Abstract
Type 1 diabetes (T1D) results from progressive loss of pancreatic islet mass through autoimmunity targeted at a diverse, yet limited, series of molecules that are expressed in the pancreatic β cell. Identification of these molecular targets provides insight into the pathogenic process, diagnostic assays, and potential therapeutic agents. Autoantigen candidates were identified from microarray expression profiling of human and rodent pancreas and islet cells and screened with radioimmunoprecipitation assays using new-onset T1D and prediabetic sera. A high-ranking candidate, the zinc transporter ZnT8 (Slc30A8), was targeted by autoantibodies in 60–80% of new-onset T1D compared with <2% of controls and <3% type 2 diabetic and in up to 30% of patients with other autoimmune disorders with a T1D association. ZnT8 antibodies (ZnTA) were found in 26% of T1D subjects classified as autoantibody-negative on the basis of existing markers [glutamate decarboxylase (GADA), protein tyrosine phosphatase IA2 (IA2A), antibodies to insulin (IAA), and islet cytoplasmic autoantibodies (ICA)]. Individuals followed from birth to T1D showed ZnT8A as early as 2 years of age and increasing levels and prevalence persisting to disease onset. ZnT8A generally emerged later than GADA and IAA in prediabetes, although not in a strict order. The combined measurement of ZnT8A, GADA, IA2A, and IAA raised autoimmunity detection rates to 98% at disease onset, a level that approaches that needed to detect prediabetes in a general pediatric population. The combination of bioinformatics and molecular engineering used here will potentially generate other diabetes autoimmunity markers and is also broadly applicable to other autoimmune disorders.
Keywords: autoantibody, zinc transport, prediabetes
Type 1 diabetes (T1D) is a T cell-dependent tissue-specific autoimmune disease, characterized in animal models by the selective destruction of the β cells of the pancreatic islets of Langerhans (1). In the nonobese diabetic (NOD) mouse, B lymphocytes also play a role in pathogenesis (2), although their role in human T1D is less clear (3). Nevertheless, the number and levels of circulating autoantibodies in man provide important predictive markers for the underlying autoimmunity that may precede clinical disease by many years, during which time, therapeutic intervention may be effective (4–7). The histological determination of islet cytoplasmic autoantibodies (ICA) (8) or the combined biochemical measurement of antibodies to insulin (IAA) (9), the 65-kD form of glutamate decarboxylase (GADA) (10) and the protein tyrosine phosphatase IA2 (IA2A) (11) can identify 80% or more of patients at disease onset or at risk of developing disease. Such autoantibody measurements in subjects with a family history of autoimmune diabetes have been instrumental in selection of patients for entry into clinical trials (12) but currently fall short of the high sensitivity and specificity required for detection of (pre)diabetes in the general population where the prevalence is of the order of 0.3% even when genetic susceptibility markers are also included (13).
Recent progress toward the development of immunological-based therapies for T1D and other autoimmune disorders in humans (14) highlights the need for development of further markers of B and T cell autoimmunity in diabetes. These can be used to identify at-risk subjects during the prodromic phase when immune intervention is most likely to be effective and may also be therapeutic agents in their own right. We report the identification of such a marker based on a multidimensional analysis of microarray data, subsequent development of sensitive immunoprecipitation assays to conformational epitopes in the molecule, and their application to study of humoral autoimmunity in new-onset and prediabetic patients.
Results
Microarray Analyses.
ZnT8 was originally identified as an EST chosen from a list of 68 candidate islet autoantigens that was compiled from multidimensional analyses of microarray mRNA expression profiling experiments. The screening process was initiated by interrogation of public domain multitissue custom arrays (Gene Atlas V2; Novartis, http://symatlas.gnf.org) (15) using three criteria of enrichment of gene expression in islets, resulting in the acceptance of 300 genes. One hundred sixty of these were excluded on the basis of their being more abundant in an αTC1-6 glucagonoma cell line than βTC3 insulinoma cell line or being expressed at comparable levels in mPAC pancreatic cells. Another 40 were excluded on the basis that they were expressed at much lower levels in mouse islet and FACS-sorted β-cells than in the cell lines. The tissue expression of the remaining 100 genes was evaluated from the frequency with which ESTs have been sequenced from cDNA libraries from 49 normal human tissues and the pancreatic specificity derived from the pancreatic EST frequency relative to the global EST frequency. The multiplication product of these indices was used to rank the genes [supporting information (SI) Table 1]. Fig. 1 shows two-dimensional maps of pancreatic specificity relative to the pancreatic EST frequency and islet transcript abundance determined by microarray analysis.
Fig. 1.
Identification of candidate autoantigens. Pancreas specificity is plotted against the pancreatic abundance (pancreatic EST clones per all tissues ESTs) and against the level of mRNA expression in human islets from microarray data (SI Table 1). Approximate positions are shown for diabetes autoantigens (filled symbols).
The final list of 68 candidate genes included major known diabetes autoantigens, notably insulin (9), GAD65 (GAD2) (10), IA2 (11), and phogrin (PTPrN2) (16) as well as most of the other putative targets of autoimmunity (heat shock protein 90B (17), carboxypeptidase E (CPE) (18), islet glucose 6 phosphatase-related protein (IGRP) (19), islet amyloid polypeptide (IAPP) (20), pancreatitis-associated protein (humanReg3a/Mouse Reg2) (21), ICA69 (22), imogen 38 (23), peripherin (24), sox13 (25), and GAD67 (GAD1) (26). The appearance of GAD65, CPE, IA2, and phogrin on the list, although gratifying, was surprising, given their known association with neuroendocrine tissues. This was attributed to their high representation in pancreas cDNA libraries, which probably underestimates their representation in islet tissue, which represents <5% of the pancreas mass.
