Three weeks after injection, the animals were deeply anesthetized with an overdose of sodium pentobarbital and transcardially perfused with 4% paraformaldehyde in 0.1 M phosphate buffer. The 3-week survival time was selected because by this time after DNA synthesis most BrdU-labeled cells in the dentate gyrus express neuronal markers (5).
Histological Procedures.
Brains were postfixed for at least 1 week. Coronal sections (40 μm) throughout the entire rostrocaudal extent of the dentate gyrus were cut with a vibratome from half brains into a bath of 0.1 M PBS (pH 7.5). For BrdU peroxidase staining, a 1:12 series of sections were mounted onto glass slides, dried, and pretreated by heating in 0.1 M citric acid (pH 6.0). Slides were then rinsed in PBS, incubated in trypsin for 10 min, rinsed, denatured in 2 M HCl:PBS for 30 min, rinsed, and incubated overnight at 4°C in mouse monoclonal antibody against BrdU (diluted 1:250 with 0.5% Tween 20; Vector Laboratories). The next day, slides were rinsed, incubated with biotinylated anti-mouse (1:200; Vector Laboratories) for 60 min, rinsed, incubated with avidin–biotin complex (1:100; Vector Laboratories) for 60 min, rinsed, and reacted in 0.01% diaminobenzidine with 0.003% H2O2 (Sigma–Aldrich). Slides were counterstained with cresyl violet, dehydrated, cleared, and coverslipped under Permount (Fisher Scientific).
Additional sections were reacted by using immunofluorescence methods for BrdU combined with the cell-type-specific marker NeuN, a marker of mature neurons; TuJ1, a marker of immature and mature neurons; or GFAP, an astroglial marker. Sections were denatured in 2 M HCl:TBS for 30 min, rinsed in TBS, and incubated with rat anti-BrdU (1:250 with 0.5% Tween 20; Accurate Chemical) plus mouse anti-NeuN (1:500; Chemicon), mouse anti-TuJ1 (1:500; Covance), or guinea pig anti-GFAP (1:500; Advanced Immunochemical) for 2 days. Next, sections were rinsed, incubated with biotinylated anti-rat (1:250; Vector Laboratories) for 90 min, rinsed, and incubated for 30 min in the dark with streptavidin-conjugated Alexa 568 (1:1,000; Molecular Probes) to visualize BrdU and with goat anti-mouse Alexa 488 or goat anti-guinea pig Alexa 488 (1:500; Molecular Probes) to visualize TuJ1, NeuN, or GFAP. Sections were mounted onto slides, dried, and coverslipped under glycerol:TBS.
Data Analysis.
For microscopic data analysis, the slides were coded before data collection. Estimates of total numbers of BrdU-labeled cells were determined by using a modified stereology protocol (69). BrdU-labeled cells on every twelfth unilateral section throughout the entire rostrocaudal extent of the dentate gyrus (GCL, SGZ, and hilus) and SVZ were counted at ×1,000 on an Olympus BX-50 light microscope. Counts were multiplied by 24 to obtain estimates of BrdU-labeled cells in the dentate gyrus and SVZ per brain. The total volume of the GCL and SVZ were calculated from cross-sectional area measurements obtained with Image-Pro Plus software (Media Cybernetics) by using Cavalieri's principle (70). SVZ data were expressed as the number of BrdU-labeled cells per mm3.
For double labeling, the percentage of BrdU-labeled cells in the dentate gyrus (GCL and SGZ) that expressed NeuN, TuJ1, or GFAP was determined by using a Zeiss Axiovert confocal laser scanning microscope (LSM 510; lasers, Argon 458/488 and HeNe 543). For each brain, 25 randomly selected BrdU-labeled cells per marker were analyzed. Optical stacks of 1-μm-thick sections were obtained through putatively double-labeled cells. To verify double labeling throughout the extent of the cell, orthogonal planes were examined.
Statistical Analysis.
Animals were assigned to one of two age groups according to whether their age fell above or below the median age of 3.5 years: 1.5–3 years (n = 8) or 3.5–7 years (n = 9). No differences were observed between group and individually housed animals, so these animals were grouped together. No significant sex differences emerged, so results were collapsed across this variable. Data were analyzed with two-tailed unpaired t tests and linear regression analysis using Prism 4.0 (GraphPad).
Acknowledgments
This work was supported by the National Institutes of Health, the National Alliance for Research on Schizophrenia and Depression (E.G.), and National Research Service Award fellowships (to B.L. and Y.K.).
