Abstract
SUMOylation of transcription factors often attenuates transcription activity. This regulation of protein activity allows more diversity in the control of gene expression. Interferon regulatory factor-1 (IRF-1) was originally identified as a regulator of IFN-α/β, and its expression is induced by viral infection or IFN stimulation. Accumulating evidence supports the theory that IRF-1 functions as a tumor suppressor and represses the transformed phenotype. Here we report that the level of SUMOylated IRF-1 is elevated in tumors. Site-directed mutagenesis experiments disclose that the SUMOylation sites of IRF-1 are identical to the major ubiquitination sites. Consequently, SUMOylated IRF-1 displays enhanced resistance to degradation. SUMOylation of IRF-1 attenuates its transcription activity, and SUMOylated IRF-1 inhibits apoptosis by repression of its transcriptional activity. These data support a mechanism whereby SUMOylation of IRF-1 inactivates its tumor suppressor function, which facilitates resistance to the immune response.
Keywords: tumor suppressor, Ubc9, SENP1
SUMOylation is a posttranslation modification, in which the SUMO moiety (known as SMT3 in yeast) is attached to the lysine residues of target proteins. Although SUMOylation shares many common features with ubiquitination, its role in cellular metabolism is diverse (1). Ubiquitination is generally, but not always, involved in proteasomal protein degradation, whereas SUMOylation affects numerous processes, including subcellular localization, modulation of transcriptional activity, and enhanced protein stability (1, 2). A striking characteristic is that a small fraction of the substrate is SUMOylated at any given time, but SUMOylation alters the long-term fate of the modified protein after rapid de-SUMOylation (3). Over the past few years, several studies have focused on the role of SUMOylation in tumorigenesis (4). Recent reports indicate that Ubc9, the single SUMO E2 ligase catalyzing the conjugation of SUMO to target protein, is overexpressed in ovarian cancer (5), and the SUMOylation status of reptin modulates the invasive activity of cancer cells with metastatic potential (6). However, at present, limited information is available on the relationship between SUMOylated proteins and tumors.
Interferon regulatory factor-1 (IRF-1) was originally identified as a regulator of IFNα/β. IRF-1 expression is dramatically up-regulated on viral infection and stimulation by the IFN family, including IFNα, IFNβ, IFNγ, and TNFα (7). Accumulating evidence supports the theory that IRF-1 functions as a tumor suppressor (8–11) and represses the transformed phenotype (10, 12–14). In human tumors, IRF-1 is inactivated to prevent apoptosis and cell cycle arrest by genetic mechanisms, such as gene deletion and exon skipping (11, 15–17). IRF-1 is a substrate of both ubiquitination and SUMOylation (18, 19). In the present work, we screened the SUMOylated proteins in tumor cells and found that the level of SUMOylated IRF-1 is significantly increased. We propose that the elevated level of SUMOylated IRF-1 in tumor cells interferes with IRF-1-mediated apoptosis.
Results
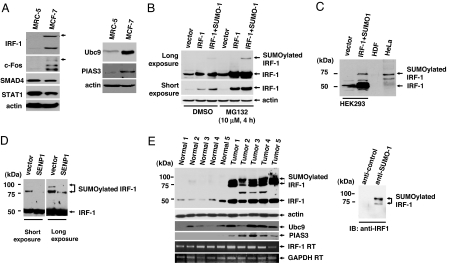
SUMOylation often affects subcellular localization, stability, and transcriptional activity of target proteins (2, 3), and it is proposed that specific SUMOylated proteins exist in tumor cells to enhance tumorigenicity. As shown previously, SUMOylated proteins migrate slowly because of the attached moiety, and are thus readily detected by immunoblot analysis. To identify the SUMOylated proteins in tumors, a series of immunoblots were performed with cell lysates extracted from MRC-5 (control) and MCF-7 (tumor sample). Because the SUMO isopeptidase is capable of readily cleaving the isopeptide bond between SUMO and the target protein, cell lysates were carefully prepared by direct boiling to inactivate the enzymes (20). Among several signaling molecules, IRF-1 displayed a shifted band in tumor samples in an anti-IRF-1 immunoblot (Fig. 1A Left). The specific modifications of IRF-1 in tumors were further confirmed by immunoblotting with other normal and tumor cell lines (data not shown). Ectopic coexpression of IRF-1 and SUMO-1 induced SUMOylation of IRF-1 in HEK293 cells (Fig. 1B). The SUMOylated IRF-1 protein comigrates with a 75-kDa band found in HeLa tumor cells (Fig. 1C). Our findings were further supported by immunoprecipitation assays with the