Abstract
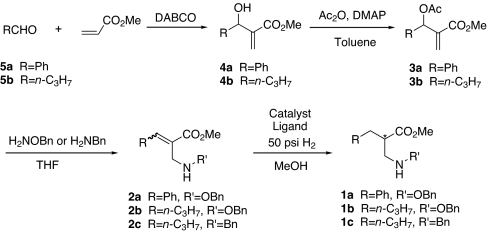
We describe highly enantioselective synthesis of β-amino acid derivatives (1a-c) using asymmetric hydrogenation of α-aminomethylacrylates (2a-c), which contain a free basic N H group, as the key step. The α-aminomethylacrylates (2a-c) were prepared using the Baylis–Hillman reaction of an appropriate aldehyde with methyl acrylate followed by acetylation of the resulting allylic alcohols (4a-b) and SN2′-type amination of the allylic acetates (3a-b).
H group, as the key step. The α-aminomethylacrylates (2a-c) were prepared using the Baylis–Hillman reaction of an appropriate aldehyde with methyl acrylate followed by acetylation of the resulting allylic alcohols (4a-b) and SN2′-type amination of the allylic acetates (3a-b).
Keywords: asymmetric catalysis, Baylis–Hillman reaction
In recent years, β-amino acids have received increasing attention as constituents of molecules with interesting biological and pharmacological activities (1–5) such as hypoglycemic and ketogenic activities. They are key moieties of a number of bioactive molecules, such as in taxol and in peptidic natural products with various enzyme inhibiting activities. Nonpeptidic β-amino acids are found in well known β-lactams. Considering their importance, asymmetric synthesis of enantiomerically pure β-amino acids has become an important challenge for organic chemists. The synthesis of enantiopure β-amino acids has been extensively studied (6–9). However, the known methods are mostly for the synthesis of β-substituted β-amino acids, and their preparation still suffers from a long synthetic sequence, low product yields, and laborious execution (10–14). For example, a recently reported synthesis of 1a involved nine steps from 3-phenylpropanoic acid (15–18). Peptide deformylase (PDF, EC 3.5.1.31), a metallopeptidase found in prokaryotic organisms, is essentially required for bacterial growth (19–21). Certain N-formyl hydroxylamine compounds were recently revealed to have good antibacterial function by means of their PDF-inhibiting capabilities. Chiral compounds 1, α-substituted β-amino acid derivatives, are key intermediates in the synthesis of this kind of compounds (15–18, 22). Their prochiral dehydro-precursors 2 could be prepared in high yields via a synthetic process shown in Scheme 1. Asymmetric hydrogenation of these substrates 2 is the simplest and most direct route to synthesize 1 because of its inherent efficiency and atom economy. In contrast to the great progress in the synthesis of β-substituted β-amino acids and derivatives via enantioselective hydrogenations (23–38), reports on the synthesis of α-substituted β-amino acids with this protocol are very limited. To the best of our knowledge, only one exceptional example has been given, very recently by Zheng and coworkers (38), using Rh-monophosphorus catalyst system for the hydrogenation of β-phthalimide acrylates. However, the activity of the catalyst was not high, and only E-isomers of substituted β-phthalimide acrylates were investigated. In fact, compounds 2 were a mixture of E- and Z-isomers formed in the synthesis, and they were not always easy to separate into single isomers. Generally, it is also difficult to achieve high activity and enantioselectivity for the system containing both isomers (23–33). In light of the successful development and preparation of α-aminomethyl acrylates 2, herein we report a highly enantioselective synthesis of β-amino acid derivatives by Rh-catalyzed asymmetric hydrogenation of α-aminomethylacrylates (2a-c), which contain a free basic N H group, as the key step. To the best of our knowledge, such an asymmetric hydrogenation of α-aminomethyl acrylates to N-unprotected amino acid derivatives has not been reported in the literature.
H group, as the key step. To the best of our knowledge, such an asymmetric hydrogenation of α-aminomethyl acrylates to N-unprotected amino acid derivatives has not been reported in the literature.
Scheme 1.
Synthesis of α-aminomethylacrylates and the asymmetric hydrogenation process.
Results and Discussion
Synthesis of α-Aminomethylacrylates 2a-c.
The success of our asymmetric hydrogenation approach depended on the development of an efficient synthesis of α-aminomethylacrylates 2a-c (Scheme 1). We rationalized that 2a-c will be easily accessible by a Baylis–Hillman reaction (39–50) of methyl acrylate with an appropriate aldehyde, followed by acetylation (51, 52) of the resulting allylic alcohols 4a-b and SN2′-type amination of the resulting acetates 3a-b. The acetates were obtained in >90% yield and were used in the next step without further purification. The final SN2′-type displacement reaction of the acetates with O-benzylhydroxylamine or benzylamine was achieved with excess amounts of these amines in THF (53–55). The reaction required 2 days at room temperature with O-benzylhydroxylamine and only 2 h with benzylamine. A mixture of E- and Z-isomers (2a-c) was formed, which could be detected by HPLC and NMR analysis. The crude products were purified by flash chromatography to remove the excess amine. However, it was not necessary to separate the E- and Z-isomers for the asymmetric hydrogenation step (23–33).
Asymmetric Hydrogenation of α-Aminomethylacrylates.
With α-aminomethylacrylates 2a-c in hand, we next focused our attention on the key enantioselective hydrogenation step. Ru and Rh were selected as the catalysts and phosphanes such as 2,2′-bis(diphenylphosphino-1,1′-binaphthyl (BINAP), (R)-[6,6′-(2S,3S-butadioxy)]-(2,2′)-bis(diphenylphosphino)-(1,1′)-biphenyl (Bu-PQ-Phos), 2,2′-O,O′-(1,1′-binaphthyl)-O,O′-dioxo-N,N-dimethylphospholidine (Monophos), 1,2-bis(phospholano)benzene (Duphos), 1,1′-di-tert-butyl-[2,2′]-diphospholanyl (Tangphos), etc., were screened as ligands (56–65). The detailed data are listed in Table 1.
Table 1.