Abbreviations
- GCL
granule cell layer
- GFAP
glial fibrillary acidic protein
- NeuN
neuronal nuclei
- SGZ
subgranular zone
- SVZ
subventricular zone
- TuJ1
class III β-tubulin.
Footnotes
The authors declare no conflict of interest.
References
- 1.Altman J, Das GD. J Comp Neurol. 1965;124:319–335. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- 2.Kaplan MS, Bell DH. J Neurosci. 1984;4:1429–1441. doi: 10.1523/JNEUROSCI.04-06-01429.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kempermann G, Kuhn HG, Gage FH. J Neurosci. 1998;18:3206–3212. doi: 10.1523/JNEUROSCI.18-09-03206.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 5.Gould E, Tanapat P, McEwen BS, Flugge G, Fuchs E. Proc Natl Acad Sci USA. 1998;95:3168–3171. doi: 10.1073/pnas.95.6.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gould E, Reeves AJ, Fallah M, Tanapat P, Gross CG, Fuchs E. Proc Natl Acad Sci USA. 1999;96:5263–5267. doi: 10.1073/pnas.96.9.5263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cameron HA, McKay RD. Nat Neurosci. 1999;2:894–897. doi: 10.1038/13197. [DOI] [PubMed] [Google Scholar]
- 8.Gross CG. Nat Rev Neurosci. 2000;1:67–73. doi: 10.1038/35036235. [DOI] [PubMed] [Google Scholar]
- 9.Kuhn HG, Dickinson-Anson H, Gage FH. J Neurosci. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Montaron MF, Petry KG, Rodriguez JJ, Marinelli M, Aurousseau C, Rougon G, Le Moal M, Abrous DN. Eur J Neurosci. 1999;11:1479–1485. doi: 10.1046/j.1460-9568.1999.00579.x. [DOI] [PubMed] [Google Scholar]
- 11.Lichtenwalner RJ, Forbes ME, Bennett SA, Lynch CD, Sonntag WE, Riddle DR. Neuroscience. 2001;107:603–613. doi: 10.1016/s0306-4522(01)00378-5. [DOI] [PubMed] [Google Scholar]
- 12.Bizon JL, Gallagher M. Eur J Neurosci. 2003;18:215–219. doi: 10.1046/j.1460-9568.2003.02733.x. [DOI] [PubMed] [Google Scholar]
- 13.Heine VM, Maslam S, Joels M, Lucassen PJ. Neurobiol Aging. 2004;25:361–375. doi: 10.1016/S0197-4580(03)00090-3. [DOI] [PubMed] [Google Scholar]
- 14.Rao MS, Hattiangady B, Shetty AK. Aging Cell. 2006;5:545–558. doi: 10.1111/j.1474-9726.2006.00243.x. [DOI] [PubMed] [Google Scholar]
- 15.Siwak-Tapp CT, Head E, Muggenburg BA, Milgram NW, Cotman CW. Neurobiol Learn Mem. 2007;88:249–259. doi: 10.1016/j.nlm.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seki T, Arai Y. NeuroReport. 1995;6:2479–2482. doi: 10.1097/00001756-199512150-00010. [DOI] [PubMed] [Google Scholar]
- 17.Jin K, Sun Y, Xie L, Batteur S, Mao XO, Smelick C, Logvinova A, Greenberg DA. Aging Cell. 2003;2:175–183. doi: 10.1046/j.1474-9728.2003.00046.x. [DOI] [PubMed] [Google Scholar]
- 18.Lemaire V, Koehl M, Le Moal M, Abrous DN. Proc Natl Acad Sci USA. 2000;97:11032–11037. doi: 10.1073/pnas.97.20.11032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nacher J, Alonso-Llosa G, Rosell DR, McEwen BS. Neurobiol Aging. 2003;24:273–284. doi: 10.1016/s0197-4580(02)00096-9. [DOI] [PubMed] [Google Scholar]
- 20.McDonald HY, Wojtowicz JM. Neurosci Lett. 2005;385:70–75. doi: 10.1016/j.neulet.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 21.Simon M, Czeh B, Fuchs E. Brain Res. 2005;1049:244–248. doi: 10.1016/j.brainres.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 22.Kronenberg G, Bick-Sander A, Bunk E, Wolf C, Ehninger D, Kempermann G. Neurobiol Aging. 2006;27:1505–1513. doi: 10.1016/j.neurobiolaging.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 23.Knuttinen MG, Gamelli AE, Weiss C, Power JM, Disterhoft JF. Neurobiol Aging. 2001;22:1–8. doi: 10.1016/s0197-4580(00)00194-9. [DOI] [PubMed] [Google Scholar]