anti-SUMO-1 antibody. MCF7 cell lysates were immunopurified with the anti-SUMO-1 antibody, and then the immunoprecipitates were probed with anti-IRF-1 antibody. Analysis of immunoprecipitates confirmed that IRF-1 is SUMOylated in tumor cells [supporting information (SI) Fig. 5]. Ectopic expression of SENP1, an SUMO isopeptidase, resulted in a dramatic reduction in shifted SUMO-IRF-1 bands. In addition, longer exposure of control HeLa cells led to multiple bands, and the level of modified forms were significantly decreased upon SENP1 expression, suggesting that IRF-1 is modified by SUMO-1 at multiple positions in tumor cells (Fig. 1D). These results were confirmed by findings from tumor tissues. It is important to note that SUMOylated proteins can be easily de-SUMOylated until inactivation of the SUMO isopeptidase by boiling. Because of the large number of steps required to homogenize tumor tissue, it was difficult to obtain clear shifted bands as obtained with the tumor cell lines. However, upon longer exposure, five of five ovarian tumors contained the shifted SUMOylated forms of IRF-1 (Fig. 1E Left), whereas normal ovarian tissues expressed low amounts of IRF-1 protein (Fig. 1E Left). To confirm that the shifted bands observed in ovarian tumor tissue lysates were SUMOylated forms of IRF-1, tumor cell lysates were immunopurified with anti-SUMO-1 antibody, followed by immunoblot analysis with anti-IRF-1 antibody (Fig. 1E Right).
Fig. 1.
SUMOylation of IRF-1 in cell lines and tumors. (A) MRC-5 and MCF-7 cells were lysed with SDS-loading buffer, and equal amounts of cell lysates were immunoblotted with the indicated antibodies. (B) IRF-1 is SUMOylated after coexpression with SUMO-1. HEK293 cells were transiently transfected with plasmids encoding IRF-1 and FLAG-SUMO-1. Treatment with the proteasomal inhibitor, MG132, blocked IRF-1 degradation. (C) The size of the shifted band in HeLa cells was similar to that of SUMOylated IRF-1. The SUMOylated IRF-1 band in HEK293 was used as a positive control. (D) SENP1 cleaved the bond between IRF-1 and SUMO-1. HeLa cells were transiently transfected with a control vector or the plasmid-encoding SENP1. (E) IRF-1 is highly SUMOylated in ovarian tumors. Western blot analysis was performed by using the indicated antibodies (Left). The star indicates a nonspecific band. RT-PCR results displayed that the amount of IRF-1 mRNA was not significantly changed between the normal cells and the tumor. Ovarian tumor cell lysates were immunopurified with either anti-SUMO-1or control antibody and then incubated with anti-IRF-1 antibody (Right).
Among components of SUMOylation machinery, Ubc9 is the sole SUMO E2 conjugation enzyme, and PIAS3 is known to be the E3 enzyme for IRF-1 SUMOylation (19). Recently, it has been reported that the levels of Ubc9 and PIAS3 were generally increased in tumor cells (5, 21). Because IRF-1 is extensively conjugated with SUMO-1, we examined the expression levels of Ubc9 and PIAS3 in tumor cells. The levels of Ubc9 and PIAS3 are elevated in the MCF7 cell line, compared with MRC5 (Fig. 1A Right). Consistent with recent findings by other investigators, we also detected the elevated levels of Ubc9 and PIAS3 in tumors (Fig. 1E).
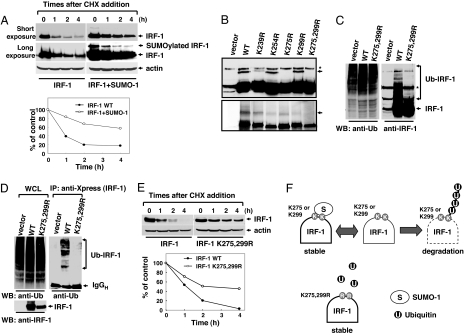
To exclude the possibility of a transcriptional increase of IRF-1 mRNA in tumor tissues, the IRF-1 mRNA level was examined by RT-PCR analysis. There was no significant difference between normal and tumor tissues (Fig. 1E), indicating that the increased expression of IRF-1 protein in tumor is independent of transcriptional control. Protein stability is often regulated by the attachment of SUMO or ubiquitin (22). Because up-regulation of IRF-1 protein is accompanied by its SUMOylation, we assumed that the level of IRF-1 protein in tumor correlates with its SUMOylation. To test this hypothesis, we examined whether SUMOylation is involved in IRF-1 protein stability. Ectopic coexpression of SUMO-1 and IRF-1 induced SUMOylation. Treatment with the proteasomal inhibitor, MG132, induced a dramatic increase in the IRF-1 protein level, suggesting that IRF-1 is primarily removed by proteasomal degradation (Fig. 1B). Next, we examined the role of SUMOylation of IRF-1 protein stability under conditions in which synthesis was blocked by cycloheximide (18). Coexpression of SUMO-1 conferred IRF protein more resistance to degradation, demonstrating that SUMOylation governs protein stability (Fig. 2A).