Asymmetric hydrogenation of α-aminomethylacrylates
| Entry* | Sub | Catalyst/ligand, mol% | S/C | Solvent | T, °C | P, psi | Time, h | Product | Isolated yield, % | ee, % (config.) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | E-2a | [Ru((S)-BINAP)(C6H6)Cl]Cl | 100 | MeOH | RT | 1000 | 20 | 1a | 15† | 45 (R) |
| 2 | E-2a | [Ru((S)-BINAP)(C6H6)Cl]Cl | 100 | EtOH | RT | 1000 | 20 | 1a | 0† | — |
| 3 | E-2a | [Ru((S)-BINAP)(C6H6)Cl]Cl | 100 | iPrOH | RT | 1000 | 20 | 1a | 0† | — |
| 4 | E-2a | [Ru((S)-BINAP)(C6H6)Cl]Cl | 100 | THF | RT | 1000 | 20 | 1a | 0† | — |
| 5 | E-2a | [Ru((S)-BINAP)(C6H6)Cl]Cl | 100 | DCM | RT | 1000 | 20 | 1a | 0† | — |
| 6 | E-2a | [Ru((S)-BINAP)(C6H6)Cl]Cl | 100 | Toluene | RT | 1000 | 20 | 1a | 0† | — |
| 7 | E-2a | [Ru((S)-BINAP)(C6H6)Cl]Cl | 100 | MeOH | 50 | 1000 | 24 | 1a | 39† | 44 (R) |
| 8 | E-2a | [Ru((RSS)-Bu-PQPhos)(C6H6)Cl]Cl | 100 | MeOH | RT | 1000 | 24 | 1a | 4† | ND |
| 9 | E-2a | Rh(COD)2BF4-((R)-Monophos) | 100 | DCM | RT | 1000 | 48 | 1a | 7† | 62 (S) |
| 10 | E-2a | (2R,5R)-[(COD)Rh(Me-Duphos)]+CF3SO3- (15%) | 7 | MeOH | RT | 50 | 48 | 1a | 99 | 95 (S) |
| 11 | E-2a | [Rh(NBD)2]BF4 (2.0%) (1R,1′R,2S,2′S)-Tangphos (2.2%) | 50 | MeOH | RT | 50 | 48 | 1a | 97 | 91 (R) |
| 12 | E-2a | [Rh((R,R)-Me-Duphos)(COD)]BF4 | 100 | MeOH | RT | 700 | 42 | 1a | 89† | 68 (S) |
| 13 | E-2a | [Rh((R,R)-Et-Duphos)(COD)]BF4 | 100 | MeOH | RT | 1000 | 24 | 1a | >99† | 92 (S) |
| 14 | E-2a | [Rh((S,S)-iPr-Duphos)(COD)]BF4 | 100 | MeOH | RT | 1000 | 97 | 1a | 20† | 72 (R) |
| 15 | E-2a | [Rh((R,R)-Et-Duphos)(COD)]BF4 | 100 | DCM | RT | 1000 | 90 | 1a | 58† | 94 (S) |
| 16 | E-2a | [Rh((R,R)-Et-Duphos)(COD)]BF4 | 100 | iPrOH | RT | 1000 | 24 | 1a | >99† | 99 (S) |
| 17 | E-2a | [Rh((S,S)-Et-Duphos)(COD)]BF4 | 100 | iPrOH | RT | 50 | 20 min | 1a | >99† | 99 (R) |
| 18 | E-2a | [Rh((S,S)-Et-Duphos)(COD)]BF4 | 100 | THF | RT | 50 | 20 min | 1a | >99† | 99 (R) |
| 19 | E-2a | [Rh((S,S)-Et-Duphos)(COD)]BF4 | 1000 | iPrOH | RT | 50 | 10 | 1a | >98 | 99 (R) |
| 20 | E-2a | [Rh((S,S)-Et-Duphos)(COD)]BF4 | 1000 | THF | RT | 50 | 10 | 1a | >98 | 99 (R) |
| 21 | E-2a | [Rh((S,S)-Et-Duphos)(COD)]BF4 | 10000 | iPrOH | RT | 50 | 78 | 1a | >98 | 99 (R) |
| 22 | E-2a | [Rh((S,S)-Et-Duphos)(COD)]BF4 | 10000 | THF | RT | 50 | 44 | 1a | 24† | 99 (R) |
| 23 | Z-2a | [Rh((S,S)-Et-Duphos)(COD)]BF4 | 100 | iPrOH | RT | 50 | 20 min | 1a | >99† | 92 (R) |
| 24 | Z-2a | [Rh((S,S)-Et-Duphos)(COD)]BF4 | 1000 | iPrOH | RT | 50 | 10 | 1a | >98 | 92 (R) |
| 25 | E/Z-2a‡ | [Rh((S,S)-Et-Duphos)(COD)]BF4 | 10000 | iPrOH | RT | 50 | 78 | 1a | >98 | 98 (R) |
| 26 | 2b | [Rh((S,S)-Et-Duphos)(COD)]BF4 | 100 | iPrOH | RT | 50 | 20 min | 1b | >99† | >99.5 (R) |
| 27 | 2b | [Rh((S,S)-Et-Duphos)(COD)]BF4 | 100 | THF | RT | 50 | 20 min | 1b | >99† | >99.5 (R) |
| 28 | 2b | [Rh((S,S)-Et-Duphos)(COD)]BF4 | 1000 | iPrOH | RT | 50 | 10 | 1b | 30† | >99.5 (R) |
| 29 | 2b | [Rh((S,S)-Et-Duphos)(COD)]BF4 | 1000 | THF | RT | 50 | 10 | 1b | 23† | >99.5 (R) |
| 30 | 2b | [Rh((S,S)-Et-Duphos)(COD)]BF4 | 500 | iPrOH | RT | 500 | 17 | 1b | 85† | >99.5 (R) |
| 31 | 2b | [Rh((S,S)-Et-Duphos)(COD)]BF4 | 500 | iPrOH | RT | 50 | 17 | 1b | 86† | >99.5 (R) |
| 32 | 2b | [Rh((S,S)-Et-Duphos)(COD)]BF4 | 500 | iPrOH | RT | 50 | 24 | 1b | >98 | >99.5 (R) |
| 33 | 2b | [Rh((S,S)-Et-Duphos)(COD)]BF4 | 500 | THF | RT | 50 | 24 | 1b | >98 | >99.5 (R) |
| 34 | 2b | [Rh((S,S)-Et-Duphos)(COD)]BF4 | 1000 | iPrOH | 50 | 50 | 7 | 1b | >98 | >99.5 (R) |
| 35 | 2b | [Rh((S,S)-Et-Duphos)(COD)]BF4 | 1000 | iPrOH | 70 | 50 | 5 | 1b | >98 | >99.5 (R) |
| 36 | 2b | [Rh((S,S)-Et-Duphos)(COD)]BF4 | 1000 | iPrOH | 90 | 50 | 5 | 1b | >98 | >99.5 (R) |
| 37 | 2b | (S,S,S,S)-[(COD)Rh(Et-FerroTANE)]+BF4- (13.6%) | 7 | MeOH | RT | 50 | 72 | 1b | — | no rxn |
| 38 | 2b | [Rh(NBD)2]BF4 (1.0%) (R)-(S)-Josiphos (1.1%) | 100 | MeOH | RT | 50 | 24 | 1b | 97 | 10 (S) |
| 39 | 2b | (2R,5R)-[(COD)Rh(Me-Duphos)]+CF3SO3-(15%) (almost no rxn with 1%) | 7 | MeOH | RT | 50 | 24 | 1b | 95 | >96 (S) |
| 40 | 2b | (2S,5S)-[(COD)Rh(Me-Duphos)]+CF3SO3- (15%) | 7 | MeOH | RT | 50 | 24 | 1b | 98 | 98 (R) |
| 41 | 2b | [Rh(NBD)2]BF4 (1.0%) (1S,1′S,2R,2′R)-Tangphos (1.1%) | 100 | MeOH | RT | 50 | 24 | 1b | 94 | >96 (S) |
| 42 | 2b | [Rh(NBD)2]BF4 (1.0%) (1R,1′R,2S,2′S)-Tangphos (1.1%) | 100 | MeOH | RT | 50 | 24 | 1b | 96 | 97 (R) |
| 43 | 2c | (2R,5R)-[(COD)Rh(Me-Duphos)]+CF3SO3- (15%) | 7 | MeOH | RT | 50 | 96 | 1c | 98 | 85 (S) |
| 44 | 2c | [Rh(NBD)2]BF4 (1.0%) (1S,1′S,2R,2′R)-Tangphos (1.1%) | 100 | MeOH | RT | 50 | 72 | 1c | — | no rxn |
| 45 | 2c | [Rh(NBD)2]BF4 (5.0%) (1S,1′S,2R,2′R)-Tangphos (5.5%) | 20 | MeOH | RT | 50 | 48 | 1c | 94 | 86 (S) |
| 46 | 2c | [Rh(NBD)2]BF4 (5.0%) (1R,1′R,2S,2′S)-Tangphos (5.5%) | 20 | MeOH | RT | 50 | 48 | 1c | 97 | 89 (R) |
| 47 | 6a | (2R,5R)-[(COD)Rh(Me-Duphos)]+CF3SO3- (15%) | 7 | MeOH | RT | 50 | 72 | 7a | 20† | ND |
| 48 | 6a | [Rh(NBD)2]BF4 (5.0%) (1S,1′S,2R,2′R)-Tangphos (5.5%) | 20 | MeOH | RT | 50 | 72 | 7a | 35† | ND |
| 49 | 6b | (2R,5R)-[(COD)Rh(Me-Duphos)]+CF3SO3- (15%) | 7 | MeOH | RT | 50 | 120 | 7b | 97 | <1 (S) |
| 50 | 6b | [Rh(NBD)2]BF4 (3.0%) (1S,1′S,2R,2′R)-Tangphos (3.3%) | 33 | MeOH | RT | 50 | 72 | 7b | 98 | 7 (R) |
| 51 | 6c | (2R,5R)-[(COD)Rh(Me-Duphos)]+CF3SO3-(15%) (no rxn with 1%) | 7 | MeOH | RT | 50 | 120 | 7c | 75† | 19 (S) |
