Abstract
Neurotrophins are potent survival factors for developing and injured neurons. However, they are not being used to treat neurodegenerative diseases because of difficulties in administration and numerous side effects that have been encountered in previous clinical trials. Their biological activities use Trk (tropomyosin-related kinase) transmembrane tyrosine kinases. Therefore, one alternative approach is to use transactivation pathways such as adenosine 2A receptor agonists, which can activate Trk receptor signaling independent of neurotrophin binding. However, the relevance in vivo and applicability of these transactivation events during neurodegenerative and injury conditions have never been extensively studied. Here we demonstrate that motoneuron survival after facial nerve lesioning is significantly enhanced by transactivation of Trk receptor tyrosine kinases by adenosine agonists. Moreover, survival of motoneurons directly required the activation of the BDNF receptor TrkB and an increase in Akt (AKT8 virus oncogene cellular homolog) activity. The ability of small molecules to activate a trophic response by using Trk signaling provides a unique mechanism to promote survival signals in motoneurons and suggests new strategies for using transactivation in neurodegenerative diseases.
Keywords: brain-derived neurotrophic factor, transactivation
Neurotrophins are prominent regulators of neuronal development, growth, and plasticity in the vertebrate nervous system (1, 2). The actions of neurotrophins are mediated by two classes of cell surface receptors: tropomyosin-related kinase (Trk) receptor tyrosine kinase and p75NTR (where NTR stands for neurotrophin receptor), a member of the tumor necrosis factor receptor superfamily (3, 4). Nerve growth factor binds exclusively to TrkA, BDNF and neurotrophin-4 bind to TrkB, and neurotrophin-3 binds to TrkC, whereas all neurotrophins bind to p75NTR. After binding to Trk receptors, neurotrophins and their receptors undergo internalization and transport from axon terminals to neuronal cell bodies (5–7), where a number of transcriptional and enzymatic activities occur. Engagement of Trk receptors results in increases in cAMP response element-binding protein and ERK5 activities (8–10), as well as phosphatidylinositol lipid phosphorylation and activation of GTPases, such as Ras (rat sarcoma) and Rap1 (Ras-related protein 1) (11).
Another mode of tyrosine kinase receptor activation is transactivation of receptor tyrosine kinases in response to G-protein-coupled receptor (GPCR) signaling (12–14). For example, receptors for epidermal growth factor, insulin-like growth factor-1, and platelet-derived growth factor can be transactivated through GPCRs to give proliferative and MAPK responses. Hence, transactivation can lead to effects on cell proliferation and differentiation. The TrkA and TrkB receptors can be activated by specific ligands of the GPCR family, in the absence of nerve growth factor or BDNF, respectively (15–17). The ligands include the nucleoside adenosine or adenosine agonists, such as 2-p-(2-carboxyethylamine-5′-N-ethylcarboxamide)adenosine (CGS21680) (16), and pituitary adenylate cyclase-activating peptide (18). Adenosine and CGS21680 recognize the A2A adenosine receptor, A2A-R (19). Transactivation of Trk receptors leads not only to increased tyrosine kinase activity but also to increased phosphorylation of several key effectors of the Trk tyrosine kinases (15–18). Treatment of pheochromocytoma 12 (PC12) cells or primary neurons with adenosine or adenosine agonists specific to the A2A-R promoted the phosphorylation of Shc (Src homology collagen) adaptor proteins as well as phospholipase Cγ, similar to the induction observed with neurotrophin application (15, 16). Activation of phosphatidylinositol 3-kinase and Akt (AKT8 virus oncogene cellular homolog) accounts for neuroprotective effects afforded by transactivation by adenosine. The effects of adenosine can be specifically blocked by K252a, an inhibitor of Trk tyrosine kinase activity (15, 20).
In the present study, we analyzed the action of A2A-R in a commonly used nerve lesion model, the facial nerve transection model in newborn mice (21). The A2A-R is expressed in motoneurons and can be activated in vivo with CGS21680 or inhibited with the specific A2A-R inhibitor 4-(2-[7-amino-2-(2-furyl)[1,2,4]triazolo[2,3-a][1,3,5]triazin-5-ylamino]ethyl)phenol (ZM241385). The results indicate that activation of the adenosine receptor by using CGS21680 leads to survival of lesioned facial motoneurons in vivo. Isolated and enriched motoneurons from the lumbar spinal cord show increased survival with CGS21680. Immunocytochemical detection of phospho-Akt in motoneurons of the lesioned side showed that the Akt kinase is activated upon treatment with the A2A-R agonist CGS21680. Significantly, the survival response to CGS21680 was abolished in isolated trkB−/− motoneurons, indicating that TrkB plays an essential role in survival responses of motoneurons after activation of A2A-R.
