Abstract
To further elucidate the roles of germ cells in the sex differentiation of gonads, we have used the medaka, a teleost fish, to generate mutants that lack germ cells from the onset of gonadogenesis by the morpholino-mediated knockdown of cxcr4. The resulting germ-cell-deficient medaka show female-to-male sex reversal of their secondary sex characteristics, accompanied by increased levels of androgen and reduced levels of estrogen. A failure to maintain granulosa cells or estrogen-producing cells also occurs at early stages of sex differentiation in the cxcr4 morphants, before the initiation of gonadal morphogenesis. In contrast, androgen-producing cells are unaffected in germ-cell-deficient medaka of either sex. In addition, a single tube-like gonad that expresses male-specific genes is formed in these mutants irrespective of the genetic sex. Significantly, each of these mutant phenotypes occurs in a somatic cell-autonomous manner, suggesting that gonadal somatic cells are predisposed toward male development in the absence of germ cells. This highlights the importance of germ cells in the sexual dimorphism of the gonads.
Keywords: sex differentiation, steroidogenic cells, sox9, foxl2, aromatase
The vertebrate gonad consists of both germ cells and gonadal somatic cells. When these cells ultimately form the gonadal primordium, in mammals and in the medaka, the gonads develop into either ovaries or testes, depending on the genetic program (1–5). The somatic cell lineages then develop into both granulosa cells and theca cells in the ovary and become Sertoli cells and Leydig cells in the testis. The granulosa and Sertoli cells are known as supporting cells and directly enclose germ cells, whereas theca cells and Leydig cells function as sex steroid hormone-producing cells. Although mutations and in vitro manipulations have now provided insights into the molecular pathways through which each of these lineages chooses its cell fate (6, 7), little is still known about the interplay between somatic and germ cells.
Specific cell ablation has proved a useful method to analyze the roles of specific cell lineages during organogenesis, and the effects of the loss of germ cells during gonadogenesis have been reported in a number of studies in mammals (8–10). In the mammalian ovary, the absence of germ cells (oogonia/oocytes) in the genital ridges does not appear to affect ovarian formation or early differentiation (11, 12). However, the loss of oocytes in the follicles inhibits the maturation of granulosa cells and induces their transdifferentiation to Sertoli cells, which harbor tubule-like structures (13–15). This is followed by the appearance of cells that produce male steroid hormones within the interstitial regions of the gonad (16–18). On the other hand, germ cell loss in the testis does not affect the formation or differentiation of somatic cells in an immature state (19–21). Recently, the ablation of germ cells in zebrafish was also reported (22), and these fish develop as sterile males with no gonadal structures evident in the adult. However, the cellular processes underlying sex reversal remain to be investigated.
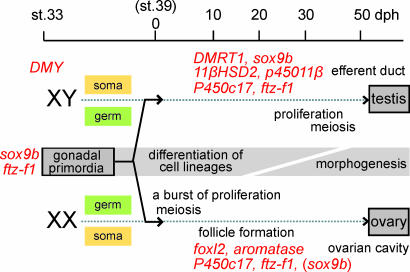
The medaka has also been established as a model organism that is particularly useful in the analysis of gonadogenesis. The medaka gonadal primordium is formed at ≈4 days postfertilization (dpf) (stage 33) (23), at which time the testis-determining gene on the Y chromosome, DMY/dmrt1bY, begins to be expressed in the male gonadal somatic cells (24) (Fig. 1). Other than DMY/dmrt1bY, all of the genes that have been shown to be expressed in the gonads show no sex-specific expression until ≈10 days posthatching (dph). Moreover, the gonadal primordia of both sexes express sox9b and ftz-f1 and are almost morphologically indistinguishable until around the time of hatching (23, 25). The germ cells in the female medaka gonads then begin to dramatically increase in number (25–27), which is not observed in the male gonads (25, 28–31). Ovarian follicles with diplotene oocytes become evident at ≈20 dph (29), and the expression of the sex-dependent genes becomes evident by 10 dph. DMRT1 and sox9b are preferentially expressed in the male gonads, whereas foxl2 and cytochrome P450 aromatase (hereafter, aromatase) are expressed in the female gonads (25, 32, 33).
Fig. 1.
Schematic representation of medaka gonadal development from stage 33 (4 dpf) to adulthood. Morphological events are indicated by black characters. Red characters indicate both the timing and location of the gene expression tested in this study. The early stages of gonadal development include the period of cell differentiation, which should be discriminated from later morphogenic stages. Secondary sex characteristics in the medaka manifest after 40–50 dph in both sexes.
Steroid-producing cells expressing genes encoding steroidogenic enzymes such as P450c17 (cytochrome P450 hydroxylase and c17 lyase) are also detectable by 20 dph in both sexes. The male germ cells enter meiosis at ≈50 dph. During the period between 30 and 50 dph, gonadal morphogenesis initiates in both sexes. The ovarian cavity in the ovary and an efferent duct and lobule structures in the testis are then formed, and secondary sex characteristics gradually become apparent under the influence of the sex steroid hormones.