Fig. 2.
IRF-1 is stabilized by SUMOylation. (A) SUMOylated IRF-1 displayed more resistance to protein degradation. HEK293 cells were transiently transfected with IRF-1 in the presence or absence of SUMO-1 and treated with 20 μM cycloheximide for the indicated periods. The stability of IRF-1 alone (filled circles) and IRF-1 with the coexpression of SUMO-1 (open circles) was evaluated under conditions in which synthesis was blocked. (B) Generation of SUMO-deficient IRF-1 mutants. Cells were transfected with IRF-1, IRF-1 K254R, IRF-1 K275R, IRF-1 K299R, and IRF-1 K275,299R with SUMO-1 (Upper). The arrow specifies SUMOylated IRF-1. These constructs were used for the in vitro SUMOylation assay (Lower). The star indicates a nonspecific band. (C) SUMO and ubiquitin target the same lysine residues of IRF-1. HEK293 cells were transiently transfected with IRF-1 or IRF-1 (K275,299R) and treated with 10 μM MG132 for 12 h. Cell lysates were subjected to immunoblotting with antiubiquitin (Left) and anti-IRF-1 antibodies (Right). (D) Cells were transfected with plasmids encoding Xpress-tagged IRF-1 or Xpress-tagged IRF-1 mutant (K275,299R). The proteins were immunoprecipitated with anti-Xpress antibody and then immunoblotted with antiubiquitin antibodies (Right). Whole-cell lysates (WCL) were subjected to immunoblotting with antiubiquitin (Left) and anti-IRF-1 antibodies (Right). (E) The IRF-1 mutant (K275,299R) displayed greater resistance to protein degradation. The stability of wild-type IRF-1 (filled circles) and IRF-1 mutant (K275,299R) (open circles) was evaluated. (F) Schematic model showing antagonistic effects of SUMO and ubiquitin on IRF-1 protein stability.
To provide further evidence for the role of SUMOylation on IRF-1 stability, we determined the SUMOylated sites on IRF-1. An in vitro SUMOylation assay was performed with IRF-1 splicing variants lacking some combination of exons 7, 8, and 9 (SI Fig. 6). SUMOylation sites were mapped within the C-terminal domain (SI Fig. 6), and K275 was identified as the major in vivo SUMOylation target by site-directed mutagenesis of lysine residues in the C-terminal domain (Fig. 2B). The IRF-1 double mutant (K275,299R) was not SUMOylated in vitro and in vivo (Fig. 2B). Because SUMO often competes with ubiquitin for the same lysine residues, we examined whether the ubiquitination and SUMOylation sites in IRF-1 overlap. An in vivo ubiquitination assay disclosed significantly diminished ubiquitination of IRF-1 in SUMO-deficient mutants (Fig. 2 C and D), and the coexpression of IRF-1 with SUMO-1 decreased the level of ubiquitinated IRF-1 (SI Fig. 7). The data suggest that the major ubiquitination and SUMOylation sites are identical. Finally, we determined the stability of wild-type and mutant IRF-1 proteins. The SUMO-deficient mutant (K275,299R) displayed higher resistance to degradation, indicating that both these lysine residues control the stability of IRF-1 (Fig. 2 E and F). Taken together, our results suggest that SUMOylation of IRF-1 contributes to the enhanced protein stability in tumors.
