Abstract
A tradeoff between growth and reproduction, often inferred from an inverse correlation between these two variables, is a fundamental paradigm of life-history evolution. Oak species provide a unique test of this relationship because different species mature acorns either in the year of pollination or in the year after pollination. This difference allows for an interspecific comparison testing whether the apparent tradeoff is causal or the result of confounding factors influencing growth and reproduction independently. Based on 13 years of data on five California oak species, we found significant negative correlations between radial growth and seed production in the three species that produce acorns the same year in which pollination occurs, but not in two species that mature acorns the year after pollination. Rainfall, which correlates positively with radial growth and correlates negatively with acorn production (based on the year of pollination), appears to be driving this pattern. We conclude that the observed negative correlations are not causal, but rather a consequence of growth and reproduction being dependent, in opposite ways, on environmental conditions. Thus, contrary to the current consensus, growth and reproduction in these species are apparently largely independent of each other. In contrast, tradeoffs between current and future reproduction appear to be much more important in the life-history evolution of these long-lived plants. We also conclude that a negative correlation does not necessarily imply a causal mechanism and should not be used as the only evidence supporting a tradeoff.
Keywords: allocation, cost of reproduction, life-history evolution, reproductive effort, masting
Many long-lived plant species exhibit strong temporal variation among years in seed production. This phenomenon, known as masting or mast fruiting (1), is often correlated with decreased radial trunk growth in years of high seed production (2–4). The standard tradeoffs hypothesis for this relationship is that a resource is limiting and is allocated either to reproduction or growth (5), leading to a fundamental tradeoff between current growth and reproduction (2, 5, 6).
A key assumption underlying the tradeoffs hypothesis is that reproduction is costly and competes with growth for resources. However, allocation to reproduction may be relatively small (2), reproduction may be constrained by pollination (7), reproductive structures may supply a large part of the resources required (8), photosynthetic rates may increase if the demand for carbon increases (9, 10), and predator satiation might be driving annual variability in reproduction (11). Any of these factors might make the cost of reproduction relatively small or driven by factors other than direct resource competition with growth. This problem is particularly vexing in trees where long life spans make manipulations difficult.
The evidence for tradeoffs between growth and reproduction in plants is mixed (2, 3, 5, 12–15), and tradeoffs often have been inferred from correlational studies (4, 12, 15–17). However, correlational studies can be misleading because of confounding factors (13, 14, 18), and negative correlations between growth and reproduction may not reflect a causal tradeoff if both growth and reproduction are independently influenced by the same environmental variables (the weather hypothesis). In most species, however, investment in reproduction and growth occur simultaneously, making it impossible to determine whether a negative correlation between these variables reflects a causal tradeoff or is incidental to confounding environmental factors.
Oaks provide a unique way to test these alternatives because they occur in 1-year species (in which each cohort of seeds matures the same year they are pollinated) and 2-year species (in which maturation, and thus the majority of a cohort's reproductive investment, occurs the year after pollination). In both types, warm, dry weather during the winter correlates with similar conditions during the spring pollination period, conditions that facilitate pollination and ultimately result in larger acorn crops (1, 19). Reproductive structures of oaks exhibit large annual variability. Investment in reproductive structures ranges from 2–27% of total above-ground productivity, and nitrogen and phosphorus allocation to reproduction closely matches biomass allocation (based on litterfall collected from 1992–1996 and estimates of trunk increment) (J.M.H.P. and W.D.K., unpublished data). Reproductive structures of oaks have a low photosynthetic rate and do not contribute much energy to developing seeds, as can be the case for other tree species (8). Thus, seed production in oaks is costly, and the majority of reproductive investment occurs in the year of acorn maturation, which differs among species.