In our current study, we have generated a medaka strain with a germ-cell-deficient gonad by inhibiting primordial germ cell migration. The resulting adult germ-cell-deficient medaka displays a female-to-male secondary sex reversal phenotype. By analysis of the germ-cell-deficient gonads, we demonstrate that the germ cells exert multiple effects upon gonad formation and sex differentiation. The differentiation of female-specific cell lineages was found to be impaired during the early stages of gonadal development, whereas cells expressing male-specific genes were observed to develop in both the female and male germ-cell-deficient gonads. Furthermore, adult germ-cell-deficient gonads from both sexes were found to possess a single tube-like structure with an organization resembling both the ovarian follicles and seminiferous tubules of wild-type medaka gonads.
Results
Masculinization Occurs in a Germ-Cell-Deficient Medaka.
To investigate the roles of germ cells during gonadal development in the medaka, we generated a germ-cell-deficient strain of this fish by injection of morpholino antisense oligonucleotides that were directed against the chemoattractant receptor gene, cxcr4 (cxcr4-MO). Previously, we have shown that cxcr4 is involved in the migration of primordial germ cells in the medaka at the gastrulation stage, and that this process is severely impaired by inhibiting this gene (34). Coinjection of an RNA-encoding EGFP with the olvas-3′ UTR, which drives EGFP expression in germ cells, confirmed the mismigration of fluorescent germ cells and their subsequent loss in the gonads at stage 35 in the resulting morphants [supporting information (SI) Fig. 5]. The morphant embryos were further allowed to grow for 2 months until they reached sexual maturity.
At the adult stage, we reexamined the absence of germ cells in the cxcr4-MO embryos. Among the adult XX morphant medaka (n = 131), 68% (n = 89/131) did not possess any germ cells in the gonads. Each of these XX medaka exhibited female-to-male sex reversal of their secondary sex characteristics with well developed anal and dorsal fins, as well as the loss of urinogenital papillae (Fig. 2 A–C). Only those fish with a complete loss of germ cells were used for all subsequent analysis.
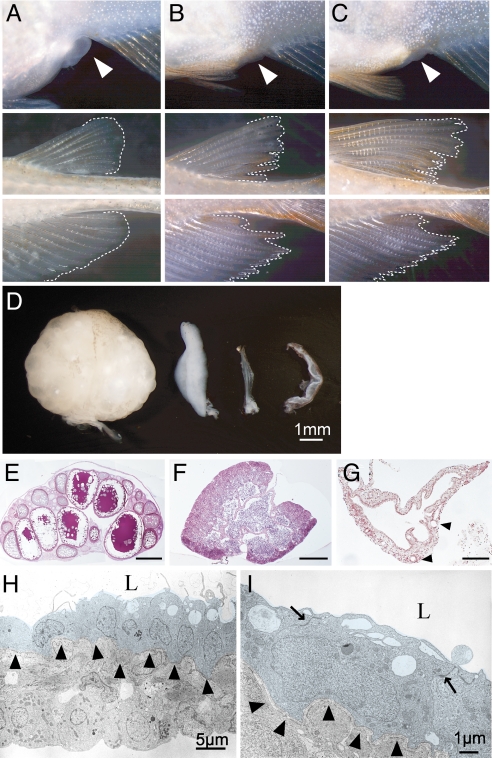
Fig. 2.
Sex reversal of germ-cell-deficient medaka and its associated gonadal morphology. (A--C) Representative images of the secondary sex characteristics of medaka adults that are genetically female (XX) (A), male (XY) (B), and cxcr4-morphant (germ-cell-deficient) female (XX) (C). (Top) External genitalia. White arrowheads indicate the anus. A developed urinogenital papilla is observed only in the wild-type female. (Middle and Bottom) Dorsal and anal fins, respectively. Phenotypic males (B and C) display a sharp and long anal fin, whereas a round-shaped anal fin is characteristic of a phenotypic female (A). (D) Dorsal views of an ovary (left), testis (second from left) and germ cell-deficient XX and XY gonads (second from right and right). (E–G), Cross-sections of ovary (E; scale bar, 500 μm), testis (F; scale bar, 200 μm), and germ-cell-deficient gonad (G; scale bar, 100 μm). A single empty lumen with several foldings is present in the germ-cell-deficient gonad. Arrowheads indicate blood vessels. (H and I) Electron micrographs of germ-cell-deficient medaka gonads. The lumen (L), basement membrane (arrowheads), and desmosomes (arrows) are indicated. A single layer of innermost cells is shaded with a pale blue color.
Thirty-two percent of the adult XX morphants (n = 42/131) possessed germ cells and were fertile, indicating in these cases that gonads containing small numbers of germ cells were present but were misidentified as germ-cell-deficient at embryonic screening, possibly because of the weak fluorescence of the germ cells. Although these fish were excluded from all subsequent analyses, it is noteworthy that 26% (11/42) of the fertile XX morphants grew as functional males, whereas the remaining 74% (31/42) developed as females.
The XY morphants all (n = 30) showed normal male secondary sex characteristics. The control wild-type adult medaka showed no sex-reversal properties (n = 46 for XX and 47 for XY).
Adult Germ-Cell-Deficient Gonads in the Medaka Exhibit a Tube-Like Structure.