| 52 | 6c | [Rh(NBD)2]BF4 (5.0%) (1S,1′S,2R,2′R)-Tangphos (5.5%) | 20 | MeOH | RT | 50 | 120 | 7c | 50† | 15 (S) |
ND, not determined; RT, room temperature.
*Entries 1–9, 12–18, and 23: substrate = 0.01 mmol, solvent volume = 0.7 ml; entries 10, 11, and 37–52: substrate = 1 mmol, solvent volume = 6 ml; entries 19, 20, 24, 28, 29, and 34–36: substrate = 0.1 mmol, solvent volume = 1.5 ml; entries 26 and 27: substrate = 0.02 mmol, solvent = 0.7 ml; entries 21, 22, and 25: substrate = 1 mmol, solvent volume = 4 ml; entries 30–33: substrate = 0.05 mmol, solvent volume = 1 ml.
†Reported as conversion based on 1H NMR spectroscopy.
‡E-2a/Z-2a = 80/20.
Because the reported synthesis of 1a was lengthy (15–18) and pure E-2a as a major isomer (E-2a/Z-2a = 80/20) could be acquired by column chromatography and recrystalization, we first studied the asymmetric hydrogenation of (E)-2a with several chiral catalyst systems. The results of using Ru-BINAP (56, 57), Bu-PQ-Phos (35, 36), and Rh-Monophos (24) complexes as catalysts were rather disappointing, showing poor reactivities and enantioselectivities (Table 1, entries 1–9). Good improvement occurred by employing Rh-(Me-Duphos) (58) or Rh-Tangphos (59) complex in this reaction. Product 1a was isolated in almost quantitative yield with excellent enantioselectivity. The enantiopurity of 1a was determined by chiral HPLC using a Daicel Chiralcel OD-H column and hexane/2-propanol (95/5) as the mobile phase. For example, complete hydrogenation of E-2a was achieved in 48 h with 15 mol% of (2R,5R)-[(COD)Rh(Me-Duphos)]+CF3SO3− (Table 1, entry 10), giving the corresponding product 1a with 95% ee. A similar result was obtained with 5.0 mol% [Rh(NBD)2]BF4 and 5.5 mol% (1R,1′R,2S,2′S)-Tangphos (Table 1, entry 11), but the enantioselectivity was somewhat lower (91% ee). As observed for E-2a, the (2R,5R)-Duphos afforded mostly the (S)-enantiomer, whereas the (1R,1′R,2S,2′S)-Tangphos gave mostly the (R)-enantiomer. Product 1a from each reaction was converted to the hydrochloride salt with HCl gas in isopropyl acetate, and the absolute configuration was determined based on reported optical rotation (15–18). Anions of the Rh complexes also showed significant influence on the enantioselectivity of 1a. Ee values decreased from 91% to 68% when CF3SO3− was replaced by BF4− (Table 1, entry 10 vs. entry 12). When we switched to the use of Rh-(Et-Duphos) for this reaction, an interesting phenomenon was noted: Et-Duphos was significantly better than Me-Duphos and iPr-Duphos (Table 1, entries 12–14, 92% vs. 68% and 72% ee, respectively). This finding implied that the substituent effect of the ligands was significant, which influenced not only the reactivity but also the enantioselectivity. The enantioselectivites were further improved to 99% using iPrOH and THF as solvents instead of MeOH and dichloromethane. Latter experiments demonstrated that the catalyst was more active in iPrOH than in THF (Table 1, entries 21, 22, 28, and 29). The reaction was very fast, it proceeded completely within 20 min at a substrate/catalyst (S/C) ratio of 100, even when the hydrogen pressure was decreased to 50 psi. It was further observed in the following cases that the hydrogen pressure of the catalyst system had little effect on the reactivity and enantioselectivity of this kind of reaction (Table 1, entries 30 and 31). This was very different from the asymmetric hydrogenation process of the E/Z mixture of β-substituted β-acylamino acrylates, in which remarkable pressure dependence was reported (23). As S/C was increased to 1,000, the reaction was completed within 10 h. Furthermore, it gave full conversion and the same high ee in iPrOH by prolonging the reaction time to 78 h even with a S/C ratio of up to 10,000. Consequentially, the hydrogenation of pure Z-2a and E/Z-2a mixtures were also tested. Rh-(Et-Duphos) as the catalyst was still effective for this reaction with very high reactivity. Although ee values decreased slightly to 92% in the hydrogenation of Z-2a (minor isomer), the hydrogenation of E/Z-2a mixture gave 1a with 98% ee (Table 1, entries 23–25). The chirality of product 1a was determined by the ligand's configuration regardless of the double bond configuration in substrate 2a. This means that the costly separation of the isomers can be avoided. After the success in the asymmetric hydrogenation of 2a, we put our effort to alkyl-substituted substrate 2b (1:1 mixture of (E) and (Z)-isomers; Table 1, entries 26–42). The result was also satisfactory by using [Rh-(Et-Duphos)(COD)]BF4 catalyst although the activity decreased a little in comparison with 2a (Table 1, entries 26–33). At room temperature and under 50 psi of H2, the reaction was complete within 24 h in iPrOH and THF with S/C up to 500, giving 1b in >98% yield and with >99.5% ee (Table 1, entries 32 and 33). The configuration of the major enantiomer was determined by further converting 1b to an advanced intermediate in our synthesis with known configuration and comparing the retention time of R and S enantiomers. The reaction rate increased as the reaction temperature was raised. Interestingly, no decrease of enantioselectivity for product 2b was found (Table 1, entries 34–36). The S/C ratio could be further increased to 1,000 at 50°C. The reaction was complete in 7 h under these conditions. Full