Results
The A2A-R Agonist CGS21680 Promotes Survival of Lesioned Facial Motoneurons.
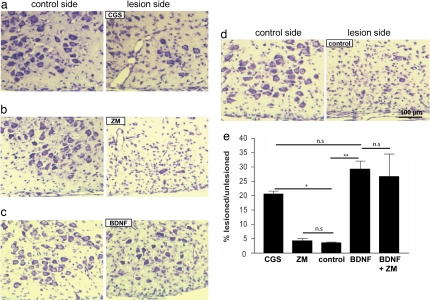
Treatment of PC 12 cells or hippocampal neurons with adenosine receptor agonists resulted in activation of Trk receptors and increased cell survival in the absence of neurotrophins (15, 17). To determine whether this response also occurs in a physiological context in vivo, we transected the facial nerve in 1-day-old mice and investigated the survival response of facial motoneurons to an adenosine receptor agonist that was locally applied to the nerve lesion site. The A2A-R agonist CGS21680 and the antagonist ZM241385 were applied in gel foam to the proximal nerve stump of the transected facial nerve and compared with BDNF treatment, using established methods (21, 22). After 7 days, when >95% of facial motoneurons were lost on the lesion side in untreated mice, the adenosine receptor agonist CGS21680 specifically enhanced survival of lesioned motoneurons to 21 ± 1% (P = 0.02131), whereas the antagonist ZM241385 did not provide protection of motoneurons in vivo after nerve lesioning (Fig. 1). For comparison, local application of BDNF supported 29 ± 3% of the lesioned motoneurons (Fig. 1 c and e), consistent with previous studies (22).
Fig. 1.
The A2A-R agonist CGS21680 rescues motoneurons from cell death after facial nerve lesioning. Survival of lesioned facial motoneurons of 1-day-old mice is enhanced in the presence of the adenosine receptor agonist CGS21680. (a, b, c, and d) The facial nerve of 1-day-old mice was lesioned unilaterally, and CGS21680 (a), ZM241385 (b), BDNF (c), or buffer alone (d) was applied to the proximal nerve stump in gel foam. Mice were killed 7 days later, and motoneurons in the facial nucleus were counted in serial sections on both the lesioned side and the unlesioned control side. (e) Values show mean ± SEM of survival rates relative to the unlesioned control side in the individual groups (CGS group: n = 6; ZM group: n = 3; control group: n = 3; BDNF group: n = 4; BDNF + ZM group: n = 4). P < 0.05 (*, P = 0.02131; **, P = 0.00342; n.s, not significant).
Transactivation of TrkB in Facial Motoneurons After Axotomy.
Transactivation of TrkA receptors after A2A-R activation with CGS21680 was previously established in several cell lines (16, 17). To test whether the survival effect of A2A-R activation in facial motoneurons after nerve lesioning involved TrkB, we first determined whether TrkB phosphorylation was enhanced after CGS21680 application. After the facial nerve was lesioned in 1-day-old postnatal mice, CGS21680 or control substances were locally applied, and the facial nucleus was microdissected 6 h later, on both the lesioned and unlesioned control sides. Protein extracts were prepared, and TrkB was immunoprecipitated and analyzed by using Western blotting with a phosphotyrosine antibody (Fig. 2). Treatment with CGS21680 enhanced TrkB phosphorylation ≈2-fold in comparison with the PBS control (P = 0.03205). Increased phosphorylation of TrkB was detected after application of CGS21680, similar to what was observed with BDNF treatment (P = 0.02973). The ZM241385 antagonist did not show any alterations of TrkB phosphorylation in comparison with controls. These results indicate that adenosine agonists can activate TrkB receptors in the facial nucleus after nerve lesioning.
Fig. 2.
The A2A-R agonist CGS21680 activates the TrkB receptor after facial nerve lesioning. After unilateral facial nerve transection at postnatal day 1 and local application of CGS21680 to the lesion site, facial nuclei were microdissected 6 h later and processed for immunoprecipitation of TrkB. (a) The precipitates were subjected to Western blot analysis for TrkB and phosphotyrosine. Band intensities were measured by using the AIDA software. (b) The ratio of phosphotyrosine to TrkB was determined for each animal, and quantification and statistical analysis are shown. au, absorbance units. Results reflect mean ± SEM from three independent experiments with n ≥ 3 in each group. *, P < 0.05.
Cultured Embryonic Motoneurons Survive in the Presence of A2A-R Agonist CGS21680.