In California, 1- and 2-year oak species are frequently sympatric. Thus, within the same community, 1-year species invest energy in growth and reproduction simultaneously with the environmental conditions affecting both of these variables. In contrast, in the 2-year species, seed maturation and the majority of reproductive investment in a cohort are delayed, taking place the year after the environmental factors influencing pollination. As a result, the tradeoffs and weather hypotheses predict mutually exclusive interspecific patterns. Under the tradeoffs hypothesis, investment in growth should always be negatively correlated with reproductive investment the same year, regardless of when pollination takes place, and thus should not differ between 1- and 2-year species. In contrast, the weather hypothesis predicts that correlated weather variables influence growth and reproduction in the same year for 1-year species, but should be lagged by 1 year in 2-year species, where environmental conditions affecting growth in year x do not affect reproduction until year x + 1.
Results and Discussion
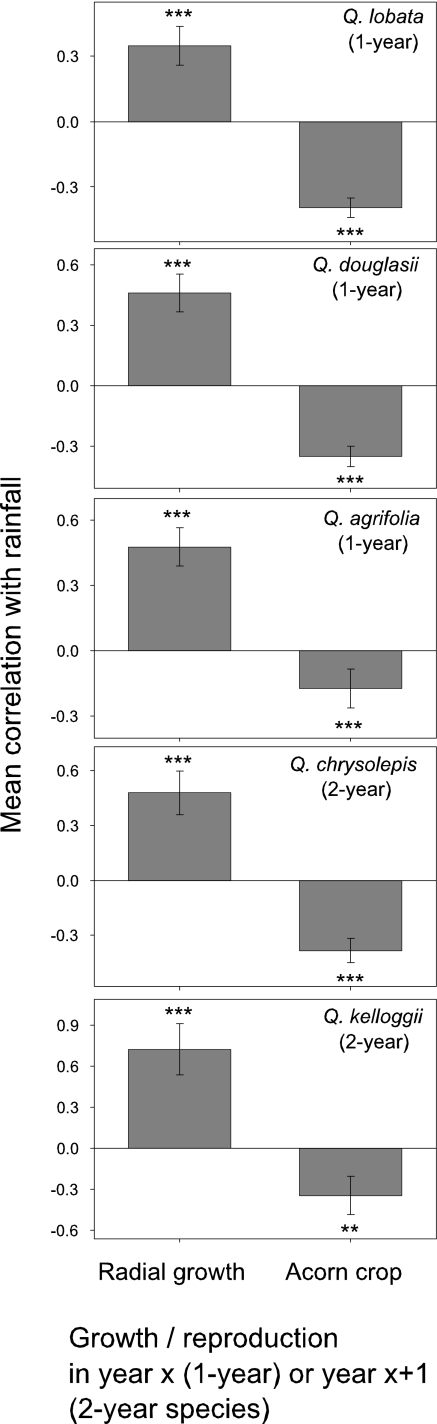
As expected in the water-limited Mediterranean climate of the study area, higher rainfall results in greater water availability to the trees (20) and increased radial growth in all five species (Fig. 1). Conversely, all species exhibited significant negative correlations between rainfall in year x and the seed crop from acorns pollinated in year x, which is consistent with the hypothesis that seed production in these wind-pollinated species is in part controlled by environmental conditions during pollination (Fig. 1) (1, 7, 21) most likely by wet conditions limiting pollen flow and fertilization. Thus, for all five species, high rainfall significantly enhances radial growth, but depresses production of seeds pollinated the same year.
Fig. 1.
Annual rainfall versus growth and reproduction in five California oak species. Plotted are mean r values ± 95% C.I. of annual rainfall versus radial growth (Left) and for annual rainfall versus the acorn crop (Right) for individual trees of five oak species, the first three 1-year species requiring 1 year to mature acorns and the last two 2-year species requiring 2 years to mature acorns. For all species, annual rainfall and radial growth are measured in the same year. For the 1-year species, correlations of rainfall versus the acorn crop also are for the same year. However, for the 2-year species, annual rainfall in year x was correlated with the acorn crop the following year (x + 1). Data are from the 13 years between 1994 and 2006. Sample sizes (number of trees) are given in the text. Statistical tests are based on sign tests (35) using the number of individual trees, for which r values were positive versus negative. **, P < 0.01; ***, P < 0.001.