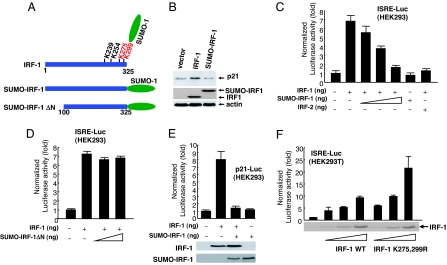
To clarify the role of IRF-1 SUMOylation in tumors, we fused SUMO-1 to the C terminus of IRF-1 (23, 24) (Fig. 3A). The chimeric protein was used as a permanently SUMOylated form of IRF-1, designated SUMO-IRF-1 (SUMOylated IRF-1). Because SUMOylation often changes the subcellular localization and transcriptional activity (1), we compared the localization and transcriptional activity of SUMO-IRF-1 with that of IRF-1 protein. IRF-1 is involved in the transcriptional induction of cell cycle inhibitor, p21 (WAF/CIP), by positively regulating the p21 promoter (25). Although the SUMO moiety did not affect the subcellular localization of IRF-1 (data not shown), p21 induction was absent in SUMO-IRF-1-expressing cells, manifesting that transcriptional activity of IRF-1 is lost in the SUMOylated form (Fig. 3B).
Fig. 3.
Inhibition of IRF-1-dependent transcription by SUMOylated IRF-1. (A) Schematic diagram of SUMO-IRF-1 and SUMO-IRF-1 ΔN. K275 and the major SUMOylation site of IRF-1 at K299. (B) SUMO-IRF-1 does not induce p21 transcription. HEK293 cells were transiently transfected with IRF-1 and SUMO-IRF1. (C) Transcription of ISRE fused to a Luc gene (ISRE-Luc) was inhibited by SUMO-IRF-1 in the presence of IRF-1 in HEK293 cells. HEK293 cells were cotransfected with 300 ng of ISRE-Luc, 300 ng of an IRF-1 expression plasmid (pcDNA3/IRF-1), and increasing concentrations of plasmid-encoding SUMO-IRF-1 (pcDNA3/SUMO-IRF-1) (10, 100, and 300 ng). IRF-2 was used as a positive control. (D) SUMO-IRF-1 ΔN does not repress transcriptional activation by IRF-1. (E) The human p21 promoter was inhibited by SUMO-IRF-1 in the presence of IRF-1. (F) The SUMOylation-deficient mutant (K275,299R) of IRF-1 increased transcriptional activity. HEK293T cells were transfected with 200 ng of ISRE-Luc and increasing concentrations of plasmid encoding IRF-1 or IRF-1 mutant (5, 10, and 25 ng) (Upper). Cell lysates were probed with anti-IRF-1 antibodies (Lower) to verify the level of IRF-1 proteins.
Using the reporter assay, we examined whether SUMO modification of IRF-1 affects IRF-1-mediated transcriptional activity. As expected, SUMO-IRF-1 repressed IRF-1-induced transcriptional activation from both interferon stimulated response element (ISRE) and p21 promoters in a dose-dependent manner (Fig. 3 C and E), in contrast to the SUMO-IRF-1 mutant lacking the DNA-binding domain (SUMO-IRF-1 ΔN) (Fig. 3D). Moreover, SUMO-IRF-1 did not repress p53-induced transcriptional activation, indicating that the antagonistic function is specific for IRF-1 (SI Fig. 8). Furthermore, a SUMOylation-deficient mutant (K275,299R) of IRF-1 is more active than wild-type IRF-1 in terms of activating transcription ISRE-luciferase (Luc) and p21-Luc reporters (Fig. 3F and SI Fig. 8). Our results suggest that SUMOylation of IRF-1 suppresses its transcriptional activity.
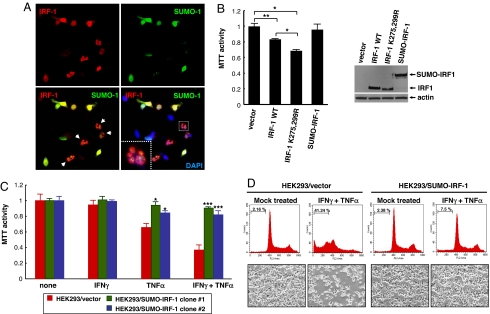
Because IRF-1 belongs to the tumor suppressor family, its expression induces cell death in some cell types (10, 26, 27). To address whether the tumor suppressor function of IRF-1 depends on its SUMOylation (28), we examined whether the SUMOylated protein inhibits IRF-1-mediated cell death. HEK293 cells were transiently transfected with plasmids encoding IRF-1 together with SUMO-1. HEK293 cells expressing high levels of IRF-1 protein and low levels of SUMO-1 induced cell blebbing, a typical phenotype of IRF-1-mediated apoptosis (indicated by arrow heads), whereas cells expressing high levels of SUMO-1 led to inhibition of IRF-1-mediated apoptosis (Fig. 4A). These findings suggest that SUMOylation of IRF-1 attenuates its tumor suppressive function. Next, we evaluated the apoptotic activity of IRF-1 and IRF-1 mutant (K275,299R). Although SUMO-IRF-1 does not change the cell proliferation, the SUMOylation-deficient mutant (K275,299R) of IRF-1 increased the apoptotic activity compared with wild-type IRF-1 (Fig. 4B), indicating that SUMOylation of IRF-1 inhibits its apoptotic activity.