conversions were achieved within 5 h at 70°C. The reaction rates were not further optimized. In contrast to the use of [Rh(Et-Duphos)(COD)]BF4 catalyst, no hydrogenation was observed with 1.0 mol% of [Rh(NBD)2]BF4 and 1.1 mol% of (S)-C4-Tunaphos (61), (S)-f-Binaphane (62), or (2S,4S)-Me-Ketalphos (63) in methanol for as long as 72 h. There was also no reaction with 1.0 mol% of (2R,5R)-[(COD)Rh(Me-DPEphos)]+CF3SO3− or 13.6 mol% of (S,S,S,S)-[(COD)Rh(Et-FerroTANE)]+BF4− (64) (entry 37) as catalyst. The hydrogenation was complete with 1.0 mol% of [Rh(NBD)2]BF4 and 1.1 mol% of (R)-(S)-Josiphos (65) in 24 h, however, the enantioselectivity was extremely poor (10% ee; Table 1, entry 38). The asymmetric hydrogenation was very slow with 1 mol% of (2R,5R)-[(COD)Rh(Me-Duphos)]+CF3SO3−, but it was complete in 24 h with ee >96% when the catalyst loading was increased to 15 mol% (Table 1, entry 39). As expected, the hydrogenation of 2b with (2S,5S)-Me-Duphos yielded 1b with 98% ee (Table 1, entry 40), but Tangphos had higher activity. The hydrogenation of 2b was complete in 24 h with only 1.0 mol% of [Rh(NBD)2]BF4 and 1.1 mol% of (1S,1′S,2R,2′R)-Tangphos, affording 1b in 94% isolated yield and >96% ee (Table 1, entry 41). Again as expected, the ligand with opposite chirality, (1R,1′R,2S,2′S)-Tangphos, afforded 1b with 97% ee (Table 1, entry 42). Overall [Rh-(Et-Duphos)(COD)]BF4 was the best catalyst in our studies of the asymmetric hydrogenation of 2b.
To study the role of the oxygen atom in the NHOCH2Ph group, we next examined the asymmetric hydrogenation of α-N-benzylaminomethyl-substituted acrylate 2c, which lacked the oxygen atom. The complete hydrogenation of 2c with 15 mol% of (2R,5R)-[(COD)Rh(Me-Duphos)]+CF3SO3− required 96 h compared with the requirement of 24 h for 2b. The enantioselectivity was also slightly lower (85% ee vs. >96% ee; see Table 1, entry 43 vs. 39). Similar results were obtained with Tangphos. In contrast to 2b, almost no reaction was observed for 2c with 1 mol% of catalyst and 1.1 mol% of ligand in 72 h (Table 1, entry 41 vs. 44). An increase in the loading of the catalyst to 5 mol% and the ligand to 5.5 mol% led to a completion of the reaction in 48 h (Table 1, entries 45 and 46). The enantiopurities of the product were also slightly lower than those from 2b. Thus, the presence of the oxygen atom in the NHOCH2h group has a positive effect on the reaction rates.
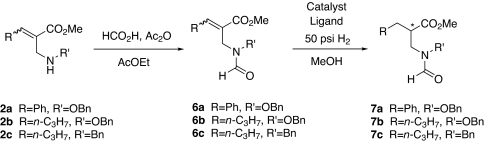
Because the N-acetyl group has been reported to coordinate to the catalyst during the synthesis of α-amino acids (66), it was of interest to study the hydrogenation of the N-acylated substrates. Thus, we synthesized N-formylated derivates 6a-c by reacting 2a-c with the mixed anhydride prepared from an excess of formic acid and acetic anhydride (Scheme 2). Surprisingly, the hydrogenation of 6b was very slow, and it required 120 h for the completion of the reaction even with 15 mol% of (2R,5R)-[(COD)Rh(Me-Duphos)]+CF3SO3− (Table 1, entry 49) and 72 h with 3.0 mol% [Rh(NBD)2]BF4 and 3.3 mol% (1S,1′S,2R,2′R)-Tangphos (Table 1, entry 50). Enantioselectivity was almost absent in both cases. Similarly, the hydrogenation of N-formylated substrate 6a also proceeded much slower. Only 20% and 35% conversions were observed with 15 mol% of Duphos and 5 mol% of Tangphos, respectively (Table 1, entries 47 and 48). The hydrogenation of 6c required 120 h with 15 mol% of Duphos or 5 mol% of Tangphos to achieve 75% and 50% conversion, respectively (Table 1, entries 51 and 52). The enantioselectivities were poor in both cases. These results were consistent with those reported for the hydrogenation of α-phthalimidomethyl-β-methylacrylate, which gave only 10% ee (67).
Scheme 2.
Synthesis of N-formylated derivates 6a-c and the hydrogenation process.
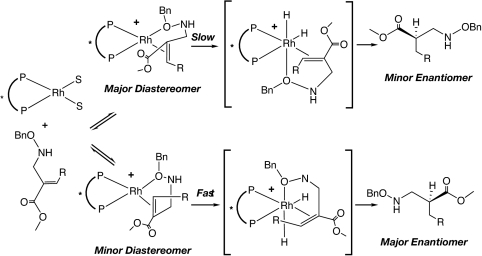
The mechanism of the Rh(I)-catalyzed asymmetric hydrogenation has been widely studied. The work by Halpern et al. (68), Brown et al. (69) and Landis et al. (70) concluded that the predominant enantiomer was derived from the minor (less stable) catalyst-substrate adduct due to its higher reactivity toward H2. Burk et al. (66) also suggested that Rh-Duphos catalysts behaved in a similar fashion. The Rh-catalyzed hydrogenation described in this paper is expected to follow the same mechanistic pathway involving a coordination of the metal with oxygen atom in the NHOCH2Ph group (Scheme 3). The configuration of the catalyst determined the product configuration and not the E/Z configuration the alkene. The higher efficiency observed for Tangphos was ascribed to its rigid backbone (59). The dramatic slow reaction rates and poor enantioselectivities with N-formylated compounds 6a-c suggested a possible different binding fashion of these substrates to the catalysts, perhaps via oxygen atom of the N CHO group. The high enantioselectivities obtained with the free basic amine derivatives are especially interesting as this avoids the need for protection and deprotection of the nitrogen in the synthesis.
CHO group. The high enantioselectivities obtained with the free basic amine derivatives are especially interesting as this avoids the need for protection and deprotection of the nitrogen in the synthesis.
Scheme 3.
Proposed mechanism for Rh-catalyzed asymmetric hydrogenation.