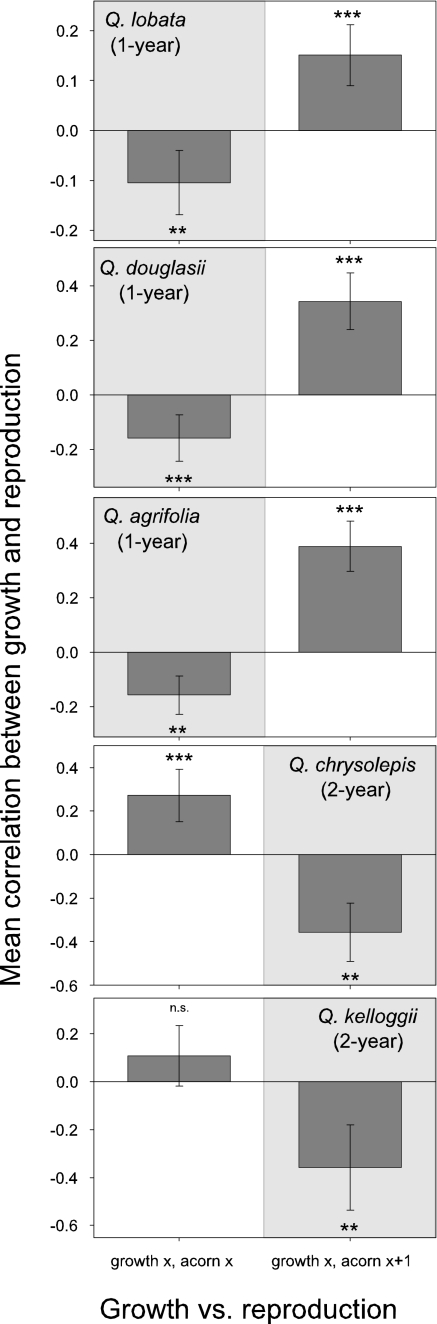
Given this relationship, both hypotheses predict that, for the 1-year species, there should be negative correlations between growth and reproduction in year x, as is indeed the case (Fig. 2). For the two 2-year species, however, correlations between growth and reproduction in year x were positive (significantly so for Quercus chrysolepis), whereas correlations between growth in year x and reproduction the following year (x + 1) were significantly negative (both P < 0.01). This pattern is exactly as expected for the weather hypothesis, but does not support the tradeoff prediction.
Fig. 2.
Correlations between radial growth and acorn production in five California oak species. Plotted are mean r values ± 95% C.I. between annual radial increment of individual trees in year x and their acorn crops as estimated from visual surveys during the same year (x, Left) and the following year (x + 1, Right). Species, sample sizes, and statistics are as in Fig. 1. **, P < 0.01; ***, P < 0.001; not significant, P > 0.05.
The weather hypothesis also predicts that the observed correlations between growth and reproduction, suggestive of tradeoffs, should largely disappear when controlling for the relevant environmental factors. In support of this prediction, partial correlations between radial growth and acorn production the same year (controlling for annual rainfall) were not statistically significant for any of the three 1-year species (Table 1). Partial correlations between growth and reproduction the same year for the 2-year species remained nonsignificant for Q. kelloggii and significantly positive (the opposite of the pattern predicted by the tradeoffs hypothesis) for Q. chrysolepis.
Table 1.
Partial correlations between growth (annual radial increment) and reproduction controlling for rainfall
| Species | Type | Number of trees where partial correlation is |
P value (sign test) | Mean r value | 95% C.I. | |
|---|---|---|---|---|---|---|
| Positive | Negative | |||||
| Q. lobata | 1-year | 47 | 35 | 0.224 | 0.048 | 0.089 |
| Q. douglasii | 1-year | 29 | 25 | 0.683 | 0.007 | 0.103 |
| Q. agrifolia | 1-year | 26 | 33 | 0.435 | −0.107 | 0.089 |
| Q. chrysolepis | 2-year | 16 | 5 | 0.027 | 0.159 | 0.112 |
| Q. kelloggii | 2-year | 10 | 8 | 0.815 | 0.078 | 0.152 |
The lack of negative correlations between growth and reproduction the same year in the 2-year species, combined with the nonsignificant partial correlations between growth and reproduction the same year (controlling for rainfall) in the 1-year species, unambiguously rejects the tradeoffs hypothesis. Instead, growth and reproduction are largely or entirely independent of each other, and the inverse correlations suggestive of tradeoffs found in the 1-year species are apparently spurious, determined by correlated environmental factors, rather than being causal. Thus, contrary to most previous conclusions of long-lived trees (15, 22), a growth/reproduction tradeoff is not a defining life-history pattern in these oak species.