- 24.Frick KM, Stearns NA, Pan JY, Berger-Sweeney J. Learn Mem. 2003;10:187–198. doi: 10.1101/lm.50703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moore TL, Killiany RJ, Herndon JG, Rosene DL, Moss MB. Neurobiol Aging. 2006;27:1484–1493. doi: 10.1016/j.neurobiolaging.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 26.Gould E. Nat Rev Neurosci. 2007;8:481–488. doi: 10.1038/nrn2147. [DOI] [PubMed] [Google Scholar]
- 27.Abbott DH, Barnett DK, Colman RJ, Yamamoto ME, Schultz-Darken NJ. Comp Med. 2003;53:339–350. [PubMed] [Google Scholar]
- 28.Geula C, Nagykery N, Wu CK. Acta Neuropathol (Berlin) 2002;103:48–58. doi: 10.1007/s004010100429. [DOI] [PubMed] [Google Scholar]
- 29.Rose S, Nomoto M, Jackson EA, Gibb WR, Jaehnig P, Jenner P, Marsden CD. Eur J Pharmacol. 1993;230:177–185. doi: 10.1016/0014-2999(93)90800-w. [DOI] [PubMed] [Google Scholar]
- 30.Harada T, Tokuriki M, Tanioka Y. Hear Res. 1999;128:119–124. doi: 10.1016/s0378-5955(98)00201-9. [DOI] [PubMed] [Google Scholar]
- 31.Berkovitz BK, Pacy J. Arch Oral Biol. 2000;45:987–995. doi: 10.1016/s0003-9969(00)00067-4. [DOI] [PubMed] [Google Scholar]
- 32.Tropepe V, Craig CG, Morshead CM, van der Kooy D. J Neurosci. 1997;17:7850–7859. doi: 10.1523/JNEUROSCI.17-20-07850.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Molofsky AV, Slutsky SG, Joseph NM, He S, Pardal R, Krishnamurthy J, Sharpless NE, Morrison SJ. Nature. 2006;443:448–452. doi: 10.1038/nature05091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sapolsky RM. Neurobiol Aging. 1992;13:171–174. doi: 10.1016/0197-4580(92)90025-s. [DOI] [PubMed] [Google Scholar]
- 35.Lupien SJ, de Leon M, de Santi S, Convit A, Tarshish C, Nair NP, Thakur M, McEwen BS, Hauger RL, Meaney MJ. Nat Neurosci. 1998;1:69–73. doi: 10.1038/271. [DOI] [PubMed] [Google Scholar]
- 36.Mirescu C, Gould E. Hippocampus. 2006;16:233–238. doi: 10.1002/hipo.20155. [DOI] [PubMed] [Google Scholar]
- 37.Cameron HA, McEwen BS, Gould E. J Neurosci. 1995;15:4687–4692. doi: 10.1523/JNEUROSCI.15-06-04687.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nacher J, Rosell DR, Alonso-Llosa G, McEwen BS. Eur J Neurosci. 2001;13:512–520. doi: 10.1046/j.0953-816x.2000.01424.x. [DOI] [PubMed] [Google Scholar]
- 39.Nacher J, McEwen BS. Hippocampus. 2006;16:267–270. doi: 10.1002/hipo.20160. [DOI] [PubMed] [Google Scholar]
- 40.Cameron HA, Tanapat P, Gould E. Neuroscience. 1998;82:349–354. doi: 10.1016/s0306-4522(97)00303-5. [DOI] [PubMed] [Google Scholar]
- 41.Banasr M, Hery M, Printemps R, Daszuta A. Neuropsychopharmacology. 2004;29:450–460. doi: 10.1038/sj.npp.1300320. [DOI] [PubMed] [Google Scholar]
- 42.Nyakas C, Oosterink BJ, Keijser J, Felszeghy K, de Jong GI, Korf J, Luiten PG. J Chem Neuroanat. 1997;13:53–61. doi: 10.1016/s0891-0618(97)00025-2. [DOI] [PubMed] [Google Scholar]
- 43.Stemmelin J, Lazarus C, Cassel S, Kelche C, Cassel JC. Neuroscience. 2000;96:275–289. doi: 10.1016/s0306-4522(99)00561-8. [DOI] [PubMed] [Google Scholar]
- 44.Enwere E, Shingo T, Gregg C, Fujikawa H, Ohta S, Weiss S. J Neurosci. 2004;24:8354–8365. doi: 10.1523/JNEUROSCI.2751-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hattiangady B, Rao MS, Shetty GA, Shetty AK. Exp Neurol. 2005;195:353–371. doi: 10.1016/j.expneurol.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 46.Shetty AK, Hattiangady B, Shetty GA. Glia. 2005;51:173–186. doi: 10.1002/glia.20187. [DOI] [PubMed] [Google Scholar]