We next examined the gonadal morphology of adult morphant fish. Germ-cell-deficient gonads from both XX and XY morphants show a translucent and single tube-like structure (Fig. 2 D and G and SI Fig. 5C). As shown by electron microscopy, a single layer of the innermost cells bordering the lumen of the tube-like gonad is lined with cells that are separated by a basement membrane from a region of outer stromal cells (Fig. 2 H and I). In some of the tube-like gonads, the innermost cells also develop blebs and conspicuous microvillous processes on their apical surfaces, characteristic of germinal epithelia (surface epithelia in mice) (35). These innermost cells invariably contact their neighboring cells to form desmosomes at the apicolateral regions, contain numerous mitochondria, and develop an endoplasmic reticulum. The outer stromal layer comprises a mixture of heterologous cells, including steroidogenic cells and cells of the vasculature. This organization was observed irrespective of the genetic sex of the morphants. These results demonstrate that gonadal somatic cells can intrinsically form this structure irrespective of their genetic sex in the absence of germ cells.
Androgen Is Synthesized in the Adult cxcr4-Morphants of both Sexes.
The tube-like gonad and the sex-reversal phenotype of our cxcr4 morphants raised the possibility that the production of sex steroids is severely impaired in the gonads of these germ-cell-deficient animals. Both female and male secondary sex characteristics develop via the activity of sex steroid hormones secreted from the gonads, estradiol-17β and 11-ketotestosterone, respectively. In the ovary, the theca cells produce testosterone via steroidogenic enzymes such as P450c17, which is immediately converted into estradiol-17β in the granulosa cells by the action of aromatase (36–39). In the testis, Leydig cells produce 11-ketotestosterone from testosterone by the action of two enzymes, 11β-hydroxydehydrogenase 2 (11β-HSD2) and P45011β. The administration of 11-ketotestosterone has been shown to cause phenotypic female-to-male sex reversal in the medaka (40).
To examine whether the production of sex steroids was altered in the cxcr4 morphants, we isolated the gonads from both normal and morphant adult fish and measured the levels of sex steroid hormones by enzyme immunoassays. We found that germ-cell-deficient XX morphant gonads contained 11-ketotestosterone at 2.3-fold greater levels than wild-type ovaries (Table 1) and at ≈50% of the amounts present in wild-type testes. This steroid was also detected in other tissues, such as the head kidney-containing interrenal cells (the adrenal equivalent in fish) and liver, at equivalent levels in XX morphants and wild-type males. This may suggest that 11-ketotestosterone is produced in the gonads and circulates with serum. Estradiol-17β, which is an abundant steroid in normal ovaries, was found to be below the detection threshold in XX morphant gonads.
Table 1.
Measurement of sex steroid hormone contents in wild-type and cxcr4-morphant adult medaka (picogram per organ, mean ± SEM)
| Wild type |
cxcr4-morphant |
|||
|---|---|---|---|---|
| XX | XY | XX | ||
| 11-keto testosterone | Gonad | 4.4 ± 0.6 (n = 5: 31.3 ± 2.1 mg) | 16.5 ± 2.9 (n = 5: 4.2 ± 0.4 mg) | 9.4 ± 1.7 (n = 5: 2.6 ± 0.3 mg) |
| Head-kidney | 0.4> ± 1.5 (n = 4: 8.8 ± 0.5 mg) | 14.8 ± 4.4 (n = 5: 8.8 ± 0.8 mg) | 17.2 ± 2.6 (n = 5: 17.2 ± 2.7 mg) | |
| Liver | 1.3 ± 0.2 (n = 4: 17.1 ± 2.6 mg) | 22.2 ± 4.4 (n = 4: 14.8 ± 0.6 mg) | 27.5 ± 5.3 (n = 5: 27.6 ± 4.5 mg) | |
| Estradiol-17β | Gonad | 499.1 ± 107.6 (n = 6: 35.3 ± 8.1 mg) | 2.0> ± 0.7 (n = 6: 4.7 ± 0.3 mg) | 0.8> ± 0.2 (n = 6: 2.3 ± 0.3 mg) |
The number of samples and their average weights are indicated in parentheses.
These endocrinological profiles demonstrate that the emergence of male secondary sex characteristics in the XX morphants results from the increased production of 11-ketotestosterone and reduced levels of estradiol-17β.
The Female Steroidogenic Cell Lineage Is Absent in Adult Germ Cell-Deficient Medaka Gonads of both Sexes.