The response of lesioned facial motoneurons to the A2A-R agonist could be mediated through transactivation of TrkB receptor through A2A-R signaling or involving other cell types that produce BDNF, leading to activation of its TrkB receptor. To test whether CGS21680 can also promote survival of motoneurons in the absence of other cell types, motoneurons were isolated from the lumbar spinal cord of 12.5-day mouse embryos by enrichment using immunopanning with an antibody against p75NTR. The neurons were cultured in the presence of CGS21680, BDNF, or glia-derived neurotrophic factor (GDNF) as controls. After 7 days in culture with neurobasal medium, B27 supplement, and 2% horse serum, the number of surviving motoneurons was determined. As shown in Fig. 3 a and c, application of CGS21680 significantly enhanced survival of isolated motoneurons in comparison with untreated controls. We noted that it was important to keep the motoneurons in low concentrations of serum because standard conditions with 10% horse serum reduced the survival effect of CGS21680 (data not shown). The A2A-R antagonist ZM241385 had no effect on motoneuron survival (data not shown). In addition, there was no additive effect of BDNF and CGS21680. Survival rates did not differ in comparison with cultures that were treated with either BDNF or CGS21680 alone (Fig. 3a).
Fig. 3.
The A2A-R agonist CGS21680 supports survival of isolated embryonic motoneurons from lumbar spinal cord of 12.5-day mouse embryos. Enriched motoneurons from lumbar spinal cord were treated with CGS21680 (CGS), the A2A-R antagonist ZM241385 (ZM), and BDNF as a positive control. After 7 days in culture, surviving motoneurons were counted. Data shown represent the percentage (mean ± SEM) of survival of originally plated cells. (a) Treatment with BDNF and the A2A-R agonist CGS21680 resulted in enhanced survival in comparison with cultures that were treated with CGS21680 resulted in enhanced survival in comparison with cultures that were treated with no factor. There was no additive effect on survival when BDNF and CGS21680 were added in combination to motoneuron cultures. (b) Motoneurons isolated from the lumbar spinal cord of 12.5-day trkB+/− intercross single mouse embryos were treated with BDNF, CGS21680, or GDNF. Survival with CGS21680 or BDNF but not with GDNF is reduced to background levels in trkB−/− motoneurons. (c) When a neutralizing antiserum against BDNF was added at a dilution of 1:1,000 to the motoneuron cultures, the effect of BDNF was reduced to background levels. P < 0.05, (***, P = 0.00076). In contrast, the BDNF antibodies did not reduce the survival effect of CGS21680, indicating that BDNF is not mediating the effects of CGS21680 on survival of motoneurons via TrkB. Each experiment was performed at least three times, with n > 3 for each group. (d) Immunoprecipitation of Trk receptors from cultured motoneurons and detection of TrkB reveals that the BDNF antiserum blocks activation of TrkB when cultured motoneurons are stimulated with BDNF but does not block activation of TrkB in the presence of CGS21680.
The survival response to CGS21680 could be dependent upon the activation of TrkB or to a GPCR-mediated pathway independent of TrkB. To distinguish between these possibilities, lumbar spinal motoneurons were isolated from TrkB−/− 12.5-day embryos obtained from TrkB+/− intercrosses (23). TrkB−/− motoneurons did not survive in the presence of BDNF (Fig. 3b), but they could be maintained with GDNF, which exerts a survival effect via GDNF receptor α and the ret tyrosine kinase receptor (24). When TrkB−/− motoneurons were treated with CGS21680, the survival effect was abolished (Fig. 3b). Similarly, a combination of BDNF and CGS21680 had no effect on motoneuron survival, whereas GDNF was capable of providing a survival signal (Fig. 4 a and b). This result indicates that TrkB is essential for the survival effects of A2A-R activation in motoneurons. Thus, transactivation of TrkB appears necessary for the survival effects observed both in vivo after facial nerve lesioning and in vitro with isolated motoneurons after application of the A2A-R agonist CGS21680.
Fig. 4.
Akt kinase is activated by the A2A-R agonist CGS21680 in facial motoneurons lesioned on postnatal day 1. The brainstem containing the facial nuclei from the lesioned mice was prepared and processed for immunohistochemical detection of phospho-Ser-473-Akt. Staining is yellow in these micrographs. (a, b, and e) Akt is specifically phosphorylated when the facial nerve stumps were treated with CGS21680 (b) or BDNF (f). (a–f) Cells from the unlesioned control side corresponding to CGS21680-treated motoneurons (a), lesioned side containing CGS21680-treated motoneurons (b), unlesioned side corresponding to ZM241385-treated motoneurons (c), lesioned side containing the ZM241385-treated motoneurons (d), untreated controls (secondary antibody only) (e), and BDNF-treated motoneurons (f). (Scale bar: 20 μm.) Activation of Akt was enhanced after application of BDNF or CGS21689 and was reduced to low or undetectable levels when the nerve stump was treated with ZM241385 (d).