Slc30A8 (ZnT8) ranked fourth in the hierarchy of candidate genes based on its pancreas and islet specificity SI Table 1) and 28th with respect to islet expression level. The islet specificity is consistent with previous studies based on PCR analysis (27) and our own quantitative PCR analyses (data not shown). The only other tissue showing significant expression was testis (seven clones from 337,730 ESTs), although the frequency of appearance is still 20-fold lower than the whole pancreas. Of the more abundantly expressed genes in human pancreas, only INS, GAD2, and IGRP ranked higher, and all of these are prominent T1D autoantigens.
ZnT8 Autoantibodies and Identification of Regional Epitopes.
A radioimmunoprecipitation assay for ZnT8 autoantibodies was developed by using [35S]methionine-labeled in vitro translation products of different fragments of human ZnT8 (Fig. 2). Results with new-onset T1D sera using the 369-aa ORF were encouraging (25% sensitivity, 98% specificity); however, a nonspecific binding of ≥5% and unacceptable false-positive rate precluded its use for patient screening (Fig. 2A). Approximately half of the ZnT8 structure lies within six membrane-spanning regions (Fig. 2B) that are unlikely to be accessible to antibodies and probably impede its folding in an aqueous environment. Assays were therefore developed to a series of fragments of the predicted luminal and cytosolic regions as either individual segments or fusion constructs. Autoreactivity to amino acids 1–74 of the N terminus (Fig. 2A) broadly correlated with that of the ORF results (R2 = 0.605, P < 0.001, n = 186), but the assay showed low sensitivity (8.0% sensitivity, 98% specificity, n = 223). In contrast, a C-terminal construct spanning amino acids 268–369 produced a robust and sensitive assay (50% sensitivity, 98% specificity). A synthetic molecule that combined both N- and C-terminal sequences in a single-chain construct performed more reliably than the ORF construct (Fig. 2C), and the levels of antibodies were correlated (R2 = 0.494, P < 0.001; SI Fig. 7), suggesting that the transmembrane regions and the short cytoplasmic and luminal connecting peptides were not major contributors of ZnT8 autoantibody epitopes. The N/C construct, however, did not incorporate all of the epitopes recognized by C-terminal reactive sera (SI Fig. 7). Assays of C-terminal and N- and C-terminal fusion proteins (N/C) thus complemented one another, leading to the detection of 63% of diabetic individuals. Sensitivity at this level is comparable to GADA, IA2A, and IAA, the current standard biochemical autoantibodies used to diagnose T1D autoimmunity.
Fig. 2.
ZnT8 autoantibody assays in new-onset T1D subjects. (A) The levels of autoreactivity to the ORF (aa1–369), N-terminal (amino acids 1–74), C-terminal (amino acids 264–369) constructs, and a single-chain fusion of the N and C termini (B). (C) The overlap in autoantibody prevalence to each construct.
Specificity and Uniqueness of ZnT8 Autoreactivity.
ZnT8 is a member of the large cation efflux family (10 mammalian homologues; almost 100 family members) of which at least 7 are expressed in islets (27–29) raising the question of whether autoreactivity to ZnT8 in T1D might be directed at other family members. Immunoprecipitation assays performed with nine strongly ZnT8-positive diabetic sera with C-terminal constructs of ZnT3 (Slc30A3) (42% identity) and ZnT5 (Slc30A5) (22% identity) were negative (data not shown), whereas preincubation of the same sera with 10 μg of recombinant His-tagged C-terminal ZnT8 reduced binding to ZnT8 by 93 ± 2.4% (n = 7) (data not shown).
Three individuals (37.5%) from a group of eight T1D subjects who were negative for GADA, IA2A, and IAA but positive for histological islet cytoplasmic antibody (ICA), showed ZnT8A (data not shown). Thirty five from a group of 133 (26.3%) young (mean age 13, range 3–23) insulin-treated patients who were also ICA-negative were reactive to ZnT8, some quite strongly (Fig. 3). In contrast, only 1 of 30 type 2 diabetes patients had ZnT8A, probably misclassified because he also had GADA (data not shown).
Fig. 3.
Uniquiness of ZnTA. ZnT8 C-terminal autoreactivity was measured in GADA-, IA2A-, IAA-, and ICA-negative new-onset T1D subjects, nondiabetic subjects who were 21-hydroxylase antibody-positive with or without Addison's disease and transglutaminase antibody-positive relatives of T1D patients with celiac disease.
Autoreactivity to islet cell proteins is often associated with other tissue-specific immune disorders such as Graves, Addison's, and celiac disease (30–32). Accordingly, ZnT8A were observed in 3 of 35 (8.6%) individuals with established Addison's disease without symptoms of diabetes (Fig. 3) along with GADA (7 of 35, 20%), IA2A (4 of 35, 11.4%), and IAA (1 of 19, 5.2%). Two subjects from a group of 15 who were 21-hydroxylase antibody-positive but without clinical Addison's disease were also ZnT8A-positive (13.3%). Similarly, 12 of 39 (30.8%) nondiabetic, tissue-transglutaminase autoantibody-positive individuals related to T1D patients with celiac disease showed ZnT8A (Fig. 3). ZnT8A measurements on a group of 25 systemic lupus patients with nucleic acid antibodies and a group of 50 rheumatoid arthritis patients, however, were negative (data not shown).