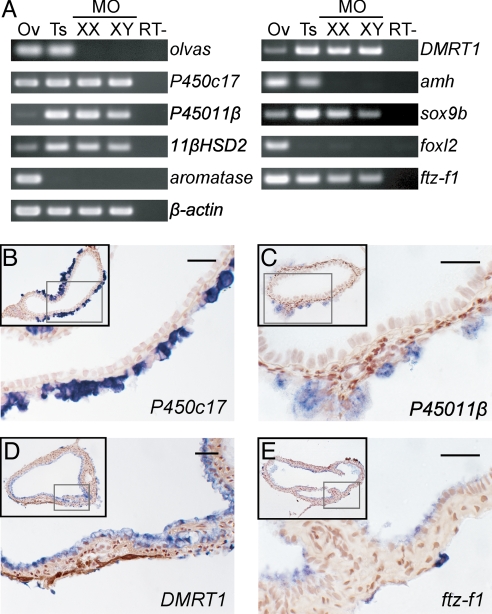
The alteration in the sex steroid profiles of the cxcr4 morphants suggests there is aberrant expression of steroidogenic enzymes in corresponding gonads. To test this possibility, we examined the gene expression patterns of steroidogenic enzymes by both RT-PCR and in situ hybridization in adult germ-cell-deficient medaka gonads. As expected, aromatase was undetectable in both the XX and XY morphant gonads, whereas the P45011β and 11βHSD2 transcripts necessary for the production of 11-ketotestosterone were detectable (Fig. 3A). In addition, P450c17, which is essential for the production of the precursor steroids for estrogen and androgen, was found to be expressed in the germ-cell-deficient gonads of both sexes.
Fig. 3.
Gene expression analysis of germ-cell-deficient gonads in the adult medaka. (A) RT-PCR analysis of the indicated genes in normal ovaries (Ov), normal testes (Ts), and germ-cell-deficient gonads from sex-reversed genetically XX and XY medaka (MO). RT−, control reaction without reverse transcriptase. (B–E) Gene expression patterns of P450c17 (B), P45011β (C), DMRT1 (D), and ftz-f1 (E) analyzed by in situ hybridization. Nuclei are counterstained with neutral red. Insets show the whole section in each case. (Scale bars, 20 μm.)
In the medaka normal testis, P450c17, P45011β, and 11βHSD2 transcripts are expressed in the Leydig cells of the stromal regions (SI Fig. 6 A–C). In situ hybridization analysis showed these transcripts could also be detected in an outer region of the germ-cell-deficient XX morphant gonads (Fig. 3 B and C and data not shown), but there was no detectable expression of aromatase (data not shown), consistent with the earlier RT-PCR data. In the normal ovary, the expression levels of P45011β and 11βHSD2 are so low that these transcripts cannot be detected by in situ hybridization (data not shown). These results are consistent with the fact that the androgen, 11-ketotestosterone, is produced in the germ-cell-deficient gonads of both sexes.
The Supporting Cell Lineage in the Adult Germ Cell-Deficient Medaka Gonad Is Masculine.
Our initial results indicated that steroidogenic cells are masculinized in germ-cell-deficient medaka gonads regardless of the genetic sex. We thus examined whether the gene expression profile of the supporting cell lineages is also affected in these adult morphants. In the normal medaka testis, the male supporting cells, known as Sertoli cells, express DMRT1, amh, and sox9b (Fig. 1, SI Fig. 6 D–F, and data not shown). In the medaka ovary, however, the female supporting cells, the granulosa cells, express amh and foxl2 (27, 41). A weak DMRT1 signal in the wild-type ovary is detectable in small oocytes only by in situ hybridization (data not shown) (42). By RT-PCR analysis, we found that in both the XX and XY cxcr4 morphant gonads, the Sertoli cell markers, DMRT1 and sox9b, were highly expressed, whereas the granulosa cell marker foxl2 was undetectable (Fig. 3A). Interestingly, amh was also found to be undetectable in either the XX or XY morphant gonads (Fig. 3A), suggesting that a reciprocal cross-talk between the germ cells and gonadal somatic cells is required for expression of this gene in both sexes (43). In addition, the medaka orthologue of mammalian Ad4BP/SF1, ftz-f1, which is expressed in the steroidogenic cells and supporting cells of both sexes (SI Fig. 6 G and H), was also detectable in both the wild-type and morphant gonads (Fig. 3A).
By in situ hybridization analysis, we found that DMRT1 is strongly expressed in the innermost cells (Fig. 3D), and that ftz-f1 was detectable in both the innermost cells and in some of the cell populations in the outer stromal region (Fig. 3E). In contrast, the expression of sox9b, amh, and foxl2 was barely detectable by in situ hybridization (data not shown). This expression profile was observed in both the XX and XY morphant gonads, suggesting that, although the supporting cells may not be fully differentiated, their lineages are present and have acquired more masculinized characteristics.
The Somatic Cell Lineages Do Not Develop Properly During the Early Stages of Sex Differentiation in the cxcr4 Morphant Gonads.