The activation of TrkB after addition of CGS21680 to motoneurons, both in vivo and in vitro, does not rule out the possibility that A2A-R activation leads to up-regulation of BDNF production in motoneurons and that the subsequent activation of TrkB is not caused by transactivation of this receptor but through activation by BDNF. To exclude this possibility, we used a neutralizing antiserum against BDNF in primary motoneuron cultures. A rabbit antiserum raised against recombinant BDNF was applied at a dilution of 1:1,000. This treatment abolished the survival effect of 10 ng/ml BDNF. Under the same conditions, the blocking antibodies did not reduce the survival effect of CGS21680 (Fig. 3c). The A2A-R agonist ZM241385 did not influence BDNF-mediated survival or TrkB phosphorylation under the same conditions. Taken together with the observation that the survival effect of CGS21680 on motoneurons requires TrkB (Fig. 3b), these data indicate that the addition of CGS21680 leads to transactivation of TrkB and thus to survival of motoneurons.
Activation of Akt Kinase in Lesioned Motoneurons by Transactivation.
The mechanism of adenosine effects on motoneuron survival through the use of TrkB is unknown. To test whether Akt, a principal downstream mediator of TrkB, is involved in the survival pathway that protects motoneurons from lesion-induced cell death after application of the A2A-R agonist CGS21680, sections of the facial nuclei from CGS21680-, ZM241385-, or BDNF-treated animals were immunostained with an antibody that specifically recognizes the phospho-Ser-473 (pS473)-specific form of Akt. pS473-Akt immunoreactivity was enhanced in motoneurons in comparison with that from the unlesioned control side (Fig. 4a) when CGS21680 (Fig. 4b) was applied to the proximal nerve stump. Levels of pS473-Akt immunoreactivity in the lesioned motoneurons were similar to those in BDNF-treated lesioned motoneurons (Fig. 4f). The application of the A2A-R antagonist ZM241385 did not enhance pS473-Akt immunoreactivity (Fig. 4d) in comparison with the unlesioned control side (Fig. 4c). These results indicate that Akt activation is involved in the actions of A2A-R agonists that mediate the survival of lesioned motoneurons after nerve lesioning in newborn mice.
Discussion
Facial nerve axotomy has been frequently used to demonstrate the ability of neurotrophic factors to promote the survival of motoneurons, and it has been a standard assay to verify the effects of ciliary neurotrophic factor (21), GDNF (25), and neurotrophins (22). However, the ability of small ligands that interact with GPCRs to transactivate trophic responses in motoneurons has never been directly tested in this model. Here we show that a well established A2A-R agonist, CGS21680, is capable of rescuing motoneurons from cell death. The survival effects were a consequence of transactivation of TrkB receptors in motoneurons. Motoneurons deficient in TrkB receptors were not responsive to adenosine transactivation. Moreover, increased Akt kinase activity as a result of TrkB signaling appeared responsible for promoting the survival of motoneurons when CGS21680 was used. Transactivation of Trk receptors by using adenosine receptor action therefore provides an alternative mechanism for stimulating trophic effects in the nervous system. The exact sequence of events that leads from the A2A-R to Trk activation is incompletely understood (16, 17). Transactivation is independent of neurotrophin binding but requires several intracellular events, including increased Src (v-src sarcoma [Schmidt-Ruppin A-2] viral oncogene homolog) family member activity such as Fyn (FYN oncogene related to SRC, FGR, YES) (26), mobilization of Ca2+ (15), and increases in biosynthetic machinery (17). In contrast with that of other tyrosine kinase receptors, transactivation of Trk receptors exhibits a delayed time course and is sustained over many hours and days. An unexpected feature of the transactivation by using CGS21680 is the intracellular site of activation of Trk receptors. Biotinylation and immunocytochemical experiments indicated that the majority of transactivated Trk receptors reside in intracellular membranes, which are found frequently associated with the Golgi complex (17, 26).
The sustained effects of adenosine upon survival signaling pathways may explain why neuronal losses in mice that lack functional TrkB (23) are higher in some populations of mice than in mice lacking BDNF and neurotrophin-4 (27). Furthermore, the transactivation of TrkB by using A2A-R signaling could explain why many populations of central neurons are unaffected by the lack of neurotrophins in knockout mouse experiments (1, 28), an effect that appears particularly striking for motoneurons. Neither BDNF, nor neurotrophin-4-knockout mice, nor the double-knockout mice show any reduction in motoneuron numbers (27, 29), in contrast with sensory neurons and other populations of peripheral neurons, which are severely affected. This effect may be the result of the ability of GPCR ligands to provide a survival function through TrkB neurotrophin receptor signaling.