Association of ZnT8 with Other T1D Autoantibodies.
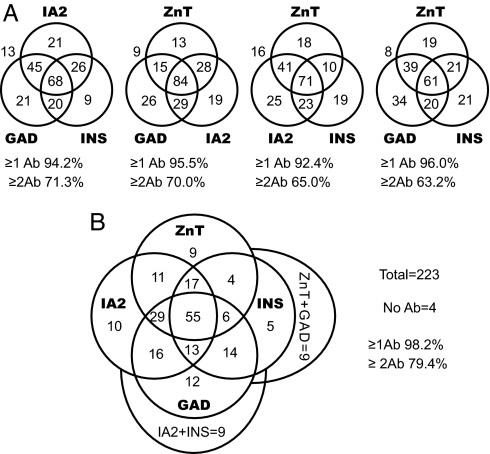
Given their high prevalence, ZnT8A obviously overlap with GADA, IA2A, and IAA at disease onset. Analyzed in terms of the levels of reactivity, ZnT8 N/C antibodies correlated weakly with IA2A (R2 = 0.095, P < 0.0001; SI Fig. 7) but not with IAA or GADA. Given also that ZnT8A can be present in otherwise antibody-negative individuals (Fig. 3), we conclude that ZnT8 autoimmunity is likely an independent T1D marker. This is also evident from the association between each of the autoantibody markers based on prevalence at disease onset (Fig. 4). Individually, IA2, GAD, INS, and ZnT8 antibodies were detected in 72%, 68%, 55%, and 63% of new-onset patients (n = 223). The combined measurement of GADA, IA2A, and IAA, the current gold standard, raised the detection of autoimmunity to 94%. ZnT8A measurements, if substituted individually for GADA, IA2A, or IAA, detected a similar number of diabetic patients; however, the real value of ZnT8A lies as an fourth measurement (Fig. 4B); inclusion of the ZnT8 assays reduced the number of diabetic autoantibody-negative individuals from 5.8% to 1.8% and increased the number who tested positive for two or more autoantibodies from 72% to 82% (n = 223, P = 0.013). Previous longitudinal studies indicate that the prevalence of multiple diabetic autoantibodies is a better index of disease progression than prevalence or titer of antibodies directed at individual antigens (4, 5, 33). This may indicate intermolecular epitope spreading and, hence, worsening autoimmunity, although it should be noted that seropositivity to two antigens has a higher specificity (100%) than one (92–98%) simply because control individuals seldom show two autoantibodies.
Fig. 4.
Overlapping prevalence of ZnT8A, GADA, IA2A, and IAA at onset. (A) Seropositive individuals evaluated with three-autoantibody standard or with ZnT8A substituted for GADA, IA2A, or IAA. The ZnT8A assay incorporates both C-terminal and N/C assays in the one measurement. (B) Seropositive individuals evaluated with four-autoantibody standard.
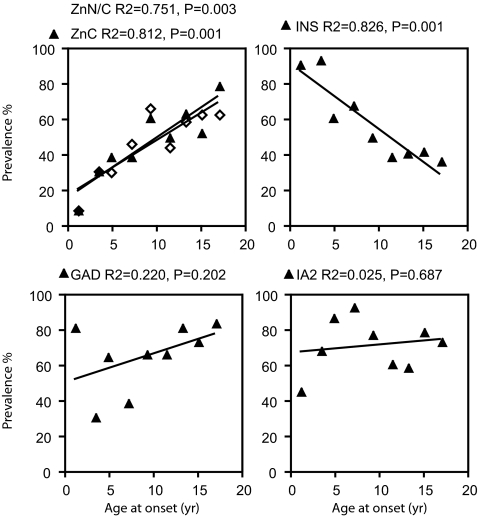
Stratification of the results with subject age at diabetes onset showed that both the prevalence (Fig. 5) of ZnT8 C-terminal and ZnT8 N/C antibodies were low in younger individuals but increased dramatically from ≈3 yr onwards. Prevalence of ZnTA peaked at 80% in late adolescence and tended to decline thereafter (58% in a 23- to 30-yr-old group, n = 19). ZnT8 C-terminal and ZnT8 N/C reactivity changed in parallel and overlapped in specificity to a similar extent at all ages. In contrast to ZnT8A, the prevalence of IAA decreased at older ages, thus emphasizing the utility of ZnT8 antibodies as an additional marker in older subjects. The levels as well as the prevalence of ZnTA increased with age (SI Fig. 8).
Fig. 5.
Relationship of ZnT8A to age at diabetes onset. New-onset T1D subjects ranging from <1 yr to 18 yr old were grouped in 2-yr intervals to illustrate the prevalence of antibodies to ZnT C-terminal and N/C constructs in relation one another and to GADA, IA2A, and IAA.
Longitudinal Studies of Prediabetic Autoimmunity and Risk Prediction.
The question of when ZnT8 autoimmunity emerges in relation to clinical disease was addressed by using samples from the Diabetes Autoimmunity Study in Youth (DAISY) (34). These included 30 first-degree relatives of T1D patients and 13 individuals from the general population selected for high-risk HLA genotypes (DR3 and/or 4) followed prospectively from <1 yr of age to diabetes [median age at onset 6.8 yr (1.9–14.3)]. At onset, 95.3% had antibodies to at least one of the four antigens; 57.9% for ZnT8, 61.9% for GAD, 53.5% for IA2, and 59.1% for INS, which was consistent with the new-onset data (SI Fig. 8 and Figs. 4 and 5), considering that this ongoing prospective study is presently biased toward disease emergence at younger ages.