Fig. 4.
(A) Coexpression with SUMO-1 attenuates IRF-1-induced cell death. HEK293 cells were transiently transfected with plasmids encoding IRF-1 and GFP-SUMO-1. At 36 h after transfection, cells were fixed and immunostained with anti-IRF-1 antibody. Whereas IRF-1-expressing cells displayed apoptosis, coexpression of SUMO-1 blocked cell death. The level of SUMO expression in blebbing cells is low. (B) The SUMOylation-deficient mutant (K275,299R) of IRF-1 has increased apoptotic activity. HEK293 cells were transiently transfected with wild-type IRF-1, IRF-1 mutant (K275,299R), or SUMO-IRF-1. Thirty-six hours after transfection, cell viability was assessed by MTT assay. (C) SUMO-IRF-1 interferes with cytokine-induced apoptosis. HEK293/vector and HEK293/SUMO-IRF-1 cells were treated with a combination of 100 units/ml IFNγ and 10 ng/ml TNFα, and cell viability was assessed by MTT assays after treatment with cytokines for 48 h. (D) Flow-cytometric analysis of cytokine-treated cells. SUMO-IRF-1-expressing and control cells were stained with propidium iodide and analyzed by flow cytometry. Images were obtained for the control treated with cytokine, which displayed a high percentage of subG1 population (Upper) and cell morphology (Lower). HEK293/vector versus HEK293/SUMO-IRF-1. *, P < 0.01; **, P < 0.05; ***, P < 0.001.
Earlier reports show that treatment with IFNγ and TNFα triggers IRF-1 expression, which in turn mediates cell death (29). We examined whether SUMO-IRF-1 interferes with IRF-1-induced cell death. We established HEK293 cell lines stably expressing SUMO-IRF-1, and we treated the combination of 100 units/ml IFNγ and 10 ng/ml TNFα. Treatment of IFNγ and TNFα induced IRF-1 expression in both control cells and SUMO-IRF-1-expressing cells and did not change the level of SUMO-IRF-1 (data not shown). Forty-eight hours after cytokine treatment, cell proliferation was measured by both 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay and flow-cytometric analysis. Although IFNγ and TNFα stimulated dramatic apoptosis in HEK293 control cells, SUMO-IRF-1-expressing cells failed to induce apoptosis by cytokine treatment (Fig. 4C). Flow-cytometry analysis using propidium iodide staining revealed a typical apoptotic pattern. The sub-G1 population of cells with fragmented DNA was 41.24% for control cells and 7.5% for SUMO-IRF-1-expressing cells after cytokine treatment (Fig. 4D). Taken together, our results are consistent with our hypothesis that SUMOylation inhibits IRF-1-mediated apoptosis.
Discussion
Here we demonstrate that the level of SUMOylated IRF-1 is elevated in tumor cell lines and tumor tissues. Site-directed mutagenesis experiments demonstrate that SUMOylation and ubiquitination sites overlap, and SUMOylated IRF-1 displays enhanced resistance to degradation. The SUMOylated protein represses IRF-1-mediated transcriptional activation and apoptosis. The SUMOylation sites of IRF-1 were determined as two lysines, K275 and K299. Our in vivo findings reveal that lysine at position 275 appears to be the major SUMOylation site, and this residue lies within the SUMOylation consensus sequence (CKEE/ψKXE). Coexpression of IRF-1 and SUMO-1 in HEK293 cells resulted in a single SUMOylated band. SUMOylation of IRF-1 under these conditions was completely suppressed upon the introduction of a K275R mutation. However, IRF-1 appears to be SUMOylated at multiple positions in tumors because several tumor-specific SUMOylated bands were detected and de-SUMOylated by SENP1. Mutant analyses confirmed that two lysines, K275 and K299, are involved in SUMO ylation of IRF-1. However, it is possible that other lysines provide acceptor sites for additional SUMOylation.