Other activities such as synaptic plasticity may also be influenced by transactivation. Indeed, dopamine GPCR transactivation of platelet-derived growth factor receptors provided an acute effect upon NMDA ion channel activity (30), and CGS21680 can augment the effects of BDNF on hippocampal synaptic transmission (31).
A previous report indicated that activation of adenosine receptors also enhances vulnerability to glutamate toxicity (32). In mixed spinal cord cultures containing glia, inhibition of TrkB and adenosine receptors actually enhanced survival of motoneurons treated with kainic acid (33). This counterintuitive observation stands in contrast to many other observations that TrkB activation normally supports the survival of many central neurons, including motoneurons (22, 34). This discrepancy can be accounted for by the culture conditions and the state of synapse formation. When motoneurons are cultured with astrocytes, there is enhanced formation of synapses (35) and greater sensitivity of glutamatergic synapses to excitotoxicity, which can be enhanced when TrkB is activated. When motoneurons are cultured at a purity of > 90% and at low density (36), the formation of synapses or autapses between motoneurons is low, such that motoneuron survival is not affected by kainate or NMDA receptor activation (37). This result explains why activation of TrkB can support survival of purified motoneurons, whereas in mixed spinal cord cultures containing astrocytes, inhibition of TrkB or adenosine receptors resulted in a reduction of kainate-mediated toxicity (32, 33).
Adenosine has been proposed as a potential treatment for several neurological disorders, including Parkinson's disease, ischemia, and other neurodegenerative conditions (38–40). In contrast, neurotrophic factors have resulted in many difficulties in such clinical applications, in particular with respect to side effects and adverse pharmacokinetic properties such as problems in overcoming the blood–brain barrier (41). An alternative pathway of activating Trk receptor functions is possible through GPCR signaling. Our findings using the facial nerve axotomy model indicate that transactivation by small molecules is a promising approach to ameliorate neurodegeneration by targeting specific populations of neurons coexpressing A2A-R and Trk receptors.
The experimental results in this study provide a rationale for investigating other GPCR ligands for neuroprotection through transactivation. Because adenosine has demonstrated both beneficial and harmful effects in the cardiovascular and nervous systems, other molecules should be considered for further therapeutic development. The finding that pituitary adenylate cyclase-activating peptide also triggers transactivation of Trk receptors in basal forebrain neurons with a time course and pattern similar to those of adenosine (18) suggests that there are other ligands that may participate in this pathway. Furthermore, there is evidence from the cellular localization and signal transduction (42) and the genetics and pharmacology of GPCR transactivation that this process represents a pathway that is both physiologically important in the nervous system and promising for further therapeutic development.
Materials and Methods
Mouse Embryonic Motoneuron Cultures.
Cultures of spinal motoneurons from embryonic day 12.5 mice were prepared by using a panning technique with a monoclonal rat anti-p75NTR antibody (Abcam, Cambridge, U.K.) (43). TrkB mutant mice (23) were obtained through The Jackson Laboratory (Bar Harbor, ME). The ventrolateral parts of individual lumbar spinal cords were dissected and transferred to Hanks's balanced salt solution. After treatment with trypsin (0.05%, 10 min), cell suspensions were generated by trituration. The cells were plated on a rat anti-p75NTR-coated culture dish (24 well; Greiner, Nürtingen, Germany) and left at room temperature for 30 min. The individual wells were subsequently washed three times with Hanks's balanced salt solution, and the attaching cells were then isolated from the plate with depolarizing saline (0.8% NaCl, 35 mM KCl). Cells were plated at a density of 2,000 cells per cm2 in four-well culture dishes (Greiner) that were precoated with poly(dl-ornithine) and laminin as described (44). Cells were grown in neurobasal medium (Invitrogen, Karlsruhe, Germany) with B27 supplement, 2% horse serum, and 500 μM Glutamax (Invitrogen) at 37°C in a 5% CO2/95% air atmosphere. We have reduced the serum content to a minimum because serum also contains adenosine (45) and thus could interfere with the effects of A2A-R agonists. Eighty percent of the medium was first replaced at day 1 and then every second day. Motoneurons were treated with neurotrophic factors (1 ng/ml BDNF or 10 ng/ml GDNF) as indicated in the figures and treated additionally with either 5 μM ZM241385 or 5 μM CGS21680 (Sigma, Saint Louis, MO). To some cultures, an antiserum was added that was generated by immunization of a rabbit with recombinant BDNF. The specificity of the serum was tested by using Western blot analysis with recombinant neurotrophins and tissue extracts, and it was tested in cell cultures of chick and mouse primary neurons for its activity in inhibiting BDNF activity. At a dilution of 1:1,000, this serum completely blocked the effect of 10 ng/ml BDNF.