Life-table analysis of the antibody appearance with time (Fig. 6 A–C) showed that ZnT8A usually preceded disease by many years and frequently appeared by 3 yr of age. IAA was more prevalent than ZnT8A between the ages of 1.5–3 yr (χ2, P = 0.035), and a similar trend was observed between ZnT8A and GADA (χ2, P = 0.134). IA2A, like ZnT8A, was less prevalent than IAA (χ2, P = 0.002) at early time points and showed a similar trend relative to GADA (χ2, P = 0.106). The levels of ZnT8A (SI Fig. 9) were typically low before 2 yr of age but increased progressively in the following 2 yr. A similar pattern was followed by IA2, whereas GAD and INS reached a plateau at 2 yr. The differences between levels recorded at year 3 and diagnosis were significant in the case of ZnT8A (P = 0.006, Mann–Whitney parametric test, n = 31) and IA2 (P = 0.034) but not GAD (P = 0.636) or INS (P = 0.769).
Fig. 6.
ZnT8A in prediabetes. (A–C) The cumulative incidence of autoantibodies in 27 subjects followed to T1D is shown for ZnT C-terminal autoreactivity relative to GADA, IA2A, and IAA. (D) ZnT8 C-terminal autoantibodies were determined on the first serum samples to test positive for GADA, IA2, or IAA. Survival analysis compares the disease outcome (mean ± SEM) for ZnT8A-seropositive versus -negative individuals. Subjects remaining in both studies at each time point are indicated.
The progression of autoreactivity in 10 individuals is illustrated in SI Fig. 10. ZnT8A were observed in 17 of the 27 cases (62.9%) before diabetes, GADA in 19 (70.3%), IA2A in 14 (51.8%), and IAA in 23 (85.1%). The corresponding numbers at disease onset were 17 (62.9%), 15 (55.5%), 13 (48.1%), and 19 (70.3%), indicating some dropout of GADA and IAA. Individual profiles varied with respect to when antibodies first appeared, the levels attained, and the sequence in which they appeared. The median age of appearance of GADA (2.0 yr, range 1.26–8.87) and IAA (2.2 yr, range 0.8–8.9) tended to be earlier than that of IA2A (2.7yr, range 1.9–8.7) and ZnT8A (3.6 yr, range 1.3–8.9), concordant with the life-table analysis (Fig. 6 A–C). However, there was no strict order of appearance of antibodies, and in two individuals, ZnT8A were the only ones observed. Two other individuals developed diabetes with only IAA and one with only IA2A.
ZnT8A persisted to diagnosis, falling only marginally in six individuals. Such stability contrasted to GADA and IAA, which frequently declined with age, often leading to dropout. The interval separating ZnT8A appearance and disease varied widely, in two cases, within a year, in others up to 7 yr. In a pair-wise comparison, diabetes emerged sooner after ZnT8 seropositivity than GADA (4.14 ± 2.62 vs. 5.17 ± 2.85 yr, paired t test P = 0.009, n = 12), sooner than IAA (3.62 ± 2.15 vs. 4.56 ± 2.41 yr, P = 0.01, n = 14) but at approximately the same time relative to IA2A appearance (3.72 ± 2.33 vs. 3.65 ± 1.49 yr, P = 0.889, n = 11). These differences reflect the earlier onset of GADA and IAA relative to ZnT8 (Fig. 6 A and B), and it is concluded that ZnT8 autoantibodies, although arising later, do not predict imminent progression to clinical disease any more than GADA, IA2A, or IAA.
To test whether the determination of ZnT8 autoantibodies enhances the ability to predict disease in the presence of other autoantibodies, an analysis was performed on the first serum samples from each of 88 DAISY subjects that had tested positive for a single biochemical antibody (GADA, IA2A, or IAA). The study focused on individuals who were older than 3 when sampled and whose follow-up was >1 year. No consideration was given to the initial antibody type nor to subsequent emergence of other autoantibodies. Of this group, 36.8% (7 of 19) of individuals with ZnT8 antibodies progressed to clinical diabetes compared with 7.3% (5 of 69) of those without (P < 0.003, two-tailed Fisher exact test). Life-table analysis (Fig. 6D) confirmed this observation and showed that the presence of ZnT8A significantly increased the probability that an individual otherwise classified as low risk would progress to clinical diabetes within 5 yr (P = 0.007). A previous prospective study restricted to IAA, GADA, and IA2A (33) has similarly shown that the risk of development of diabetes within 5 yr increased from 15% with one autoantibody to 44% with two.
Discussion
The ZnT8 transporter was pursued as a candidate diabetes autoantigen based on a bioinformatic screen of genes aimed at testing the hypothesis that tissue-specific autoimmunity is often directed at molecules that are expressed in a tissue-specific manner at moderate-to-high abundance. It fulfilled another postulate in being associated with the regulated pathway of secretion (27), a feature common to many β-cell and thyroid autoantigens (35). ZnT8 is thought to be responsible for the concentration of Zn2+ in the granule lumen (27), although this is currently unproven because the β-cell expresses other more widely distributed Slc30A family members such as ZnT2, 4, and 5, some with granule localization (29). We found no evidence that autoreactivity to ZnT8 was shared by the most-closely related family members and, in general terms, pointed to epitopes being confined to ZnT8. A recent genome-wide association study demonstrated an association of a series of ZnT8 polymorphisms with human type 2 diabetes (36). The chromosomal region where Slc30A8 is localized (8q24) has not, however, been implicated in human T1D.