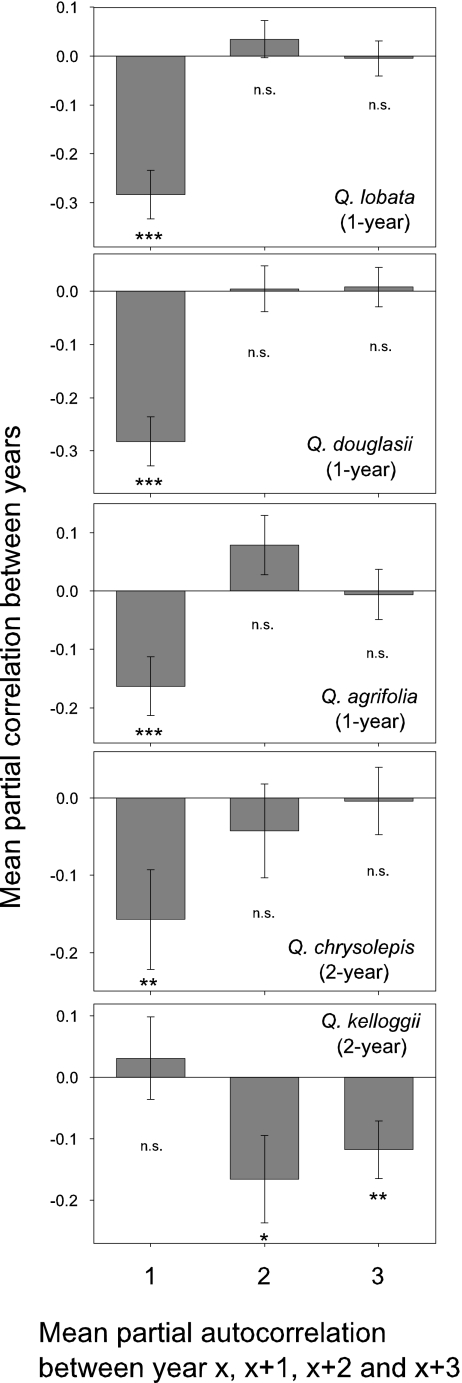
This conclusion does not imply that resources are unlimited or that investment in reproduction does not involve tradeoffs. All five oak species exhibit highly significant negative autocorrelations between acorn crop sizes at 1-year (four species) or 2- and 3-year time lags (one species) (Fig. 3). This finding suggests that the major tradeoff in resource allocation is between current and future reproduction, rather than between growth and reproduction. In addition, reproduction may be strongly limited by pollination in many years, and tradeoffs between growth and reproduction might only occur in years when conditions are favorable for pollination success. However, most years favorable for pollination also are low in total rainfall, thus limiting vegetative growth. Future studies can potentially address this notion by examining years of high-acorn crops that differ in the timing of annual rainfall, which require more years of data.
Fig. 3.
Autocorrelations between years in reproduction in five California oak species. Graphs are the mean partial autocorrelations ± 95% C.I. of the log-transformed acorn crop at lags of 1–3 years for each of the five oak species. Data are based on visual acorn surveys between 1980 and 2006 (27 years). Species, sample sizes, and statistics are as in Fig. 1. *, P < 0.05; **, P < 0.01; ***, P < 0.001; not significant, P > 0.05.