Facial Nerve Lesion.
All manipulations with living animals were approved by the local authorities at the University of Würzburg. One-day-old mice were anesthetized by hypothermia on ice, and then the right facial nerve was exposed as it exits from the foramen stylomastoideum and transected with microscissors. Then CGS21860 (1 μM), ZM241385 (1 μM), or BDNF (5 μg) was applied in a gel foam (Spongostan; Johnson & Johnson, Norderstedt, Germany). Wounds were sealed, and the animals recovered on a 37°C warm plate until they were given back to the mothers for either 6 h or 7 days. After 6 h, some of the animals were killed and the right and left facial nuclei were microdissected for immunoprecipitation experiments, and after 7 days, the brains of those animals were dissected for preparation of cryosections of the facial nuclei and immunohistochemical detection of phospho-Ser-473-Akt. Other animals were perfused with 4% paraformaldehyde at 7 days after the lesioning and then their brainstems were isolated and processed for preparation of 7-μm serial sections. These sections were Nissl stained for counting of motoneurons, applying established methods (21, 46). Motoneurons in the facial nuclei were counted in every fifth section as described in ref. 47.
Immunhistochemical Detection of Phospho-Ser-473-Akt.
Cryosections of the brain stems were fixed in ice-cold methanol for 10 min and then blocked with 5% BSA in Tris-buffered saline (TBS, pH 7.4), and the phospho-Ser-473-Akt antibody (Cell Signaling, Heidelberg, Germany) was incubated at a dilution of 1:200 in TBS/1% BSA overnight in a humidified chamber at room temperature. The sections were washed three times with TBS, and phospho-Ser-473Akt was detected with a goat anti-rabbit IgG Cy3-coupled antibody (20 ng/ml) in TBS/1% BSA. The sections were observed under a fluorescence microscope, and pictures were taken with a TCS4 Confocal microscope (Leica, Wetzlar, Germany). Embryonic motoneurons were fixed with 4% paraformaldehyde in PBS (pH 7.4) for 10 min at room temperature and subsequently processed further for immunohistochemistry as described earlier (36).
Immunoprecipitation and Western Blotting.
Dissected facial nuclei or cultured motoneurons (100,000 per well) were lysed, and the immunoprecipitation was performed with the immunoprecipitation catch-and-release kit from Biomol/Upstate (Hamburg, Germany) according to the supplier's instructions. In brief, the lysates were diluted with the lysis/wash buffer, 4 μg of TrkB antibody (Santa Cruz, Heidelberg, Germany) was added, and the solution was then added to the prewarmed and equilibrated column. After three washing steps, the proteins were eluted in 30 μl of elution buffer. The eluates were mixed with 1 vol of 2× reducing sample buffer, loaded on a 6% polyacrylamide gel, separated by electrophoresis, and transferred to a nitrocellulose membrane. The nitrocellulose membrane was used for Western blot detection with the TrkB antibody and a phosphotyrosine antibody (Cell Signaling, Hamburg, Germany) or a phospho-specific TrkB antibody (Tyr-706/707; Cell Signaling). Detection was performed with peroxidase-coupled secondary antibodies after ECL chemiluminescence detection. The signal intensities were measured with the AIDA software program (LaVision, Göttingen, Germany).
Statistical Analysis.
Results of neuronal counts were expressed as mean and SEM. Statistical significance was assessed by using a t test or ANOVA followed by Bonferonni's test, using the Prism software (GraphPad, San Diego, CA).
Acknowledgments
We are grateful to Jennifer Rath, Michaela Pfister, and Katrin Kuebert for technical assistance. This work was supported by the Deutsche Forschungsgemeinschaft, Grant Sonderforschungsbereich 487, C4 (to M.S.).