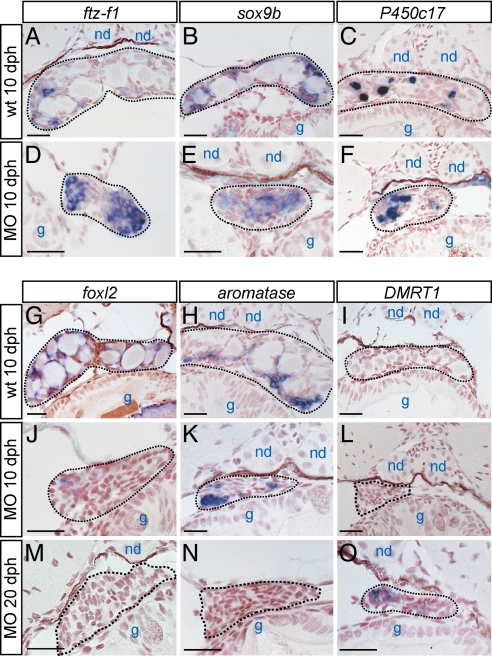
To investigate cell lineage differentiation at the earlier stages of gonadal development (10–20 dph) in germ-cell-deficient medaka, in situ hybridization was performed by using the developing gonads of the XX morphants. In the medaka, ftz-f1 and sox9b are established markers of the gonadal somatic cell precursors in undifferentiated gonads (23). Both ftz-f1 and sox9b were found in our current experiments to be expressed in the germ-cell-deficient gonads of the XX morphants at 10 dph (Fig. 4 D and E), indicating that the gonadal somatic cell precursors can form normally in the absence of germ cells. At a later stage, ftz-f1 expression was observed to be confined mainly to both the steroidogenic and granulosa cells, as reported in mammals (44), whereas sox9b becomes preferentially detectable in the Sertoli cells (32, 33). In normal female gonads at 10 dph, sox9b expression can be observed in the somatic cells surrounding relatively small oocytes, suggesting these sox9b-expressing cells have not fully differentiated into granulosa cells at this stage (Fig. 4B) (25).
Fig. 4.
Gene expression analysis of both wild-type and germ-cell-deficient developing gonads. The XX gonads of 10 dph wild-type (A–C and G–I), 10 dph morphant (D–F and J–L), and 20 dph morphant (M–O) were analyzed by in situ hybridization for the marker genes, which are indicated on the top of columns. Nuclei are counterstained with neutral red. The gonad in each image is enclosed by a black dotted line. Nephric duct (nd), gut (g). (Scale bars, 20 μm.)
In the wild-type XX medaka gonads, foxl2 expression is detectable in the granulosa cells around the oocytes (Fig. 4G), and aromatase is expressed near the ventral regions (Fig. 4H). In germ-cell-deficient XX gonads, however, foxl2 is only weakly expressed in the interstitial region (Fig. 4J) and then diminishes by 20 dph (Fig. 4M). Aromatase is detected in the ventral region of germ-cell-deficient XX gonads at 10 dph (Fig. 4K). However, this expression also diminishes at 20 dph (Fig. 4N). The eventual loss of aromatase and foxl2 expression is thus consistent with the results of our RT-PCR analysis of adult morphant gonads. These results indicate that the germ cells are required for the maintenance of both aromatase and foxl2 expression.
Androgen-producing cells were detectable by monitoring the expression of P450c17 at 10 dph in both wild-type and germ-cell-deficient XX gonads (Fig. 4 C and F). These cells persist into adulthood (Fig. 3 A and B). The Sertoli cell marker, DMRT1, also begins to be expressed at 10–20 dph in the XX morphant gonads (Fig. 4 L and O). The loss of foxl2 expression and concomitant induction of DMRT1 expression in the developing gonads of germ-cell-deficient XX morphants thus suggest that the female supporting cell lineage has been transdifferentiated into a male cell lineage. Alternatively, male supporting cells could newly develop in these tissues during the early stages of gonadal development before the initiation of gonadal morphogenesis.
Discussion
The results of our current study show that germ-cell-deficient XX medaka morphants manifest a female-to-male sex reversal of their secondary sex characteristics, which are induced by androgen and estrogen, respectively. The sex reversal phenotype of our XX morphants is therefore consistent with the increasing amounts of 11-ketotestosterone and reduced levels of estradiol-17β that we measured in their gonads. Female-specific estrogen-secreting cells were impaired in the XX morphant gonads.
In addition to the loss of female-specific steroidogenic cells that we observe in our morphants, we find that the germ-cell-deficient gonads are partly masculinized in terms of their gene expression profile, although the number of genes examined was small. Significantly, this event occurs in the absence of germ cells and thus in a cell-autonomous manner in the soma. In this regard, our present results suggest that the gonadal somatic cells are predisposed to male development in the medaka.
In contrast to mammals, the ovaries in teleost fish contain not only developing follicles but also cyst-forming mitotic and gonia-like germ cells (30, 45, 46). A previously reported sex-reversal experiment in the medaka suggested that the gonia-like germ cells are sexually indifferent, and that the branching point toward either sex occurs either before or somewhere during the process of meiosis (47). This finding raises the possibility that a functional sex-reversal event could arise in fertile XX morphant gonads with a small number of undifferentiated gonia-like germ cells that are under the influence of the gonadal somatic cells with a masculine predisposition. This may also be related to the sex differentiation of zebrafish, known as “juvenile hermaphroditism,” in which all of the individuals initially develop ovaries, but the oocytes are lost in the presumptive male gonads by apoptotic cell death, leading to testicular development (48). In mammals also, the loss of oocytes induces the transdifferentiation of granulosa cells into Sertoli-like cells (15), indicating that normal ovarian development requires the presence of developing oocytes. These phenomena support our contention that somatic cells are predisposed to developing male characteristics, but that the presence of oocytes canalizes them into female development pathways.