Conclusions
We have developed a highly enantioselective method for the synthesis of β-amino acid derivatives (1a-c), in which Rh-catalyzed asymmetric hydrogenation of the E/Z mixtures α-aminomethylacrylates (2a-c) containing a free basic NH group was used as the key step. The α-aminomethylacrylates (2a-c) were prepared using the Baylis–Hillman reaction of an appropriate aldehyde with methyl acrylate followed by acetylation of the resulting allylic alcohols (4a-b) and SN2′-type amination of the allylic acetates (3a-b). The easy approach and high S/C ratio provide a practical route for the practical preparation of β-amino acids and their derivatives via asymmetric hydrogenation under mild reaction conditions.
Materials and Methods
All of the reagents were purchased from commercial suppliers and used without further purification. (S)-C4-Tunaphos, (S)-f-Binaphane, (2S,4S)-Me-Ketalphos, (1S,1′S,2R,2′R) and (1R,1′R,2S,2′S)-Tangphos were obtained from Chiral Quest (Monmouth Junction, NJ). BINAP, Monophos, iPr-Duphos, Me-Duphos, [Rh(COD)2]BF4, (2R,5R)-[(COD)Rh(Me-Duphos)]+CF3SO3−, (2S,5S)-[(COD)Rh(Me-Duphos)]+CF3SO3−, [Rh((S,S)-Et-Duphos)(COD)]BF4 and [Rh((R,R)-Et-Duphos)(COD)]BF4 were purchased from Strem Chemicals (Newburyport, MA). [Rh(NBD)2]BF4 was obtained from Johnson Matthey (London, U.K.). 1H and 13C NMR spectra were recorded on a Bruker FT-NMR spectrometer at 300 and 75 MHz or a Varian 500 spectrometer at 500 and 125 MHz, respectively. High-resolution mass spectroscopy (HRMS) was carried out on a ThermoFinnigan MAT-900 (Finnigan MAT, Bremen, Germany) in electrospray mode. Other mass spectra were obtained on a Micromass LCT in electrospray mode. Opticals rotations were recorded on a PerkinElmer 341 (Manchester, U.K.) polarimeter in a 10-cm cell. Melting points were measured on a Büchi 533 melting point apparatus (Flawil, Switzerland). The enantiopurities of 1a-c were determined by chiral HPLC on a Rainin Dynamax (Woburn, MA) system or an Agilent 1100 series using Daicel Chiralcel OD-H column (4.6 × 250 mm) and a mixture of hexane/2-propanol (95/5) as the mobile phase (isocratic at a flow rate of 0.5 ml/min and UV detector at 220 nm). The retention times of (R)-1a and (S)-1a (free base) were 21.9 and 24.3 min, respectively. The retention time of (R)-1b and (S)-1b was 11.4 and 13.0 min, respectively. The retention time of (R)-1c and (S)-1c was 11.2 and 11.8 min, respectively. The enantiopurities of 7b and 7c were determined by N-deformylating them to 1b and 1c with HCl and analysis by chiral HPLC. 3-Hydroxy-3-phenyl-2-methylenepropanoic acid methyl ester (4a) and 3-hydroxy-2-methylenehexanoic acid methyl ester (4b) are known compounds and were prepared by reacting methyl acrylate with butanal, benzal, and pentanaldehyde, respectively, for 7 days at room temperature in the presence of catalytic DABCO (39–46, 51, 52).
(E/Z)-2-[(Benzyloxyamino)methyl]-3-Phenylacrylic Acid Methyl Ester (2a).
A mixture of 3-acetoxy-3-phenyl-2-methylenepropanoic acid methyl ester (3a) (35.14 g, 150 mmol) and O-benzylhydroxylamine (55.42 g, 450 mmol) in THF (100 ml) was allowed to stir at room temperature under N2 for 2 days. The reaction mixture was concentrated under reduced pressure (20 mbar) until no further solvent distilled. The residue was dissolved in ethyl acetate (500 ml) and washed with saturated aqueous sodium bicarbonate (250 ml). The ethyl acetate layer was concentrated under reduced pressure (20 mbar) until no further solvent distilled to afford a colorless liquid (87.4 g). The crude material was purified by column chromatography (silica gel, 5% ethyl acetate in heptane) to afford an ≈80:20 mixture of (E) and (Z)-(2a) (39.72 g, 89%) as a semisolid: 1H NMR (300 MHz, CDCl3) δ 3.64 (s, 0.2 × 3H), 3.80 (s, 0.8 × 3H), 3.83 (bs, 0.2 × 2H), 3.96 (bs, 0.8 × 2H), 4.71 (s, 0.2 × 2H), 4.72 (s, 0.8 × 2H), 6.08 (bs, 0.8 × 1H), 6.86 (bs, 0.2 × 1H), 7.25–7.4 (m, 8H), 7.45–7.55 (m, 2H), 7.87 (s, 1H); 13C NMR (75 MHz, CDCl3) δ 49.05, 52.09, 52.55, 57.04, 76.36, 76.68, 127.84, 128.19, 128.24, 128.42, 128.58, 128.70, 128.77, 128.83, 128.87, 128.92, 129.45, 130.02, 130.68, 135.23, 135.91, 137.98, 138.23, 138.45, 144.26, 168.63, 169.61; MS(ESI) 298.15 (MH+). The semisolid can be recrystallized from heptane to afford (E)-(2a) as a white solid (30.34 g, 68%): mp 47–49°C; 1H NMR (300 MHz, CDCl3) δ 3.80 (s, 3H), 3.96 (d, 2H, J = 5.7 Hz), 4.72 (s, 2H), 6.08 (bs, 1H), 7.25–7.4 (m, 8H), 7.45–7.55 (m, 2H), 7.87 (s, 1H); 13C NMR (75 MHz, CDCl3) δ 49.05, 52.55, 76.36, 127.84, 128.19, 128.77, 128.83, 128.92, 129.45, 130.02, 135.23, 138.45, 144.26, 168.63; MS(ESI) 298.15 (MH+). The residual part in the mother liquid was concentrated and purified again by column chromatography to give oily (Z)-(2a). 1H NMR (500 MHz, CDCl3) δ 3.66 (s, 3H), 3.84 (s, 2H), 4.72 (s, 2H), 6.88 (s, 1H), 7.25–7.38 (m, 11H); 13C NMR (125 MHz, CDCl3) δ 51.67, 56.58, 76.24, 127.81, 128.13, 128.26, 128.33, 128.42, 128.47, 130.18, 135.45, 137.61, 137.76, 169.18; MS(ESI) 298.15 (MH+).
(E/Z)-2-[(Benzyloxyamino)methyl]-2-Hexenoic Acid Methyl Ester (2b).