ZnT8, like another diabetes autoantigen, IGRP (19, 37), is a multispanning transmembrane protein that presents a challenge to development of assays for humoral and cell-mediated autoreactivity because of the difficulty of maintaining its tertiary structure outside of a membrane environment. Sensitive and specific ZnT8A assays nevertheless were developed by using radioimmunoprecipitation of in vitro-translated products generated from soluble domains of the protein and construction of single-chain molecules fusing C- and N-terminal domains. Most structural prediction models place both the C and N termini in the cytoplasm and are thus unlikely to be exposed on the cell surface during granule exocytosis. A similar topology for three other membrane-associated diabetes autoantigens, GAD65, IA2, and phogrin, has been inferred, leading to speculation that these antigens might encounter immune surveillance only as alternatively spliced forms (38, 39), after apoptosis of β-cells early in postnatal life (40) or after immune-mediated destruction of islets targeted at other cell-surface or secreted autoantigens. As a protein that is initially incorporated into the endoplasmic reticulum (ER), it might also be delivered to the MHC processing compartment by premature termination of translation products or after ER stress (35). The observation that high levels of ZnT8A emerged late relative to IAA and GADA fits the idea that ZnT8 antigen presentation occurs secondary to immune damage. However, the absence of hierarchy of autoantibody emergence in the individuals followed prospectively suggests that either the initiating antigen is unknown or that there exist multiple pathways and diverse forms of human T1D from an immunological perspective. The complex kinetic series might also be accounted for by independent etiological events triggered by different environmental factors.
ZnT8A were persistent in the prediabetic phase and proved a useful independent marker of autoimmunity either alone in antibody-negative subjects or in conjunction with IAA, GADA, or IA2A, where it increases the likelihood of detecting a prognostic second autoantibody (6). ZnT8A should be especially useful in older individuals in whom insulin autoantibodies wane with age. Unlike GAD and IA2, ZnT8 is highly β-cell-specific, and thus ZnT8A measurements may be useful in monitoring islet destruction after onset and in evaluating therapeutic interventions that limit β-cell-specific autoreactivity or restore β-cell mass. IAA measurements in these circumstances are precluded because insulin administration itself induces insulin antibodies.
The bioinformatics approach that identified ZnT8 as a candidate autoantigen can potentially be extended to other tissue-specific autoimmune disorders. In the case of T1D, with a prevalence of 0.3% in the Caucasian population, the development of further robust assays for humoral autoreactivity in combination with genetic screening raises the potential of pediatric screening for T1D susceptibility in the general population. Such undertakings, however, are not trivial, given that many of the autoantigen epitopes are conformational, requiring structural evaluation and molecular engineering strategies to develop robust assays.
Methods
Human islets of 75–80% purity and 76–96% viability were obtained from the National Institutes of Health Islet Cell Resource (ICR) program. Total RNA extracted with TRIzol reagent was quantified (Agilent Bioanalyzer; Agilent Technologies, Palo Alto, CA) and processed for hybridization to Affymetrix U133A Ver 2.0 oligonucleotide microarrays by using standard procedures (Affymetrix, Santa Clara, CA). Results from five different islet preparations were normalized by the GC robust multi-array average (GCRMA) procedure.
The Novartis custom oligonucleotide array of 79 human tissues (Gene Atlas V2; Novartis) (15) was queried by using three criteria: fold enrichment of genes in islets versus whole pancreas, fold enrichment in islets versus median expression on the array, and tissue specificity based on the calculation of Shannon entropy (41). A compilation of 300 genes was then compared with the corresponding mouse genes on MOE40 microarrays of pancreatic tumor cell lines (αTC1–6 glucagonoma, βTC3 insulinoma, and mPAC ductal cell lines) to filter out housekeeping genes and genes expressed at higher levels (>2.5-fold) in non-β islet cells. The 140 genes that passed these criteria were evaluated by the Unigene EST profile viewer in 49 normal human tissues based on the frequency with which ESTs have been observed in cDNA libraries. The pancreatic abundance (pancreatic clones per million cDNAs) and pancreatic specificity (pancreatic clonal frequency per global frequency) were calculated and the product used as a score to rank the genes (SI Table 1). The data and programs used in these analyses can be accessed through http://genespeed.uchsc.edu/development_1, a public domain database geared to transcriptional and cell biological analyses focused on pancreatic islet development (42).
The human ZnT8 (Slc30A8) ORF (ORF) was amplified by PCR from human islet cDNA prepared with an iScript kit (Bio-Rad, Hercules CA) using the primers; forward FL-CACCATGGAGTTTCTTGAAAG and reverse FL-CTAGTCACAGGGGTCTTCAC. An N-terminal construct used the same forward primer and the N-terminal reverse primer GAGTTTCCCACTTGGCATAGGC and a C-terminal construct the C-forward CACCATGAAGGACTTCTCCATCTTACTC and the reverse FL primer. The N/C construct was made by combining a N-terminal PCR product generated with the forward FL primers and a linker N/C reverse primer (GTAAGATGGAGAAGTCCTTTCCGCCACCAGAACAGAGTTTCCACTTGGC) and a C-terminal PCR product generated with a N/C forward primer (GCCAAGTGGAAACTCTGTTCTGGTGGCGGAAAGGACTTCTCCATCTTAC) and reverse FL primer. The products were gel purified, mixed and reamplified with the FL primer set to generate a single-chain N/C fusion sequence with a AKWKLCSGGGKDFSIL junction. All constructs were cloned into pCDNA3.1 directional TOPO vector (kit 45–0158; Invitrogen, Carlsbad, CA), and sequence verified.