The level of SUMOylated IRF-1 is relatively high compared with other SUMOylated molecules (3). However, our immunoblot data with tumor cell lysates indicate that the remaining IRF-1 is unmodified in tumors. Our experience with other SUMOylated proteins suggests that the SUMOylation level of a given protein is dependent on the sample preparation protocol, thus special care is required to obtain intact SUMOylated proteins. Immunoblotting with tumor samples revealed that a fraction of IRF-1 is SUMOylated in tumors. However, it is possible that IRF-1 can be partially de-SUMOylated during sample preparation, and completely SUMOylated IRF-1 is not detected in tumors because of technical problems. Another explanation for unmodified IRF-1 in tumors is that SUMOylation alters the long-term fate of the modified protein after rapid de-SUMOylation (3). Moreover, because it is quite difficult to SUMOylate a single protein of interest in vivo, SUMO-fused IRF-1 (SUMO-IRF-1) was prepared to demonstrate the role of SUMOylated IRF-1 on IRF-1-mediated transcriptional activity. SUMO-IRF-1 lost the transcriptional activity (Fig. 3 B and C). This approach facilitates the elucidation of the role of SUMOylated IRF-1 (23, 24). However, the SUMOylation process is reversible and dynamic, and the SUMO fusion protein does not behave exactly the same as a native SUMOylated protein. To further clarify the function of SUMOylated IRF-1, the SUMOylation-deficient mutant (K25,299R) also was used to support the functional inactivation of IRF-1 tumor suppressor by SUMOylation. Further investigation is required for a better understanding of SUMOylated IRF-1.
SUMOylation of IRF-1 can enhance the tumor cell evasion of the immune system. It is known that IRF-1 is inactivated in some tumors to prevent apoptosis and cell cycle arrest by genetic mechanisms, such as gene deletion and exon skipping (11, 15–17). We demonstrate that SUMOylated IRF-1 inhibits cytokine-mediated apoptosis by competing with unmodified IRF-1. Thus, SUMOylation of IRF-1 attenuates the tumor suppressor function that facilitates resistance to the immune response, such as that during cytokine treatment. Further investigation is required to elucidate the role of SUMOylated IRF-1 in tumorigenesis, cancer progression, or drug resistance in clinical situations.
Several recent studies have reported the relationship between SUMOylation and human diseases (23, 30). However, little is known about SUMOylation in cancer. Screening of signaling proteins that are preferentially SUMOylated in tumors led us to identify a tumor suppressor protein that is extensively SUMOylated. Other signaling molecules preferentially SUMOylated in tumors possibly exist. Further studies are required to identify other SUMOylated proteins in tumors and define their roles in tumorigenesis.
Materials and Methods
Cell Culture and Cell Proliferation Assay.
MRC-5, HDF, MCF-7, and HeLa cells were grown in DMEM supplemented with 10% FBS. Transfection of HeLa and HEK293 cells was performed by using Fugene 6 (Roche Diagnostics, Indianapolis, IN). Cell growth was measured by using the MTT assay according to the method described in ref. 31. Cells were seeded onto 24-well plate and then treated with specific cytokines for the indicated time periods. Human IFNγ and TNFα were purchased from Sigma–Aldrich (St. Louis, MO).
Plasmid Construction.
To generate SUMO-IRF-1 chimeric protein, amplified SUMO-1 cDNA was cleaved with XhoI and XbaI and subcloned into pcDNA3. IRF-1 cDNA was subcloned into the EcoRI and XhoI sites of pcDNA3/SUMO-1 and designated as pcDNA3/SUMO-IRF-1. Point mutations of IRF-1 were generated by using the QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA), and each mutant was completely sequenced to verify the mutation of the intended sequences. The reporter plasmid, ISRE-Luc, and p21-Luc were kindly provided by J. Choe (Korea Advanced Institute of Science and Technology, Daejeon, Korea).
Immunoblotting and Immunoprecipitation.
For immunoblotting, cells were harvested and resuspended in lysis buffer [150 mM NaCl, 50 mM Hepes (pH 8.0), 0.5% Nonidet P-40] containing a protease inhibitor mixture (Roche Diagnostics). Immunoblot detection was performed with a 1:1,000 or 1:2,000 dilution of primary antibody and an ECL system (Amersham Biosciences, Chicago, IL). To detect the SUMOylated protein, cells were washed with PBS and directly lysed with SDS-loading buffer [100 mM Tris·HCl (pH 6.8), 20% glycerol, 4% SDS, 0.001% bromophenol blue] supplemented with 20 μM N-ethylmaleimide (NEM; Sigma–Aldrich), which is an inhibitor of desumoylation enzymes. Finally, cell lysates were boiled for 5 min, centrifuged for 10 min at 15,700 × g, and analyzed by SDS/PAGE. To confirm the SUMOylated IRF-1 protein, cells were washed with cold PBS supplemented with 20 μM NEM and lysed by boiling for 5 min in 150 mM Tris·HCl (pH 6.7) buffer containing 5% SDS, 30% glycerol, and 20 μM NEM. Total lysates were diluted 20-fold with lysis buffer containing NEM and a complete protease inhibitor mixture, incubated with anti-SUMO-1 antibody or anti-IgG control antibody, and then immunoprecipitated with protein A-agarose (Peptron, Daejeon, South Korea). Immune complexes were washed three times with lysis buffer containing NEM and then subjected to SDS/PAGE analysis, followed by immunoblotting with anti-IRF-1 antibody. Antibodies for IRF-1, ubiquitin, and SUMO-1 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA) and Cell Signaling Technology (Danvers, MA). Ovarian tumor and normal ovary tissues were obtained from Samsung Medical Center tumor tissue bank with the approval of the Institutional Review Board.