To generate germ-cell-deficient medaka, the migration of primordial germ cells was blocked by the inhibition of the cxcr4 gene by morpholino injection (34). The effects of morpholinos generally persist for 3 days at most after injection into fertilized eggs. Given that the onset of gonadal formation initiates at 3–4 dpf in the medaka, and that ovarian cavity formation initiates at ≈30 dph, it is thus highly unlikely that the development of male somatic cells and the tube-like structure is due to the secondary effects of the injected cxcr4-MO.
In support of this contention, we have also generated germ-cell-deficient medaka via a morpholino knockdown of medaka nanos, a gene that is essential for the maintenance of germ cell identity (49). These nanos morphants also exhibited sex reversal of their secondary sex characteristics and a tube-like structure of the gonad (SI Fig. 7). These findings are also consistent with a previous study in zebrafish showing that a germ cell deficiency generated by the inhibition of deadend, a gene involved in primordial germ cell development, causes sex reversal (22).
Ovaries and testes are composed of structurally similar units (fundamental units) that comprise a layer of supporting cells surrounding the germ cell(s), which is separated from the outer stromal region by a basement membrane. This structure is evident in ovarian follicles and testicular tubules in the normal ovary and testis, respectively. In contrast to previous reports (8, 11, 19–21, 50), however, we show in our current study that medaka gonads with impaired cell lineages resulting from the forced absence of germ cells develop a single tube-like structure comprising this fundamental unit in terms of both histology and gene expression. Moreover, we find that this occurs irrespective of the genetic sex. Our findings thus indicate that gonadal somatic cells can form this fundamental unit without the function of the germ cells, whereas germ cells play an essential role in the generation of sexual dimorphism, possibly by organizing this unit in a sex-specific manner.
In conclusion, we have herein characterized some of the principal cellular mechanisms underlying sex reversal in the medaka model organism and have revealed the importance of germ cells in the process of sex differentiation in the gonads of this species. These findings thus provide insights into the properties of gonadal somatic cells and germ cells in vertebrates.
Materials and Methods
PCR conditions and primer sets are published as SI Tables 2–4.
Generation of Germ-Cell-Deficient Medaka.
The inbred medaka strain, cab, was used in these experiments. The microinjection and generation of medaka with fluorescent germ cells, and cxcr4 and nanos morphants, have been described (34). The absence of fluorescent germ cells in the gonads was confirmed between stages 35 and 38 by fluorescence microstereoscopy. Forty-four percent of the cxcr4-MO embryos and 50% of the nanos-MO embryos were identified as germ-cell-deficient. Germ cell deficiency was also confirmed by immunostaining. The discrimination of secondary sex characteristics has been described in ref. 51.
Measurement of Steroid Hormones.
Steroid hormones were extracted in a solution containing chloroform and methanol (2:1) and then assayed by using an 11-ketotestosterone or estradiol EIA Kit (Cayman Chemical, Ann Arbor, MI).
In Situ Hybridization and Histology.
Whole-mount in situ hybridization was performed as reported (23), after which the samples are carefully selected to confirm the complete absence of germ cells. Briefly, the samples showing gene expression signals were sectioned entirely from one end to the other and subjected to immunostaining with anti-medaka OLVAS (vasa homologue) antibodies. This procedure determined whether the samples contained germ cells, and only those lacking germ cells were selected for further histological analyses.
Electron Microscopy.
Gonad specimens were fixed overnight at 4°C in 2% glutaraldehyde, followed by 2% OsO4 in cacodylate buffer. The tissue was then embedded in EPON812 (TAAB). Ultrathin sections were stained with a saturated solution of uranyl acetate. Imaging was performed with a JEM-1200EX electron microscope (JEOL, Tokyo, Japan).
Supplementary Material
Acknowledgments
We thank Drs. K. Naruse (National Institute for Basic Biology), A. Kanamori (Nagoya University, Nagoya, Japan), and M. Matsuda (Utsunomiya University, Utsunomiya City, Japan) for providing cDNAs and ESTs and C. Morinaga (Solution Oriented Research for Science and Technology, Kondoh Research Team, Japan Science and Technology Agency, Kyoto, Japan) for technical advice on genotyping. We are also grateful to Ms. Ichikawa for fish maintenance. This work was supported in part by Grants-in-Aid for Scientific Research (17052027, 17370083, and 19040027, to M.T.; 18570064, to D.S.).
Abbreviations
- P450c17
cytochrome P450 hydroxylase and c17 lyase
- 11β-HSD
11β-hydroxysteroid dehydrogenase
- dph
days posthatching
- dpf
days postfertilization.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database [accession nos. EF025509 (P45011β) and DQ991146 (11βHD2)].
This article contains supporting information online at www.pnas.org/cgi/content/full/0609932104/DC1.