Plasmid DNA (2 μg) was incubated in a 100 μl of an in vitro-coupled transcription/translation reticulocyte lysate reaction (TNTQuick T7 promoter; Promega, San Luis Osbispo, CA) with 20 μCi (1 Ci = 37 GBq) of [35S]methionine (1,000 Ci/mmol; Amersham Bioscience, Pittsburgh, PA) and the products gel-filtered on G25 Sephadex (NAP5 column; GE Healthcare, Piscataway, NJ). Radioactivity incorporated into protein was determined by precipitation with 5% (wt/vol) trichloroacetic acid after alkaline hydrolysis of any [35S]Met tRNA left in the sample with 1M NaOH (percent incorporation: C-terminal 10.0 ± 2.1; N/C 10.6 ± 0.4). Human serum samples (5 μl) were incubated overnight at 4°C with 25,000 cpm of the above translation product in 50 μl of PBS containing 0.1%(wt/vol) BSA, 0.15% Nonidet P-40, and 0.02% NaN3 in a 96-well plate. Immobilized Protein A (10 μl of 50% (vol/vol) slurry (Protein A Sepharose 4 Fast Flow 17–5280; Amersham Bioscience) was added to each well, and 45 min later, the beads were recovered by filtration (MADVNOB50 96-well plates; Millipore, Billerica, MA) and bound radioactivity determined by liquid scintillation counting (Wallac 1450 Microbeta Trilux counter; PerkinElmer, Waltham, MA). GADA IA2A and IAA assays followed similar standard protocols (43).
Each ZnT8A assay was run with 16 matched control samples and an eight-step doubling dilution of a high-titer T1D sera standard prepared from a pool of nine new-onset sera, which at a 1:10 dilution, precipitated 102.5 ± 2.3% (n = 25) of the added radioactivity. Half-maximal binding occurred at a 1:50 dilution. In the standard C-terminal assay, blanks (no sera) averaged 150 ± 26 (seven experiments) and control sera 259 ± 58 cpm (22 experiments). Intrassay coefficient of variation (CV) on controls ranged from 8–16% (n = 16) and interassay CV 21% (n = 30). Cutoff values were calculated as the mean ± 4 SD of the within-assay controls and globally relative to 368 DAISY control sera at a 98% cutoff. Normalization was achieved by using the immunoprecipitation index (sample − control)/(high standard − control) that gave a cutoff value = 0.010–0.012. In the case of the longitudinal study (SI Fig. 10), results were expressed as the log10 fold SD to enable compare IAA data from assays with different formats. The ZnT N/C assay blanks were 215 ± 40 cpm (n = 16), control values 370 ± 40 cpm (n = 268) and 99th percentile cutoff index (0.015). The C-terminal assay was validated against blinded sera from 50 newly diagnosed diabetic patients (aged 18–28) and 100 age-matched controls provided by the Diabetes Autoantigen Standardization Program (DASP) within the Centers for Disease Control (44). The assay showed a 58% sensitivity at 100% specificity and 64% sensitivity at 95% specificity.
Subjects were drawn from newly diagnosed T1D patients at the Barbara Davis Center, their relatives, and from DAISY participants. The new-onset populations were comprised as follows: T1D n = 277; mean age 12.1 (range 1–46), 87% Caucasian, 6.3% Hispanic; Control n = 150; mean age 13.1 (range 1–55), 72% Caucasian, 15.1% Hispanic; T1D but all Ab-negative n = 133: mean age 13.0 (3–23), 71% Caucasian 16.7% Hispanic; Nondiabetic 21-hydroxylase Ab-positive n = 35; mean age 38.7 (range 16–72); Addison's n = 15 mean age 33.4 (range 6–79) 100% Caucasian; nondiabetic tTG Ab-positive first-degree relatives of tTG T1D n = 39, mean age 12.2 (4–21) 100% Caucasian. Patients from the DAISY cohort were selected in the following groups: First positive antibody study n = 88: mean age 4.5 (range 3–14), 77% Caucasian 13.0% Hispanic; DAISY followed to diabetes 43 subjects, 441 samples: median age 4.9 (range 0.6–11), 100% Caucasian; and DAISY controls n = 268: mean age 6.8 (range 1.2–46), 66.5% Caucasian. The latter were healthy volunteers, the parents and children in the newborn cohort of the DAISY study, and parents of the DAISY sibling/offspring cohort. The male/female gender ratio over all of the groups ranged from 0.8 to 1.4. Informed consent was obtained from participants and/or parents under approved Institutional Review Board oversight. Statistical analyses were performed with the Prism 4 software package www.graphpad.com. Results are expressed as mean ± SD unless otherwise specified.
Supplementary Material
Acknowledgments
We thank Kathy Barriga, Jennifer Barker, Michael Holers, and Teri Aly for their advice and assistance in retrieving archived serum samples. This work was supported by the Children's Diabetes Foundation in Denver, CO, the University of Colorado Health Sciences Center Diabetes and Endocrinology Research Center [National Institutes of Health (NIH) Grant P30 DK57516], the Beta Cell Biology Consortium (NIH Grant U19 DK61248), and the Juvenile Diabetes Research Foundation.
Abbreviations
- GADA
glutamate decarboxylase antibodies
- IAA
antibodies to insulin
- IA2A
IA2 antibodies
- ICA
islet cytoplasmic autoantibodies
- T1D
type 1 diabetes
- ZnTA
ZnT8 antibodies.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0705894104/DC1.