Abbreviations
- A2A-R
adenosine type 2A receptor
- CGS21680
2-p-(2-carboxyethylamine-5′-N-ethylcarboxamide)adenosine
- GDNF
glia-derived neurotrophic factor
- GPCR
G-protein-coupled receptor; ZM241385 4-(2-[7-amino-2-(2-furyl)[1,2,4]triazolo[2,3-a] [1,3,5]triazin-5-ylamino]ethyl)phenol.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Huang EJ, Reichardt LF. Annu Rev Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chao MV. Nat Rev Neurosci. 2003;4:299–309. doi: 10.1038/nrn1078. [DOI] [PubMed] [Google Scholar]
- 3.Teng KK, Hempstead BL. Cell Mol Life Sci. 2004;61:35–48. doi: 10.1007/s00018-003-3099-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang EJ, Reichardt LF. Annu Rev Biochem. 2003;72:609–642. doi: 10.1146/annurev.biochem.72.121801.161629. [DOI] [PubMed] [Google Scholar]
- 5.Hendry IA, Stach R, Herrup K. Brain Res. 1974;82:117–128. doi: 10.1016/0006-8993(74)90897-x. [DOI] [PubMed] [Google Scholar]
- 6.Distefano PS, Friedman B, Radziejewski C, Alexander C, Boland P, Schick CM, Lindsay RM, Wiegand SJ. Neuron. 1992;8:983–993. doi: 10.1016/0896-6273(92)90213-w. [DOI] [PubMed] [Google Scholar]
- 7.Bronfman FC, Tcherpakov M, Jovin TM, Fainzilber M. J Neurosci. 2003;23:3209–3220. doi: 10.1523/JNEUROSCI.23-08-03209.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Riccio A, Pierchala BA, Ciarallo CL, Ginty DD. Science. 1997;277:1097–1100. doi: 10.1126/science.277.5329.1097. [DOI] [PubMed] [Google Scholar]
- 9.Watson FL, Porcionatto MA, Bhattacharyya A, Stiles CD, Segal RA. J Neurobiol. 1999;39:323–336. doi: 10.1002/(sici)1097-4695(199905)39:2<323::aid-neu15>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 10.Watson FL, Heerssen HM, Bhattacharyya A, Klesse L, Lin MZ, Segal RA. Nat Neurosci. 2001;4:981–988. doi: 10.1038/nn720. [DOI] [PubMed] [Google Scholar]
- 11.York RD, Yao H, Dillon T, Ellig CL, Eckert SP, McCleskey EW, Stork PJ. Nature. 1998;392:622–626. doi: 10.1038/33451. [DOI] [PubMed] [Google Scholar]
- 12.Daub H, Weiss FU, Wallasch C, Ullrich A. Nature. 1996;379:557–560. doi: 10.1038/379557a0. [DOI] [PubMed] [Google Scholar]
- 13.Luttrell LM, Daaka Y, Lefkowitz RJ. Curr Opin Cell Biol. 1999;11:177–183. doi: 10.1016/s0955-0674(99)80023-4. [DOI] [PubMed] [Google Scholar]
- 14.Fischer OM, Hart S, Gschwind A, Ullrich A. Biochem Soc Trans. 2003;31:1203–1208. doi: 10.1042/bst0311203. [DOI] [PubMed] [Google Scholar]
- 15.Lee FS, Chao MV. Proc Natl Acad Sci USA. 2001;98:3555–3560. doi: 10.1073/pnas.061020198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee FS, Rajagopal R, Chao MV. Cytokine Growth Factor Rev. 2002;13:11–17. doi: 10.1016/s1359-6101(01)00024-7. [DOI] [PubMed] [Google Scholar]
- 17.Rajagopal R, Chen ZY, Lee FS, Chao MV. J Neurosci. 2004;24:6650–6658. doi: 10.1523/JNEUROSCI.0010-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee FS, Rajagopal R, Kim AH, Chang PC, Chao MV. J Biol Chem. 2002;277:9096–9102. doi: 10.1074/jbc.M107421200. [DOI] [PubMed] [Google Scholar]
- 19.Jarvis MF, Schulz R, Hutchison AJ, Do UH, Sills MA, Williams M. J Pharmacol Exp Ther. 1989;251:888–893. [PubMed] [Google Scholar]
- 20.Berg MM, Sternberg DW, Parada LF, Chao MV. J Biol Chem. 1992;267:13–16. [PubMed] [Google Scholar]
- 21.Sendtner M, Kreutzberg GW, Thoenen H. Nature. 1990;345:440–441. doi: 10.1038/345440a0. [DOI] [PubMed] [Google Scholar]
- 22.Sendtner M, Holtmann B, Kolbeck R, Thoenen H, Barde Y-A. Nature. 1992;360:757–758. doi: 10.1038/360757a0. [DOI] [PubMed] [Google Scholar]
- 23.Klein R, Smeyne RJ, Wurst W, Long LK, Auerbach BA, Joyner AL, Barbacid M. Cell. 1993;75:113–122. [PubMed] [Google Scholar]