Previous theoretical work has shown that tradeoffs between key life-history variables may be weak or nonexistent if resource acquisition is more variable than allocation (23, 24), if resource allocation occurs at an earlier hierarchical level (25–28), if genetic tradeoffs are not constant and change in different environments (29, 30), or if bet hedging is the dominant life-history strategy in the population (6). Nonetheless, a lack of evidence for growth/reproduction correlations within a population is frequently attributed to lack of power, superabundant resources, confounding annual effects, differences in individual quality (5), or failure to look at the underlying physiological mechanisms (31). This study demonstrates that even a clear negative correlation between growth and reproduction is not necessarily causal. The results also demonstrate that growth/reproduction tradeoffs are not universal at least in long-lived perennial plants such as the oaks studied here. Alternative life-history frameworks not predicated on such tradeoffs clearly warrant increased attention.
Methods
Site Description.
The study was conducted at Hastings Natural History Reservation, which is 20 km east of the Pacific Ocean in central coastal California. The climate is Mediterranean, with warm, dry summers and mild, wet winters. Annual rainfall (from September 1 to August 31) measured at reservation headquarters varied from 328 to 1,035 mm during the years of this study (1994–2006). Vegetation consists of annual grassland, oak savanna, mixed evergreen woodland, chaparral, and riparian forests (32). Five oak species commonly occur at the site, including three 1-year species (Q. lobata, Q. douglasii, and Q. agrifolia) and two 2-year species (Q. chrysolepis and Q. kelloggii). Individual trees sampled were originally selected in 1980 for a long-term study of acorn productivity.
Growth and Reproduction.
Each year between 1980 and 2006 (27 years), we estimated annual seed production in mid-September at the height of the acorn crop (33). Radial increment using dendrometers (34) fitted around the main trunk in 1993 was measured from 1994 to 2006 (13 years) at the time of the acorn survey. Measurements were made on 239 mature individuals, including 84 Q. lobata, 55 Q. douglasii, 61 Q. agrifolia, 21 Q. chrysolepis, and 18 Q. kelloggii. Visual surveys consisted of two observers each counting as many acorns as possible on each tree for 15 sec; the two counts were then summed and log-transformed [log (n + 1)] for analysis (19, 22, 33).
Testing for Tradeoffs.
Analyses involved calculating Pearson correlations by using the relevant variables for each tree over all available years. Calculations were performed on Z-transformed r values (35). Plotted are back-transformed means ± 95% confidence intervals (C.I.) (1.96 ± SE) of these correlations across individuals within species. Statistical tests are based on two-tailed sign tests using the number of individuals for which r values were >0 versus <0. In all but a few cases, P values matched those estimated by using the mean ± 95% C.I. plotted in the figures. We used annual rainfall calculated from September 1 the prior year through August 31 because rainfall occurs mainly in the winter in this Mediterranean climate. We also used the annual rainfall as a proxy for spring conditions important for pollination (36) because annual rainfall is strongly negatively correlated with the maximum April temperature (r = −0.42, P < 0.001) and positively correlated with April rainfall (r = 0.53, P > 0.001).
Acknowledgments
We thank L. Harshman, D. Pilson, K. Potter, T. Rand, B. Shaffer, and T. Zera for comments and discussion. This work was supported by the University of California's Integrated Hardwoods Range Management Program and the National Science Foundation.
Abbreviation
- C.I.