A mixture of 3-acetoxy-2-methylenehexanoic acid methyl ester (3b) (4.00 g, 20 mmol) and O-benzylhydroxylamine (7.39 g, 60 mmol) in THF (30 ml) was allowed to stir at room temperature under N2 for 2 days. The reaction mixture was concentrated under reduced pressure (20 mbar) until no further solvent distilled. The residue was dissolved in ethyl acetate (75 ml) and washed with saturated aqueous sodium bicarbonate (50 ml). The ethyl acetate layer was concentrated under reduced pressure (20 mbar) until no further solvent distilled to afford a colorless liquid (11.2 g). The crude material was purified by column chromatography (silica gel, 5% ethyl acetate in heptane) to afford an ≈1:1 mixture of (E) and (Z)-(2b) (4.01 g, 76%) as a colorless liquid: 1H NMR (300 MHz, CDCl3) (≈1:1 mixture of E/Z isomers) δ 0.89–0.96 (m, 3H), 1.38–1.53 (m, 2H), 2.19 (m, 0.5 × 2H), 2.48 (m, 0.5 × 2H), 3.65 (s, 0.5 × 2H), 3.71 (s, 0.5 × 3H), 3.72 (s, 0.5 × 3H), 3.74 (s, 0.5 × 2H), 4.67 (s, 0.5 × 2H), 4.68 (s, 0.5 × 2H), 5.88 (bs, 1H), 6.14 (t, 0.5 × 1H, J = 7.4 Hz), 6.95 (t, 0.5 × 1H, J = 7.6 Hz), 7.25–7.29 (m, 5H); 13C NMR (75 MHz, CDCl3) δ 14.21, 14.26, 22.41, 22.82, 31.02, 32.05, 48.04, 51.70, 52.13, 56.31, 76.29, 76.30, 127.89, 127.96, 128.13, 128.69, 128.77, 128.86, 138.35, 138.41, 147.97, 148.06, 168.03, 168.22; HRMS(ESI) 264.1554 (MH+, exact mass calculated for C15H22NO3 264.1600).
(E/Z)-2-[(Benzylamino)methyl]-2-Hexenoic Acid Methyl Ester (2c).
A mixture of 3-acetoxy-2-methylenehexanoic acid methyl ester (3b) (10.01 g, 50 mmol) and benzylamine (21.43 g, 80 mmol) in THF (75 ml) was allowed to stir at room temperature under N2 for 2 h. The resulting suspension was concentrated under reduced pressure (20 mbar) until no further solvent distilled. To the residue was added ethyl acetate (200 ml) and saturated aqueous sodium bicarbonate (50 ml) to afford a biphasic solution. The ethyl acetate layer was separated and washed with water (50 ml). The organic layer was concentrated under reduced pressure (20 mbar) until no further solvent distilled to afford a colorless liquid (23.1 g). The crude material was purified by column chromatography (silica gel, 20% ethyl acetate in heptane) to afford an ≈65:35 mixture of (E) and (Z)-(2c) (8.01 g, 65%) as a colorless liquid: 1H NMR (300 MHz, CDCl3) (≈65:35 mixture of E/Z isomers) δ 0.85–0.97 (m, 3H), 1.37–1.52 (m, 2H), 1.79 (bs, 1H), 2.05–2.15 (m, 0.65 × 2H), 2.4–2.5 (m, 0.35 × 2H), 3.40 (s, 0.35 × 2H), 3.45 (s, 0.65 × 2H), 3.7–3.8 (m, 5H), 6.06 (t, 0.35 × 1H, J = 7.4 Hz), 6.90 (t, 0.65 × 1H, J = 7.6 Hz), 7.2–7.4 (m, 5H); 13C NMR (75 MHz, CDCl3) δ 13.86, 14.13, 22.12, 22.58, 22.70, 30.48, 31.52, 44.27, 51.23, 51.66, 52.42, 52.86, 126.86, 128.06, 128.14, 128.30, 128.33, 129.79, 130.14, 140.27, 140.32, 144.85, 145.57, 167.93, 168.04; MS(ESI) 248.2 (MH+).
General Procedure for the Asymmetric Hydrogenation.
The appropriate catalyst prepared in situ or purchased from commercial suppliers was added to a solution of the substrate 2a-c or 6a-c and degassed solvent in a Parr bottle or a glass-lined stainless steel autoclave under nitrogen purge. After four vacuum/H2 cycles, the reaction mixture was pressurized to 50 psig H2 and maintained constantly under this pressure during the course of hydrogenation. The reaction was allowed to continue at room temperature for the time specified in Table 1. The completion of the reaction was monitored by HPLC. The reaction mixture was concentrated under reduced pressure (20 mbar) until no further solvent distilled. The residue was filtered through a short SiO2 column (≈1 g) with ethyl acetate/heptane (50/50, 10 ml) to remove the catalyst. The filtrate was concentrated under reduced pressure (20 mbar) until no further solvent distilled. The enantiomeric purity was determined by chiral HPLC.
(R)-2-[(Benzyloxyamino)methyl]-3-Phenylpropionic Acid Methyl Ester (1a).
Aymmetric hydrogenation of (E)-2-[(benzyloxyamino)methyl]-3-phenylacrylic acid methyl ester (2a) was carried out with bis(norbornadiene)rhodium(I) tetrafluoroborate (2.0 mol%) and (1R,1′R,2S,2′S)-Tangphos (2.2 mol%) at 50 psig H2 for 48 h at room temperature to afford (R)-2-[(benzyloxyamino)methyl]-3-phenylpropionic acid methyl ester (1a) (97%) as a colorless oil. The free base was added into a solution of 1 M HCl gas in isopropyl acetate (3 equiv) at room temperature to form a suspension. The solid was filtered, washed with isopropyl acetate, and dried to afford (R)-(1a) hydrochloride salt (94%) as a white solid: mp 145–147°C; ee = 91% (R); [α]d25 +26.9 (c 1.0, EtOH); spectroscopic data were in agreement with published data (15–18).
(S)-2-[(Benzyloxyamino)methyl]-3-phenyl-propionic acid methyl ester (S)-1a was also obtained by using (2R,5R)-[(COD)Rh(Me-Duphos)]+CF3SO3− (15 mol%) as catalyst in 48 h at room temperature [99%, ee = 95% (S)].
(R)-2-[(Benzyloxyamino)methyl] Hexanoic Acid Methyl Ester (1b).
Asymmetric hydrogenation of an ≈1:1 mixture of (E) and (Z)-2-[(benzyloxyamino)methyl]-2-hexanoic acid methyl ester (2b) was carried out with [Rh((S,S)Et-Duphos)(COD)]BF4 as the catalyst (S/C = 500) at 50 psig H2 for 24 h at room temperature to afford (R)-(1c) (>98% yield): oil; ee >99.5% (R); [α]d25–11.8 (c 1.0, EtOH); 1H NMR (CDCl3, 500 MHz) δ 0.90 (t, 3H, J = 7.0 Hz), 1.21–1.34 (m, 4H), 1.47–1.63 (m, 2H), 2.73–2.80 (m, 1H), 3.02–3.07 (m, 1H), 3.17–3.22 (m, 1H), 3.67 (s, 3H), 4.72 (d, 2H, J = 3.5 Hz), 5.25 (bs, 1H), 7.2–7.4 (m, 5H); 13C NMR (CDCl3, 125 MHz) δ 13.79, 22.47, 29.30, 29.97, 43.72, 51.42, 53.74, 76.13, 127.72, 128.24, 128.37, 137.66, 175.79; HRMS(ESI): calcd for C15H24NO3 (MH+) 266.1756, found 266.1757.
(R)-2-[(Benzylamino)methyl]hexanoic Acid Methyl Ester (1c).