- 47.Palmer TD, Willhoite AR, Gage FH. J Comp Neurol. 2000;425:479–494. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 48.Hattiangady B, Shetty AK. Neurobiol Aging. 2006 doi: 10.1016/j.neurobiolaging.2006.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van Praag H, Kempermann G, Gage FH. Nat Neurosci. 1999;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- 50.Kempermann G, Kuhn HG, Gage FH. Nature. 1997;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- 51.Leuner B, Gould E, Shors TJ. Hippocampus. 2006;16:216–224. doi: 10.1002/hipo.20153. [DOI] [PubMed] [Google Scholar]
- 52.Kozorovitskiy Y, Gould E. J Neurosci. 2004;24:6755–6759. doi: 10.1523/JNEUROSCI.0345-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van Praag H, Shubert T, Zhao C, Gage FH. J Neurosci. 2005;25:8680–8685. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Drapeau E, Montaron MF, Aguerre S, Abrous DN. J Neurosci. 2007;27:6037–6044. doi: 10.1523/JNEUROSCI.1031-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Drapeau E, Mayo W, Aurousseau C, Le Moal M, Piazza PV, Abrous DN. Proc Natl Acad Sci USA. 2003;100:14385–14390. doi: 10.1073/pnas.2334169100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Olariu A, Cleaver KM, Cameron HA. J Comp Neurol. 2007;501:659–667. doi: 10.1002/cne.21268. [DOI] [PubMed] [Google Scholar]
- 57.Maslov AY, Barone TA, Plunkett RJ, Pruitt SC. J Neurosci. 2004;24:1726–1733. doi: 10.1523/JNEUROSCI.4608-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Czeh B, Michaelis T, Watanabe T, Frahm J, de Biurrun G, van Kampen M, Bartolomucci A, Fuchs E. Proc Natl Acad Sci USA. 2001;98:12796–12801. doi: 10.1073/pnas.211427898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Malberg JE, Eisch AJ, Nestler EJ, Duman RS. J Neurosci. 2000;20:9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, et al. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 61.Perera TD, Coplan JD, Lisanby SH, Lipira CM, Arif M, Carpio C, Spitzer G, Santarelli L, Scharf B, Hen R, et al. J Neurosci. 2007;27:4894–4901. doi: 10.1523/JNEUROSCI.0237-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Curtis MA, Kam M, Nannmark U, Anderson MF, Axell MZ, Wikkelso C, Holtas S, van Roon-Mom WM, Bjork-Eriksson T, Nordborg C, et al. Science. 2007;315:1243–1249. doi: 10.1126/science.1136281. [DOI] [PubMed] [Google Scholar]
- 63.Bruel-Jungerman E, Rampon C, Laroche S. Rev Neurosci. 2007;18:93–114. doi: 10.1515/revneuro.2007.18.2.93. [DOI] [PubMed] [Google Scholar]
- 64.Merrill DA, Karim R, Darraq M, Chiba AA, Tuszynski MH. J Comp Neurol. 2003;459:201–207. doi: 10.1002/cne.10616. [DOI] [PubMed] [Google Scholar]
- 65.Cuppini R, Bucherelli C, Ambrogini P, Ciuffoli S, Orsini L, Ferri P, Baldi E. Hippocampus. 2006;16:141–148. doi: 10.1002/hipo.20140. [DOI] [PubMed] [Google Scholar]
- 66.Driscoll I, Hamilton DA, Yeo RA, Brooks WM, Sutherland RJ. Horm Behav. 2005;47:326–335. doi: 10.1016/j.yhbeh.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 67.Geinisman Y, Detoledo-Morrell L, Morrell F, Heller RE. Prog Neurobiol. 1995;45:223–252. doi: 10.1016/0301-0082(94)00047-l. [DOI] [PubMed] [Google Scholar]
- 68.Burke SN, Barnes CA. Nat Rev Neurosci. 2006;7:30–40. doi: 10.1038/nrn1809. [DOI] [PubMed] [Google Scholar]
- 69.West MJ, Slomianka L, Gundersen HJ. Anat Rec. 1991;231:482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- 70.Gundersen HJ, Bagger P, Bendtsen TF, Evans SM, Korbo L, Marcussen N, Moller A, Nielsen K, Nyengaard JR, Pakkenberg B, et al. Acta Pathol Microbiol Scand. 1988;96:857–881. doi: 10.1111/j.1699-0463.1988.tb00954.x. [DOI] [PubMed] [Google Scholar]