References
- 1.Koopman P, Munsterberg A, Capel B, Vivian N, Lovell-Badge R. Nature. 1990;348:450–452. doi: 10.1038/348450a0. [DOI] [PubMed] [Google Scholar]
- 2.Sinclair AH, Berta P, Palmer MS, Hawkins JR, Griffiths BL, Smith MJ, Foster JW, Frischauf AM, Lovell-Badge R, Goodfellow PN. Nature. 1990;346:240–244. doi: 10.1038/346240a0. [DOI] [PubMed] [Google Scholar]
- 3.Koopman P, Gubbay J, Vivian N, Goodfellow P, Lovell-Badge R. Nature. 1991;351:117–121. doi: 10.1038/351117a0. [DOI] [PubMed] [Google Scholar]
- 4.Matsuda M, Nagahama Y, Shinomiya A, Sato T, Matsuda C, Kobayashi T, Morrey CE, Shibata N, Asakawa S, Shimizu N, et al. Nature. 2002;417:559–563. doi: 10.1038/nature751. [DOI] [PubMed] [Google Scholar]
- 5.Nanda I, Kondo M, Hornung U, Asakawa S, Winkler C, Shimizu A, Shan Z, Haaf T, Shimizu N, Shima A, et al. Proc Natl Acad Sci USA. 2002;99:11778–11783. doi: 10.1073/pnas.182314699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brennan J, Capel B. Nat Rev Genet. 2004;5:509–521. doi: 10.1038/nrg1381. [DOI] [PubMed] [Google Scholar]
- 7.Yao HH. Mol Cell Endocrinol. 2005;230:87–93. doi: 10.1016/j.mce.2004.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McLaren A. BioEssays. 1991;13:151–156. doi: 10.1002/bies.950130402. [DOI] [PubMed] [Google Scholar]
- 9.McLaren A. Mol Cell Endocrinol. 2000;163:3–9. doi: 10.1016/s0303-7207(99)00234-8. [DOI] [PubMed] [Google Scholar]
- 10.Guigon CJ, Magre S. Biol Reprod. 2006;74:450–458. doi: 10.1095/biolreprod.105.047134. [DOI] [PubMed] [Google Scholar]
- 11.Merchant H. Dev Biol. 1975;44:1–21. doi: 10.1016/0012-1606(75)90372-3. [DOI] [PubMed] [Google Scholar]
- 12.Merchant-Larios H, Centeno B. Prog Clin Biol Res. 1981;59B:383–392. [PubMed] [Google Scholar]
- 13.Couse JF, Hewitt SC, Bunch DO, Sar M, Walker VR, Davis BJ, Korach KS. Science. 1999;286:2328–2331. doi: 10.1126/science.286.5448.2328. [DOI] [PubMed] [Google Scholar]
- 14.Vainio S, Heikkilä M, Kispert A, Chin N, McMahon AP. Nature. 1999;397:405–409. doi: 10.1038/17068. [DOI] [PubMed] [Google Scholar]
- 15.Guigon CJ, Coudouel N, Mazaud-Guittot S, Forest MG, Magre S. Endocrinology. 2005;146:2992–3004. doi: 10.1210/en.2005-0045. [DOI] [PubMed] [Google Scholar]
- 16.Dupont S, Dennefeld C, Krust A, Chambon P, Mark M. Dev Dyn. 2003;226:103–106. doi: 10.1002/dvdy.10202. [DOI] [PubMed] [Google Scholar]
- 17.Jeays-Ward K, Dandonneau M, Swain A. Dev Biol. 2004;276:431–440. doi: 10.1016/j.ydbio.2004.08.049. [DOI] [PubMed] [Google Scholar]
- 18.Heikkilä M, Prunskaite R, Naillat F, Itaranta P, Vuoristo J, Leppaluoto J, Peltoketo H, Vainio S. Endocrinology. 2005;146:4016–4023. doi: 10.1210/en.2005-0463. [DOI] [PubMed] [Google Scholar]
- 19.Handel MA, Eppig JJ. Biol Reprod. 1979;20:1031–1038. doi: 10.1095/biolreprod20.5.1031. [DOI] [PubMed] [Google Scholar]
- 20.McCoshen JA. Am J Obstet Gynecol. 1982;142:83–88. doi: 10.1016/s0002-9378(16)32288-8. [DOI] [PubMed] [Google Scholar]
- 21.Pellas TC, Ramachandran B, Duncan M, Pan SS, Marone M, Chada K. Proc Natl Acad Sci USA. 1991;88:8787–8791. doi: 10.1073/pnas.88.19.8787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Slanchev K, Stebler J, de la Cueva-Mendez G, Raz E. Proc Natl Acad Sci USA. 2005;102:4074–4079. doi: 10.1073/pnas.0407475102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakamura S, Kobayashi D, Aoki Y, Yokoi H, Ebe Y, Wittbrodt J, Tanaka M. Dev Biol. 2006;295:678–688. doi: 10.1016/j.ydbio.2006.03.052. [DOI] [PubMed] [Google Scholar]
- 24.Matsuda M. Annu Rev Genet. 2005;39:293–307. doi: 10.1146/annurev.genet.39.110304.095800. [DOI] [PubMed] [Google Scholar]
- 25.Nakamura S, Aoki Y, Saito D, Kuroki Y, Fujiyama A, Naruse K, Tanaka M. Mol Reprod Dev. 2007 doi: 10.1002/mrd.2076. [DOI] [PubMed] [Google Scholar]