References
- 1.Anderson MS, Bluestone JA. Annu Rev Immunol. 2005;23:447–485. doi: 10.1146/annurev.immunol.23.021704.115643. [DOI] [PubMed] [Google Scholar]
- 2.Silveira PA, Serreze DV, Grey ST. Front Biosci. 2007;12:2183–2193. doi: 10.2741/2221. [DOI] [PubMed] [Google Scholar]
- 3.Martin S, Wolf-Eichbaum D, Duinkerken G, Scherbaum WA, Kolb H, Noordzij JG, Roep BO. N Engl J Med. 2001;345:1036–1040. doi: 10.1056/NEJMoa010465. [DOI] [PubMed] [Google Scholar]
- 4.Bingley PJ, Christie MR, Bonifacio E, Bonfanti R, Shattock M, Fonte MT, Bottazzo GF, Gale EA. Diabetes. 1994;43:1304–1310. doi: 10.2337/diab.43.11.1304. [DOI] [PubMed] [Google Scholar]
- 5.Pihoker C, Gilliam LK, Hampe CS, Lernmark A. Diabetes. 2005;54(Suppl 2):S52–S61. doi: 10.2337/diabetes.54.suppl_2.s52. [DOI] [PubMed] [Google Scholar]
- 6.Verge CF, Gianani R, Kawasaki E, Yu L, Pietropaolo M, Jackson RA, Chase HP, Eisenbarth GS. Diabetes. 1996;45:926–933. doi: 10.2337/diab.45.7.926. [DOI] [PubMed] [Google Scholar]
- 7.Achenbach P, Warncke K, Reiter J, Williams AJ, Ziegler AG, Bingley PJ, Bonifacio E. Diabetologia. 2006;49:2969–2976. doi: 10.1007/s00125-006-0451-9. [DOI] [PubMed] [Google Scholar]
- 8.Bergerot I, Souchier C, Thivolet C. Can R Acad Sci III. 1993;316:1368–1373. [PubMed] [Google Scholar]
- 9.Palmer JP, Asplin CM, Clemons P, Lyen K, Tatpati O, Raghu PK, Paquette TL. Science. 1983;222:1337–1339. doi: 10.1126/science.6362005. [DOI] [PubMed] [Google Scholar]
- 10.Baekkeskov S, Aanstoot HJ, Christgau S, Reetz A, Solimena M, Cascalho M, Folli F, Richter-Olesen H, De Camilli P. Nature. 1990;347:151–156. doi: 10.1038/347151a0. [DOI] [PubMed] [Google Scholar]
- 11.Bonifacio E, Lampasona V, Genovese S, Ferrari M, Bosi E. J Immunol. 1995;155:5419–5426. [PubMed] [Google Scholar]
- 12.Schatz DA, Bingley PJ. J Pediatr Endocrinol Metab. 2001;14(Suppl 1):619–622. doi: 10.1515/jpem.2001.14.s1.619. [DOI] [PubMed] [Google Scholar]
- 13.Hermann R, Bartsocas CS, Soltesz G, Vazeou A, Paschou P, Bozas E, Malamitsi-Puchner A, Simell O, Knip M, Ilonen J. Diabetes Metab Res Rev. 2004;20:322–329. doi: 10.1002/dmrr.455. [DOI] [PubMed] [Google Scholar]
- 14.Chatenoud L. Int Rev Immunol. 2006;25:215–233. doi: 10.1080/08830180600743032. [DOI] [PubMed] [Google Scholar]
- 15.Su AI, Cooke MP, Ching KA, Hakak Y, Walker JR, Wiltshire T, Orth AP, Vega RG, Sapinoso LM, Moqrich A, Patapoutian A, Hampton GM, Schultz PG, Hogenesch JB. Proc Natl Acad Sci USA. 2002;99:4465–4470. doi: 10.1073/pnas.012025199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hawkes CJ, Wasmeier C, Christie MR, Hutton JC. Diabetes. 1996;45:1187–1192. doi: 10.2337/diab.45.9.1187. [DOI] [PubMed] [Google Scholar]
- 17.Qin HY, Mahon JL, Atkinson MA, Chaturvedi P, Lee-Chan E, Singh B. J Autoimmun. 2003;20:237–245. doi: 10.1016/s0896-8411(03)00035-0. [DOI] [PubMed] [Google Scholar]
- 18.Castano L, Russo E, Zhou L, Lipes MA, Eisenbarth GS. J Clin Endocrinol Metab. 1991;73:1197–1201. doi: 10.1210/jcem-73-6-1197. [DOI] [PubMed] [Google Scholar]
- 19.Lieberman SM, Evans AM, Han B, Takaki T, Vinnitskaya Y, Caldwell JA, Serreze DV, Shabanowitz J, Hunt DF, Nathenson SG, et al. Proc Natl Acad Sci USA. 2003;100:8384–8388. doi: 10.1073/pnas.0932778100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clark A, Yon SM, de Koning EJ, Holman RR. Diabet Med. 1991;8:668–673. doi: 10.1111/j.1464-5491.1991.tb01675.x. [DOI] [PubMed] [Google Scholar]
- 21.Gurr W, Shaw M, Li Y, Sherwin R. Diabetes. 2007;56:34–40. doi: 10.2337/db06-0669. [DOI] [PubMed] [Google Scholar]