Asymmetric hydrogenation of an ≈65:35 mixture of (E) and (Z)-2-[(benzylamino)methyl]-2-hexenoic acid methyl ester (2c) was carried out with bis(norbornadiene)rhodium(I) tetrafluoroborate (5.0 mol%) and (1R,1′R,2S,2′S)-Tangphos (5.5 mol%) at 50 psig H2 for 48 h at room temperature to afford (R)-(1c) (97%): oil; ee = 89% (R); [α]d25 −10.9 (c 1.0, MeOH); 1H NMR (300 MHz, CDCl3) δ 0.87 (t, 3H, J = 7.0 Hz), 1.15–1.35 (m, 4H), 1.4–1.7 (m, 3H), 2.52–2.64 (m, 1H), 2.64–2.74 (m, 1H), 2.8–2.9 (m, 1H), 3.67 (s, 3H), 3.77 (d, 2H, J = 2.6 Hz), 7.18–7.34 (m, 5H); 13C NMR (75 MHz, CDCl3) δ 13.91, 22.61, 29.54, 30.08, 46.13, 50.81, 51.48, 53.72, 126.89, 128.01, 128.34, 140.30, 176.07; MS(ESI) 250.17 (MH+); anal. calcd. for C15H23NO2: C, 72.25; H, 9.30; N, 5.62; found: C, 72.38; H, 9.45; N, 5.65.
(S)-2-[(Benzylamino)methyl]hexanoic acid methyl ester (S)-1c was prepared by using (1S,1′S,2R,2′R)-Tangphos [94%, ee = 86% (S)] or (2R,5R)-[(COD)Rh(Me-Duphos)]+CF3SO3− [98%, ee = 85% (S)] as catalyst.
The synthesis of substrates 3a-b and 6a-c and the spectra of 3a-b, 6a-c, and 7a-c are provided in supporting information (SI) Text.
Supplementary Material
Acknowledgments
We thank Dr. C. P. Mak (Global Head, CHAD) and Dr. G. Penn (Distinguished Fellow) of Novartis Pharma AG (Basel, Switzerland) for supporting this collaboration. This work was supported by Hong Kong Research Grants Council (Project Number PolyU5001/04P), the University Grants Committee Areas of Excellence Scheme in Hong Kong (AoE P/10-01), the Hong Kong Polytechnic University Area of Strategic Development Fund, and Novartis Pharmaceutical Corporation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0704461104/DC1.
References
- 1.Ojima I, Lin SN, Wang T. Curr Med Chem. 1999;6:927–954. [PubMed] [Google Scholar]
- 2.Drey CNC. In: Chemistry and Biochemistry of Amino Acids. Barret GC, editor. London: Chapman and Hall; 1992. [Google Scholar]
- 3.Seebach D, Overhand M, Kuhnle F, Martinoni B, Oberer L, Hommel U, Widmer H. Helv Chim Acta. 1996;79:913–941. [Google Scholar]
- 4.Gellman SH. Acc Chem Res. 1998;31:17–180. [Google Scholar]
- 5.Gademann K, Hintermann T, Schreiber JV. Curr Med Chem. 1999;6:905–925. [PubMed] [Google Scholar]
- 6.Juaristi E, editor. Enantioselective Synthesis of β-Amino Acids. New York: Wiley-VCH; 1997. [Google Scholar]
- 7.Cole DC. Tetrahedron. 1994;50:9517–9582. [Google Scholar]
- 8.Juaristi E, Lopez-Ruiz H. Curr Med Chem. 1999;6:983–1004. [PubMed] [Google Scholar]
- 9.Liu M, Sibi MP. Tetrahedron. 2002;58:7991–8035. [Google Scholar]
- 10.Gutiérrez-García VM, Reyes-Rangel G, Muñoz-Muñiz O, Juaristi E. Helv Chim Acta. 2002;85:4189–4199. [Google Scholar]
- 11.Jin Y, Kim DH. Synlett. 1998:1189–1190. [Google Scholar]
- 12.Juaristi E, Quintana D, Lamastsch B, Seebach D. J Org Chem. 1991;56:2553–2557. [Google Scholar]
- 13.Barnish IT, Corless M, Dunn PJ, Ellis D, Finn PW, Hardstone JD, James K. Tetrahedron Lett. 1993;34:1323–1326. [Google Scholar]
- 14.Jefford CW, Wang J. Tetrahedron Lett. 1993;34:1111–1114. [Google Scholar]
- 15.Park JD, Kim DH, Kim S-J, Woo J-R, Ryu SE. J Med Chem. 2002;45:5295–5302. doi: 10.1021/jm020258v. [DOI] [PubMed] [Google Scholar]
- 16.Kim DH, Jin Y. Bioorg Med Chem Lett. 1999;9:691–696. doi: 10.1016/s0960-894x(99)00055-4. [DOI] [PubMed] [Google Scholar]
- 17.Pratt LM, Beckett RP, Davies SJ, Launchbury SB, Miller A, Spavold ZM, Todd RS, Whittaker M. Bioorg Med Chem Lett. 2001;11:2585–2588. doi: 10.1016/s0960-894x(01)00509-1. [DOI] [PubMed] [Google Scholar]
- 18.Robl JA, Simpkins LM, Asaad MM. Bioorg Med Chem Lett. 2000;10:257–260. doi: 10.1016/s0960-894x(99)00671-x. [DOI] [PubMed] [Google Scholar]
- 19.Chang S-YP, McGary EC, Chang S. J Bacteriol. 1989;171:4071–4072. doi: 10.1128/jb.171.7.4071-4072.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meinnel T, Blanquet S. J Bacteriol. 1994;176:7387–7390. doi: 10.1128/jb.176.23.7387-7390.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mazel D, Pochet S, Marliere P. EMBO J. 1994;13:914–923. doi: 10.1002/j.1460-2075.1994.tb06335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prashad M, Kim H, Hu B, Slade J, Kapa PK, Girgis MJ. 2004. WO 2004/076053.