- 26.Suzuki A, Tanaka M, Shibata N, Nagahama Y. J Exp Zoolog A Comp Exp Biol. 2004;301:266–273. doi: 10.1002/jez.a.20027. [DOI] [PubMed] [Google Scholar]
- 27.Nakamoto M, Matsuda M, Wang DS, Nagahama Y, Shibata N. Biochem Biophys Res Commun. 2006;344:353–361. doi: 10.1016/j.bbrc.2006.03.137. [DOI] [PubMed] [Google Scholar]
- 28.Satoh N, Egami N. J Embryol Exp Morphol. 1972;28:385–395. [PubMed] [Google Scholar]
- 29.Kobayashi T, Matsuda M, Kajiura-Kobayashi H, Suzuki A, Saito N, Nakamoto M, Shibata N, Nagahama Y. Dev Dyn. 2004;231:518–526. doi: 10.1002/dvdy.20158. [DOI] [PubMed] [Google Scholar]
- 30.Saito D, Morinaga C, Aoki Y, Nakamura S, Mitani H, Furutani-Seiki M, Kondoh H, Tanaka M. Dev Biol. 2007;310:280–290. doi: 10.1016/j.ydbio.2007.07.039. [DOI] [PubMed] [Google Scholar]
- 31.Kanamori A, Nagahama Y, Egami N. Zool Sci. 1985;2:695–706. [Google Scholar]
- 32.Klüver N, Kondo M, Herpin A, Mitani H, Schartl M. Dev Genes Evol. 2005;215:297–305. doi: 10.1007/s00427-005-0477-x. [DOI] [PubMed] [Google Scholar]
- 33.Nakamoto M, Suzuki A, Matsuda M, Nagahama Y, Shibata N. Biochem Biophys Res Commun. 2005;333:729–736. doi: 10.1016/j.bbrc.2005.05.158. [DOI] [PubMed] [Google Scholar]
- 34.Kurokawa H, Aoki Y, Nakamura S, Ebe Y, Kobayashi D, Tanaka M. Dev Growth Differ. 2006;48:209–221. doi: 10.1111/j.1440-169X.2006.00858.x. [DOI] [PubMed] [Google Scholar]
- 35.Wischnitzer S. J Morphol. 1965;117:387–399. doi: 10.1002/jmor.1051170305. [DOI] [PubMed] [Google Scholar]
- 36.Gore-Langton RE, Armstrong DT. In: The Physiology of Reproduction. Knobil E, Neil D, editors. New York: Raven; 1994. pp. 571–627. [Google Scholar]
- 37.Nagahama Y. Int J Dev Biol. 1994;38:217–229. [PubMed] [Google Scholar]
- 38.Zeleznik AJ. Leung PCK, Adashi EY. The Ovary. London: Academic; 2004. pp. 45–53. [Google Scholar]
- 39.Ojeda SR, Skinner MK. Neil JD. Physiology of Reproduction. New York: Academic; 2006. pp. 2061–2126. [Google Scholar]
- 40.Hishida T, Kawamoto N. J Exp Zool. 1970;173:279–283. [Google Scholar]
- 41.Klüver N, Pfennig F, Pala I, Storch K, Schlieder M, Froschauer A, Gutzeit HO, Schartl M. Dev Dyn. 2007;236:271–281. doi: 10.1002/dvdy.20997. [DOI] [PubMed] [Google Scholar]
- 42.Winkler C, Hornung U, Kondo M, Neuner C, Duschl J, Shima A, Schartl M. Mech Dev. 2004;121:997–1005. doi: 10.1016/j.mod.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 43.Morinaga C, Saito D, Nakamura S, Sasaki T, Asakawa S, Shimizu N, Mitani H, Furutani-Seiki M, Tanaka M, Kondoh H. Proc Natl Acad Sci USA. 2007;104:9691–9696. doi: 10.1073/pnas.0611379104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morohashi K. Genes Cells. 1997;2:95–106. doi: 10.1046/j.1365-2443.1997.1060304.x. [DOI] [PubMed] [Google Scholar]
- 45.Begovac PC, Wallace RA. J Morphol. 1987;193:117–133. doi: 10.1002/jmor.1051930202. [DOI] [PubMed] [Google Scholar]
- 46.Grier H. J Morphol. 2000;243:265–281. doi: 10.1002/(SICI)1097-4687(200003)243:3<265::AID-JMOR4>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 47.Shibata N, Hamaguchi S. J Exp Zool. 1988;245:71–77. doi: 10.1002/jez.1402450111. [DOI] [PubMed] [Google Scholar]
- 48.Uchida D, Yamashita M, Kitano T, Iguchi T. J Exp Biol. 2002;205:711–718. doi: 10.1242/jeb.205.6.711. [DOI] [PubMed] [Google Scholar]
- 49.Koprunner M, Thisse C, Thisse B, Raz E. Genes Dev. 2001;15:2877–2885. doi: 10.1101/gad.212401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shinomiya A, Hamaguchi S, Shibata N. J Exp Zool. 2001;290:402–410. doi: 10.1002/jez.1081. [DOI] [PubMed] [Google Scholar]
- 51.Yamamoto T. J Exp Zool. 1958;137:227–263. doi: 10.1002/jez.1401370203. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.