- 22.Pietropaolo M, Castano L, Babu S, Buelow R, Kuo YL, Martin S, Martin A, Powers AC, Prochazka M, Naggert J, et al. J Clin Invest. 1993;92:359–371. doi: 10.1172/JCI116574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arden SD, Roep BO, Neophytou PI, Usac EF, Duinkerken G, de Vries RR, Hutton JC. J Clin Invest. 1996;97:551–561. doi: 10.1172/JCI118448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boitard C, Villa MC, Becourt C, Gia HP, Huc C, Sempe P, Portier MM, Bach JF. Proc Natl Acad Sci USA. 1992;89:172–176. doi: 10.1073/pnas.89.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rabin DU, Pleasic SM, Palmer-Crocker R, Shapiro JA. Diabetes. 1992;41:183–186. doi: 10.2337/diab.41.2.183. [DOI] [PubMed] [Google Scholar]
- 26.Luhder F, Schlosser M, Mauch L, Haubruck H, Rjasanowski I, Michaelis D, Kohnert KD, Ziegler M. Autoimmunity. 1994;19:71–80. doi: 10.3109/08916939409009534. [DOI] [PubMed] [Google Scholar]
- 27.Chimienti F, Devergnas S, Favier A, Seve M. Diabetes. 2004;53:2330–2337. doi: 10.2337/diabetes.53.9.2330. [DOI] [PubMed] [Google Scholar]
- 28.Clifford KS, MacDonald MJ. Diabetes Res Clin Pract. 2000;49:77–85. doi: 10.1016/s0168-8227(00)00141-8. [DOI] [PubMed] [Google Scholar]
- 29.Kambe T, Narita H, Yamaguchi-Iwai Y, Hirose J, Amano T, Sugiura N, Sasaki R, Mori K, Iwanaga T, Nagao M. J Biol Chem. 2002;277:19049–19055. doi: 10.1074/jbc.M200910200. [DOI] [PubMed] [Google Scholar]
- 30.Pietropaolo M, Peakman M, Pietropaolo SL, Zanone MM, Foley TP, Jr, Becker DJ, Trucco M. J Autoimmun. 1998;11:1–10. doi: 10.1006/jaut.1997.0170. [DOI] [PubMed] [Google Scholar]
- 31.Barker JM, Ide A, Hostetler C, Yu L, Miao D, Fain PR, Eisenbarth GS, Gottlieb PA. J Clin Endocrinol Metab. 2005;90:128–134. doi: 10.1210/jc.2004-0874. [DOI] [PubMed] [Google Scholar]
- 32.Bao F, Yu L, Babu S, Wang T, Hoffenberg EJ, Rewers M, Eisenbarth GS. J Autoimmun. 1999;13:143–148. doi: 10.1006/jaut.1999.0303. [DOI] [PubMed] [Google Scholar]
- 33.Verge CF, Gianani R, Kawasaki E, Yu L, Pietropaolo M, Chase HP, Eisenbarth GS. J Autoimmun. 1996;9:379–383. doi: 10.1006/jaut.1996.0051. [DOI] [PubMed] [Google Scholar]
- 34.Rewers M, Norris JM, Eisenbarth GS, Erlich HA, Beaty B, Klingensmith G, Hoffman M, Yu L, Bugawan TL, Blair A, et al. J Autoimmun. 1996;9:405–410. doi: 10.1006/jaut.1996.0055. [DOI] [PubMed] [Google Scholar]
- 35.Lieberman SM, DiLorenzo TP. Tissue Antigens. 2003;62:359–377. doi: 10.1034/j.1399-0039.2003.00152.x. [DOI] [PubMed] [Google Scholar]
- 36.Sladek R, Rocheleau G, Rung J, Dina C, Shen L, Serre D, Boutin P, Vincent D, Belisle A, Hadjadj S, et al. Nature. 2007;445:881–885. doi: 10.1038/nature05616. [DOI] [PubMed] [Google Scholar]
- 37.Arden SD, Zahn T, Steegers S, Webb S, Bergman B, O'Brien RM, Hutton JC. Diabetes. 1999;48:531–542. doi: 10.2337/diabetes.48.3.531. [DOI] [PubMed] [Google Scholar]
- 38.Park YS, Kawasaki E, Kelemen K, Yu L, Schiller MR, Rewers M, Mizuta M, Eisenbarth GS, Hutton JC. Diabetologia. 2000;43:1293–1301. doi: 10.1007/s001250051525. [DOI] [PubMed] [Google Scholar]
- 39.Ng B, Yang F, Huston DP, Yan Y, Yang Y, Xiong Z, Peterson LE, Wang H, Yang XF. J Allergy Clin Immunol. 2004;114:1463–1470. doi: 10.1016/j.jaci.2004.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Trudeau JD, Dutz JP, Arany E, Hill DJ, Fieldus WE, Finegood DT. Diabetes. 2000;49:1–7. doi: 10.2337/diabetes.49.1.1. [DOI] [PubMed] [Google Scholar]
- 41.Schug J, Schuller WP, Kappen C, Salbaum JM, Bucan M, Stoeckert CJ., Jr Genome Biol. 2005;6:R33. doi: 10.1186/gb-2005-6-4-r33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kutchma A, Quayum N, Jensen J. Nucleic Acids Res. 2007;35:D674–D679. doi: 10.1093/nar/gkl990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang J, Miao D, Babu S, Yu J, Barker J, Klingensmith G, Rewers M, Eisenbarth GS, Yu L. J Clin Endocrinol Metab. 2007;92:88–92. doi: 10.1210/jc.2006-1494. [DOI] [PubMed] [Google Scholar]
- 44.Bingley PJ, Bonifacio E, Mueller PW. Diabetes. 2003;52:1128–1136. doi: 10.2337/diabetes.52.5.1128. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.