- 24.Airaksinen MS, Saarma M. Nat Rev Neurosci. 2002;3:383–394. doi: 10.1038/nrn812. [DOI] [PubMed] [Google Scholar]
- 25.Yan Q, Matheson C, Lopez OT. Nature. 1995;373:341–344. doi: 10.1038/373341a0. [DOI] [PubMed] [Google Scholar]
- 26.Rajagopal R, Chao MV. Mol Cell Neurosci. 2006;33:36–46. doi: 10.1016/j.mcn.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 27.Liu X, Ernfors P, Wu H, Jaenisch R. Nature. 1995;375:238–240. doi: 10.1038/375238a0. [DOI] [PubMed] [Google Scholar]
- 28.Snider WD. Cell. 1994;77:627–638. doi: 10.1016/0092-8674(94)90048-5. [DOI] [PubMed] [Google Scholar]
- 29.Conover JC, Erickson JT, Katz DM, Bianchi LM, Poueymirou WT, McClain J, Pan L, Helgren M, Ip NY, Boland P, et al. Nature. 1995;375:235–238. doi: 10.1038/375235a0. [DOI] [PubMed] [Google Scholar]
- 30.Kotecha SA, Oak JN, Jackson MF, Perez Y, Orser BA, Van Tol HH, MacDonald JF. Neuron. 2002;35:1111–1122. doi: 10.1016/s0896-6273(02)00859-0. [DOI] [PubMed] [Google Scholar]
- 31.Diogenes MJ, Fernandes CC, Sebastiao AM, Ribeiro JA. J Neurosci. 2004;24:2905–2913. doi: 10.1523/JNEUROSCI.4454-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mojsilovic-Petrovic J, Arneja A, Kalb RG. Neurodegener Dis. 2005;2:160–165. doi: 10.1159/000089621. [DOI] [PubMed] [Google Scholar]
- 33.Mojsilovic-Petrovic J, Jeong GB, Crocker A, Arneja A, David S, Russell DS, Kalb RG. J Neurosci. 2006;26:9250–9263. doi: 10.1523/JNEUROSCI.1856-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Henderson CE, Camu W, Mettling C, Gouin A, Poulsen K, Karihaloo M, Rullamas J, Evans T, McMahon SB, et al. Nature. 1993;363:266–270. doi: 10.1038/363266a0. [DOI] [PubMed] [Google Scholar]
- 35.Ullian EM, Harris BT, Wu A, Chan JR, Barres BA. Mol Cell Neurosci. 2004;25:241–251. doi: 10.1016/j.mcn.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 36.Wiese S, Metzger F, Holtmann B, Sendtner M. Eur J Neurosci. 1999;11:1668–1676. doi: 10.1046/j.1460-9568.1999.00585.x. [DOI] [PubMed] [Google Scholar]
- 37.Metzger F, Wiese S, Sendtner M. J Neurosci. 1998;18:1735–1742. doi: 10.1523/JNEUROSCI.18-05-01735.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stone TW. Neurol Res. 2005;27:161–168. doi: 10.1179/016164105X21896. [DOI] [PubMed] [Google Scholar]
- 39.Ribeiro JA, Sebastiao AM, de Mendonca A. Drug News Perspect. 2003;16:80–86. doi: 10.1358/dnp.2003.16.2.740246. [DOI] [PubMed] [Google Scholar]
- 40.Fredholm BB, Cunha RA, Svenningsson P. Curr Top Med Chem. 2003;3:413–426. doi: 10.2174/1568026033392200. [DOI] [PubMed] [Google Scholar]
- 41.Thoenen H, Sendtner M. Nat Neurosci. 2002;5(Suppl):1046–1050. doi: 10.1038/nn938. [DOI] [PubMed] [Google Scholar]
- 42.Sorkin A. Sci STKE. 2005;2005:e1. doi: 10.1126/stke.2672005pe1. [DOI] [PubMed] [Google Scholar]
- 43.Wiese S, Pei G, Karch C, Troppmair J, Holtmann B, Rapp UR, Sendtner M. Nat Neurosci. 2001;4:137–142. doi: 10.1038/83960. [DOI] [PubMed] [Google Scholar]
- 44.Wiese S, Digby MR, Gunnersen JM, Götz R, Pei G, Holtmann B, Lowenthal J, Sendtner M. Nat Neurosci. 1999;2:978–983. doi: 10.1038/14777. [DOI] [PubMed] [Google Scholar]
- 45.Shipman C, Jr, Drach JC. Science. 1978;200:1163–1165. doi: 10.1126/science.206965. [DOI] [PubMed] [Google Scholar]
- 46.Masu Y, Wolf E, Holtmann B, Sendtner M, Brem G, Thoenen H. Nature. 1993;365:27–32. doi: 10.1038/365027a0. [DOI] [PubMed] [Google Scholar]
- 47.Sendtner M, Götz R, Holtmann B, Escary J-L, Masu Y, Carroll P, Wolf E, Brehm G, Brulet P, Thoenen H. Curr Biol. 1996;6:686–694. doi: 10.1016/s0960-9822(09)00450-3. [DOI] [PubMed] [Google Scholar]