Immunofluorescence and Confocal Microscopy.
Cells were grown on sterilized glass coverslips, fixed with 4% paraformaldehyde, and blocked with 0.1% BSA in PBS. Cells were incubated with 1:500 diluted primary antibody in PBS and reacted with 1:5,000 diluted Alexa 488- or Alexa 568-conjugated secondary antibody (Vector Laboratories, Burlingame, CA). Finally, slides were washed three times with PBS and mounted in mounting media (Vector Laboratories). Images were captured with a confocal microscope (Bio-Rad, Hercules, CA).
In Vitro SUMOylation Reaction.
SAE1/SAE2, Ubc9, and SUMO-1 were purchased from LAE Biotech (Rockville, MD), and the in vitro conjugation reaction was performed with in vitro-translated IRF-1 according to the manufacturer's instructions.
Supplementary Material
Acknowledgments
We thank J. Choe (Korea Advanced Institute of Science and Technology, Daejeon, Korea) and J. U. Jung (Harvard University, Cambridge, MA) for helpful discussions on the manuscript. This work was supported by an SRC grant from the Korea Science and Engineering Foundation.
Abbreviations
- IRF-1
interferon regulatory factor-1
- ISRE
interferon stimulated response element
- Luc
luciferase
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide
- NEM
N-ethylmaleimide.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0609852104/DC1.
References
- 1.Seeler JS, Dejean A. Nat Rev Mol Cell Biol. 2003;4:690–699. doi: 10.1038/nrm1200. [DOI] [PubMed] [Google Scholar]
- 2.Verger A, Perdomo J, Crossley M. EMBO Rep. 2003;4:137–142. doi: 10.1038/sj.embor.embor738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hay RT. Mol Cell. 2005;18:1–12. doi: 10.1016/j.molcel.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 4.Alarcon-Vargas D, Ronai Z. Cancer Biol Ther. 2002;1:237–242. doi: 10.4161/cbt.74. [DOI] [PubMed] [Google Scholar]
- 5.Mo YY, Yu Y, Theodosiou E, Rachel Ee PL, Beck WT. Oncogene. 2005;24:2677–2683. doi: 10.1038/sj.onc.1208210. [DOI] [PubMed] [Google Scholar]
- 6.Kim JH, Choi HJ, Kim B, Kim MH, Lee JM, Kim IS, Lee MH, Choi SJ, Kim KI, Kim SI, et al. Nat Cell Biol. 2006;8:631–639. doi: 10.1038/ncb1415. [DOI] [PubMed] [Google Scholar]
- 7.Taniguchi T, Ogasawara K, Takaoka A, Tanaka N. Annu Rev Immunol. 2001;19:623–655. doi: 10.1146/annurev.immunol.19.1.623. [DOI] [PubMed] [Google Scholar]
- 8.Moriyama Y, Nishiguchi S, Tamori A, Koh N, Yano Y, Kubo S, Hirohashi K, Otani S. Clin Cancer Res. 2001;7:1293–1298. [PubMed] [Google Scholar]
- 9.Lengyel P. Proc Natl Acad Sci USA. 1993;90:5893–5895. doi: 10.1073/pnas.90.13.5893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tanaka N, Ishihara M, Kitagawa M, Harada H, Kimura T, Matsuyama T, Lamphier MS, Aizawa S, Mak TW, Taniguchi T. Cell. 1994;77:829–839. doi: 10.1016/0092-8674(94)90132-5. [DOI] [PubMed] [Google Scholar]
- 11.Taniguchi T, Lamphier MS, Tanaka N. Biochim Biophys Acta. 1997;1333:M9–M17. doi: 10.1016/s0304-419x(97)00014-0. [DOI] [PubMed] [Google Scholar]