confidence interval.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Sork VL, Bramble J, Sexton O. Ecology. 1993;74:528–541. [Google Scholar]
- 2.Harper JL. Population Biology of Plants. London: Academic; 1977. [Google Scholar]
- 3.Woodward A, Silsee DG, Schreiner EG, Means JE. Can J Forest Res. 1994;24:1133–1143. [Google Scholar]
- 4.Koenig WD, Knops JMH. Am Nat. 2000;155:59–69. doi: 10.1086/303302. [DOI] [PubMed] [Google Scholar]
- 5.Obeso JR. New Phytol. 2002;155:321–348. doi: 10.1046/j.1469-8137.2002.00477.x. [DOI] [PubMed] [Google Scholar]
- 6.Stearns SC. The Evolution of Life Histories. Oxford: Oxford Univ Press; 1992. [Google Scholar]
- 7.Koenig WD, Ashley MV. Trends Ecol Evol. 2003;118:157–159. [Google Scholar]
- 8.Bazzaz FA, Carlson RW, Harper JL. Nature. 1979;279:554–555. [Google Scholar]
- 9.Lambers H, Chapin FS, III, Pons TL. Plant Physiological Ecology. New York: Springer; 1998. [Google Scholar]
- 10.Gehring JL, Delph LF. Int J Plant Sci. 2006;167:843–854. [Google Scholar]
- 11.Janzen DH. Am Nat. 1970;104:501–527. [Google Scholar]
- 12.Koenig W, Knops J. Nature. 1998;396:225–226. [Google Scholar]
- 13.Fox JF, Stevens GC. Ecology. 1991;72:1013–1023. [Google Scholar]
- 14.Horvitz CC, Schemske DW. Ecology. 1988;69:1741–1745. [Google Scholar]
- 15.Silvertown J, Dodd M. Am Nat. 1999;29:321–332. doi: 10.1086/303238. [DOI] [PubMed] [Google Scholar]
- 16.Reznick D. Oikos. 1985;44:257–267. [Google Scholar]
- 17.Montesinos D, De Luis M, Verdu M, Raventos J, Grarcia-Fayos P. Ann Bot (London) 2006;98:885–889. doi: 10.1093/aob/mcl172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Despland E, Houle G. Am J Bot. 1997;84:928–937. [PubMed] [Google Scholar]
- 19.Koenig WD, Mumme RL, Carmen WJ, Stanback MT. Ecology. 1994;75:99–109. [Google Scholar]
- 20.Knops JMH, Koenig WD. Madrono. 2000;47:106–108. [Google Scholar]
- 21.Knapp EE, Goedde MA, Rice KJ. Oecologia. 2001;128:48–55. doi: 10.1007/s004420000623. [DOI] [PubMed] [Google Scholar]
- 22.Koenig WD, Knops JMH. Am Sci. 2005;93:340–347. [Google Scholar]
- 23.van Noordwijk AJ, de Jong G. Am Nat. 1986;128:137–142. [Google Scholar]
- 24.Reznick D, Nunney L, Tessier A. Trends Ecol Evol. 2000;15:421–425. doi: 10.1016/s0169-5347(00)01941-8. [DOI] [PubMed] [Google Scholar]
- 25.Obeso JR. J Ecol. 1997;85:159–166. [Google Scholar]
- 26.Worley AC, Houle D, Barrett SCH. Am Nat. 2003;161:153–167. doi: 10.1086/345461. [DOI] [PubMed] [Google Scholar]
- 27.de Jong G. Funct Ecol. 1993;7:75–83. [Google Scholar]
- 28.Koelewijn HP, Hunsheid MPH. J Evol Biol. 2000;13:302–315. [Google Scholar]
- 29.Roff DA, Mostowy S, Fairbairn DJ. Evolution (Lawrence, Kans) 2002;56:84–95. doi: 10.1111/j.0014-3820.2002.tb00851.x. [DOI] [PubMed] [Google Scholar]
- 30.Sgro CM, Hoffmann AA. Heredity. 2004;93:241–248. doi: 10.1038/sj.hdy.6800532. [DOI] [PubMed] [Google Scholar]
- 31.Zera AJ, Harshman LG. Annu Rev Ecol Syst. 2001;32:95–126. [Google Scholar]
- 32.Knops JMH, Griffin JR, Royalty AC. Biol Conserv. 1995;71:115–123. [Google Scholar]
- 33.Koenig WD, Knops JMH, Carmen WJ, Stanback MT, Mumme RL. Can J For Res. 1994;24:2105–2112. [Google Scholar]
- 34.Cattelino PJ, Becker CA, Fuller LG. Nor J Appl For. 1986;3:73–75. [Google Scholar]
- 35.Sokal RR, Rohlf FJ. Biometry. New York: Freeman; 1995. [Google Scholar]
- 36.Koenig WD, Knops JMH, Carmen WJ, Stanback MT, Mumme RL. Can J For Res. 1996;26:1677–1683. [Google Scholar]