- 23.Drexler H-J, You J, Zhang S, Fischer C, Baumann W, Spannenberg A, Heller D. Org Proc Res Dev. 2003;7:355–361. [Google Scholar]
- 24.Peña D, Minnaard AJ, de Vries JG, Feringa BL. J Am Chem Soc. 2002;124:14552–14553. doi: 10.1021/ja028235t. [DOI] [PubMed] [Google Scholar]
- 25.Tang W, Zhang X. Org Lett. 2002;4:4159–4161. doi: 10.1021/ol026935x. [DOI] [PubMed] [Google Scholar]
- 26.Lee S-g, Zhang YJ. Org Lett. 2002;4:2429–2431. doi: 10.1021/ol0261884. [DOI] [PubMed] [Google Scholar]
- 27.Yasutake M, Gridnev ID, Higashi N, Imamoto T. Org Lett. 2001;3:1701–1704. doi: 10.1021/ol0158967. [DOI] [PubMed] [Google Scholar]
- 28.Heller D, Holz J, Drexler HJ, Lang J, Drauz K, Krimmer H-P, Börner A. J Org Chem. 2001;66:6816–6817. doi: 10.1021/jo010445l. [DOI] [PubMed] [Google Scholar]
- 29.Zhu G, Chen Z, Zhang X. J Org Chem. 1999;64:6907–6910. doi: 10.1021/jo990565h. [DOI] [PubMed] [Google Scholar]
- 30.Furukawa M, Okawara T, Noguchi Y, Terawaki Y. Chem Pharm Bull. 1979;27:2223–2226. [Google Scholar]
- 31.Achiwa K, Soga T. Tetrahedron Lett. 1978;19:1119–1120. [Google Scholar]
- 32.Lubell WD, Kitamura M, Noyori R. Tetrahedron. 1991;2:543–554. [Google Scholar]
- 33.Zhou Y-G, Tang W, Wang W-B, Li W, Zhang X. J Am Chem Soc. 2002;124:4952–4953. doi: 10.1021/ja020121u. [DOI] [PubMed] [Google Scholar]
- 34.Wu J, Chen X, Guo R, Yeung C-H, Chan ASC. J Org Chem. 2003;68:2490–2493. doi: 10.1021/jo026675x. [DOI] [PubMed] [Google Scholar]
- 35.Qiu L, Wu J, Chan S, Au-Yeung TT-L, Ji J-X, Guo R, Pai C-C, Zhou Z, Li X, Fan Q-H, Chan ASC. Proc Natl Acad Sci USA. 2004;101:5815–5820. doi: 10.1073/pnas.0307774101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qiu L, Kwong FY, Wu J, Lam WH, Chan S, Yu W-Y, Guo R, Chan ASC. J Am Chem Soc. 2006;128:5955–5965. doi: 10.1021/ja0602694. [DOI] [PubMed] [Google Scholar]
- 37.Hsiao Y, Rivera NR, Rosner T, Krska SW, Njolito E, Wang F, Sun Y, Armstrong JD, III, Grabowski EJJ, Tillyer RD, et al. J Am Chem Soc. 2004;126:9918–9919. doi: 10.1021/ja047901i. [DOI] [PubMed] [Google Scholar]
- 38.Huang H, Liu X, Deng J, Qiu M, Zheng Z. Org Lett. 2006;8:3359–3362. doi: 10.1021/ol0612399. [DOI] [PubMed] [Google Scholar]
- 39.Basavaiah D, Rao PD, Hyma RS. Tetrahedron. 1996;52:8001–8062. [Google Scholar]
- 40.Fort Y, Berthe MC, Caubere P. Tetrahedron. 1992;48:6371–6384. [Google Scholar]
- 41.Mateus CR, Feltrin MP, Costa AM, Coelho F, Almeida P. Tetrahedron. 2001;57:6901–6908. [Google Scholar]
- 42.Hoffmann HMR, Rabe J. J Org Chem. 1985;50:3849–3859. [Google Scholar]
- 43.Kuchholz R, Hoffmann HMR. Helv Chim Acta. 1991;74:1213–1220. [Google Scholar]
- 44.Kündig EP, Xu L-H, Romanens P. Tetrahedron Lett. 1995;36:4047–4050. [Google Scholar]
- 45.Keck GE, Welch DS. Org Lett. 2002;4:3687–3690. doi: 10.1021/ol026638s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Basavaiah D, Sharada DS, Kumaragurubaran N, Reddy RM. J Org Chem. 2002;67:7135–7137. doi: 10.1021/jo0257952. [DOI] [PubMed] [Google Scholar]
- 47.Pei W, Wei H-X, Li G. Chem Commun. 2002:1856–1857. doi: 10.1039/b204210j. [DOI] [PubMed] [Google Scholar]
- 48.Aggarwal VK, Dean DK, Mereu A, Williams R. J Org Chem. 2002;67:510–514. doi: 10.1021/jo016073y. [DOI] [PubMed] [Google Scholar]
- 49.Cai J, Zhou Z, Zhao G, Tang C. Org Lett. 2002;4:4723–4725. doi: 10.1021/ol027197f. [DOI] [PubMed] [Google Scholar]
- 50.Das B, Mahender G, Chowdhury N, Banerjee J. Synlett. 2005:1000–1002. [Google Scholar]
- 51.Annunziata R, Benaglia M, Cinquini M, Cozzi F, Raimondi L. J Org Chem. 1995;60:4697–4706. [Google Scholar]
- 52.Basavaiah D, Hyma RS. Tetrahedron. 1996;52:1253–1258. [Google Scholar]
- 53.Hbaïeb S, Latiri Z, Amri H. Synth Comm. 1999;29:981–988. [Google Scholar]
- 54.Perlmutter P, Tabone M. J Org Chem. 1995;60:6515–6522. [Google Scholar]
- 55.Drewes SE, Horn MM, Ramesar N. Synth Comm. 2000;30:1045–1055. [Google Scholar]
- 56.Ohkuma T, Kitamura M, Noyori R. In: Catalytic Asymmetric Synthesis. 2nd Ed. Ojima I, editor. Weinheim: Wiley-VCH; 2000. pp. 1–110. [Google Scholar]
- 57.Noyori R, Kitamura M, Ohkuma T. Proc Natl Acad Sci USA. 2004;101:5356–5362. doi: 10.1073/pnas.0307928100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Burk MJ. ACC Chem Res. 2000;33:363–372. doi: 10.1021/ar990085c. [DOI] [PubMed] [Google Scholar]
- 59.Tang W, Zhang X. Angew Chem Int Ed. 2002;41:1612–1614. doi: 10.1002/1521-3773(20020503)41:9<1612::aid-anie1612>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 60.Brown JM. In: Comprehensive Asymmetric Catalysis. Jacobsen EN, Pfaltz A, Yamamoto H, editors. Berlin: Springer; 1999. pp. 60–122. [Google Scholar]
- 61.Zhang Z, Qian H, Longmire J, Zhang X. J Org Chem. 2000;65:6223–6226. doi: 10.1021/jo000462v. [DOI] [PubMed] [Google Scholar]
- 62.Xiao D, Zhang X. Angew Chem Int Ed. 2001;40:3425–3428. doi: 10.1002/1521-3773(20010917)40:18<3425::aid-anie3425>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 63.Yan Y-Y, RajanBabu TV. J Org Chem. 2000;65:900–906. [Google Scholar]
- 64.Berens U, Burk MJ, Gerlach A, Hems W. Angew Chem Int Ed. 2000;39:1981–1984. doi: 10.1002/1521-3773(20000602)39:11<1981::aid-anie1981>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 65.Togni A, Breutel C, Schnyder A, Spindler F, Landert H, Tijani A. J Am Chem Soc. 1994;116:4062–4066. [Google Scholar]
- 66.Burk MJ, Feaster JE, Nugent WA, Harlow R. J Am Chem Soc. 1993;115:10125–10138. [Google Scholar]
- 67.Saylik D, Campi EM, Donohue AC, Jackson WR, Robinson AJ. Tetrahedron. 2001;12:657–667. [Google Scholar]
- 68.Landis CR, Halpern J. J Am Chem Soc. 1987;109:1746–1754. [Google Scholar]
- 69.Alcock NW, Brown JM, Derome AE, Lucy AR. J Chem Soc Chem Comm. 1985:575–578. [Google Scholar]
- 70.Giovannetti JS, Kelly CM, Landis CR. J Am Chem Soc. 1993;115:4040–4057. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.