- 12.Harada H, Kitagawa M, Tanaka N, Yamamoto H, Harada K, Ishihara M, Taniguchi T. Science. 1993;259:971–974. doi: 10.1126/science.8438157. [DOI] [PubMed] [Google Scholar]
- 13.Tanaka N, Ishihara M, Taniguchi T. Cancer Lett. 1994;83:191–196. doi: 10.1016/0304-3835(94)90318-2. [DOI] [PubMed] [Google Scholar]
- 14.Pizzoferrato E, Liu Y, Gambotto A, Armstrong MJ, Stang MT, Gooding WE, Alber SM, Shand SH, Watkins SC, Storkus WJ, Yim JH. Cancer Res. 2004;64:8381–8388. doi: 10.1158/0008-5472.CAN-04-2223. [DOI] [PubMed] [Google Scholar]
- 15.Willman CL, Sever CE, Pallavicini MG, Harada H, Tanaka N, Slovak ML, Yamamoto H, Harada K, Meeker TC, List AF, et al. Science. 1993;259:968–971. doi: 10.1126/science.8438156. [DOI] [PubMed] [Google Scholar]
- 16.Harada H, Kondo T, Ogawa S, Tamura T, Kitagawa M, Tanaka N, Lamphier MS, Hirai H, Taniguchi T. Oncogene. 1994;9:3313–3320. [PubMed] [Google Scholar]
- 17.Lee EJ, Jo M, Park J, Zhang W, Lee JH. Biochem Biophys Res Commun. 2006;347:882–888. doi: 10.1016/j.bbrc.2006.06.145. [DOI] [PubMed] [Google Scholar]
- 18.Nakagawa K, Yokosawa H. Eur J Biochem. 2000;267:1680–1686. doi: 10.1046/j.1432-1327.2000.01163.x. [DOI] [PubMed] [Google Scholar]
- 19.Nakagawa K, Yokosawa H. FEBS Lett. 2002;530:204–208. doi: 10.1016/s0014-5793(02)03486-5. [DOI] [PubMed] [Google Scholar]
- 20.Park J, Seo T, Kim H, Choe J. Mol Cell Biol. 2005;25:8202–8214. doi: 10.1128/MCB.25.18.8202-8214.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang L, Banerjee S. Oncol Rep. 2004;11:1319–1324. [PubMed] [Google Scholar]
- 22.Ulrich HD. Trends Cell Biol. 2005;15:525–532. doi: 10.1016/j.tcb.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 23.Steffan JS, Agrawal N, Pallos J, Rockabrand E, Trotman LC, Slepko N, Illes K, Lukacsovich T, Zhu YZ, Cattaneo E, et al. Science. 2004;304:100–104. doi: 10.1126/science.1092194. [DOI] [PubMed] [Google Scholar]
- 24.Ross S, Best JL, Zon LI, Gill G. Mol Cell. 2002;10:831–842. doi: 10.1016/s1097-2765(02)00682-2. [DOI] [PubMed] [Google Scholar]
- 25.Tanaka N, Ishihara M, Lamphier MS, Nozawa H, Matsuyama T, Mak TW, Aizawa S, Tokino T, Oren M, Taniguchi T. Nature. 1996;382:816–818. doi: 10.1038/382816a0. [DOI] [PubMed] [Google Scholar]
- 26.Horiuchi M, Yamada T, Hayashida W, Dzau VJ. J Biol Chem. 1997;272:11952–11958. doi: 10.1074/jbc.272.18.11952. [DOI] [PubMed] [Google Scholar]
- 27.Kirchhoff S, Hauser H. Oncogene. 1999;18:3725–3736. doi: 10.1038/sj.onc.1202704. [DOI] [PubMed] [Google Scholar]
- 28.Kirchhoff S, Schaper F, Hauser H. Nucleic Acids Res. 1993;21:2881–2889. doi: 10.1093/nar/21.12.2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suk K, Chang I, Kim YH, Kim S, Kim JY, Kim H, Lee MS. J Biol Chem. 2001;276:13153–13159. doi: 10.1074/jbc.M007646200. [DOI] [PubMed] [Google Scholar]
- 30.Li M, Guo D, Isales CM, Eizirik DL, Atkinson M, She JX, Wang CY. J Mol Med. 2005;83:504–513. doi: 10.1007/s00109-005-0645-5. [DOI] [PubMed] [Google Scholar]
- 31.Park K, Kim K, Rho SB, Choi K, Kim D, Oh SH, Park J, Lee SH, Lee JH. Cancer Res. 2005;65:749–757. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.