Abstract
Matrix metalloproteinases (MMPs) are of central importance in the proteolytic remodeling of matrix and the generation of biologically active molecules. MMPs are distinguished by a conserved catalytic domain containing a zinc ion, as well as a prodomain that regulates enzyme activation by modulation of a cysteine residue within that domain. Because nitric oxide (NO) and derived reactive nitrogen species target zinc ions and cysteine thiols, we assessed the ability of NO to regulate MMPs. A dose-dependent, biphasic regulatory effect of NO on the activity of MMPs (MMP-9, -1, and -13) secreted from murine macrophages was observed. Low exogenous NO perturbed MMP/tissue inhibitor of metalloproteinase (TIMP)-1 levels by enhancing MMP activity and suppressing the endogenous inhibitor TIMP-1. This was cGMP-dependent, as confirmed by the cGMP analog 8-bromo-cGMP, as well as by the NO–soluble guanylyl cyclase–cGMP signaling inhibitor thrombospondin-1. Exposure of purified latent MMP-9 to exogenous NO demonstrated a concentration-dependent activation and inactivation of the enzyme, which occurred at higher NO flux. These chemical reactions occurred at concentrations similar to that of activated macrophages. Importantly, these results suggest that NO regulation of MMP-9 secreted from macrophages may occur chemically by reactive nitrogen species-mediated protein modification, biologically through soluble guanylyl-cyclase-dependent modulation of the MMP-9/TIMP-1 balance, or proteolytically through regulation of MMP-1 and -13, which can cleave the prodomain of MMP-9. Furthermore, when applied in a wound model, conditioned media exhibiting peak MMP activity increased vascular cell migration that was MMP-9-dependent, suggesting that MMP-9 is a key physiologic mediator of the effects of NO in this model.
Keywords: tissue inhibitor of metalloproteinase-1, thrombospondin-1
Intracellular NO synthase (NOS) enzymes oxidize l-arginine to generate l-citrulline and the free radical NO. Although NO itself is relatively unreactive, the chemistry of NO is based on the stabilization of its unpaired electron that can occur in two ways. Low nanomolar concentrations of NO react directly with metal complexes or higher-energy radicals (1). These direct effects of NO mediate numerous physiologic and biochemical processes through NO regulation of iron-binding proteins, particularly soluble guanylyl cyclase (sGC) (1). NO binds the Fe–heme site to activate sGC, resulting in the generation of cGMP, which is known to regulate the expression of many genes, as well as diverse processes such as inflammation, vascular smooth muscle relaxation, angiogenesis, and cancer (1, 2). Alternatively, the indirect effects occur at higher micromolar concentrations of NO, where its reaction with molecular oxygen (O2) or superoxide generates reactive nitrogen species (RNS) (3). These highly labile RNS are capable of modifying bioorganic molecules and are generally thought to mediate cellular stress (3). Therefore, when combining direct and indirect effects, NO is capable of regulating numerous physiologic and disease processes through multiple mechanistic pathways.
Matrix reorganization is a complex process involving the degradation of components of extracellular matrix via matrix metalloproteinases (MMPs) (4). Matrix remodeling occurs during both normal physiological and pathological processes, including angiogenesis, wound healing, inflammation, and cancer (4–9). Toward this end, some MMPs have been associated with cancer progression (10, 11). MMPs share common functional domains, as well as similar structural requirements for activation and catalytic activity (4). MMPs are distinguished by a highly conserved catalytic domain containing a zinc ion bound by three conserved histidines and a cysteine (12–14). In addition, the prodomain maintains the enzyme in its inactive or latent form by its association with a cysteine residue coordinated to the active site zinc in a manner that blocks substrate recognition and binding (15, 16). Activation of MMPs requires either the removal or disruption of this cysteine–zinc bond, which results in the opening of the active site cleft for substrate cleavage, a process commonly referred to as the cysteine switch (15, 16). NO and RNS target zinc ions (17), as well as cysteine residues (18), which are critical to MMP activation, suggesting that NO/RNS may, in part, mediate their physiologic effects through targeting MMP regulation during matrix reorganization (19–24).
To examine the role of MMPs as molecular targets of NO, murine macrophages (25) were used because they express high levels of both inducible NO synthase (iNOS) and MMP-9. Effects of NO on MMP activation were examined in cells treated with the NO donor spermine NONOate (Sper/NO) in the presence and absence of NOS or sGC inhibitors. Our results demonstrate biphasic NO modulation of MMP-9 activity by activation/inactivation of MMP-9 in concert with down-regulation of the endogenous MMP-9 inhibitor, tissue inhibitor of metalloproteinase-1 (TIMP-1). NO exposure also resulted in similar biphasic activation of MMP-1 and MMP-13, which are both known to proteolytically activate latent MMP-9. Importantly, when applied to a tissue explant model that provides an index of wound-driven angiogenesis via the induction of vascular cell migration through a collagen matrix, the conditioned media of NO-treated macrophages with optimal MMP-9 activity mediated a 2-fold increase in vascular cell migration that was inhibited by a translational blocking antisense oligo specific for MMP-9.
Results
NO, MMP-9, and macrophages are known modulators of wound healing and pathological angiogenesis (26). Moreover, macrophages generate high levels of NO and secrete large amounts of MMP-9 (26). Herein, the role of macrophage-secreted MMP-9, as a targeted mediator of the effects of NO-induced vascular cell migration, was examined in a tissue explant model simulating wound-driven angiogenesis (27, 28). Stimulation of vascular cell migration by low NONOate concentrations (10 μM) has been demonstrated by using this explant model (28). Compared with untreated controls, explants incubated with conditioned media from 10 μM Sper/NO-treated macrophages demonstrate a >2-fold enhanced vascular cell migration (Fig. 1 A and B). Although the modest migration response associated with deactivated Sper/NO is not clear, reduction of nitrite to NO and/or nitrite signaling cannot be ruled out. NO-induced migration was suppressed (Fig. 1C) by selectively blocking MMP-9 translation in the ANA1 cells before treatment with Sper/NO, whereas a four-base mismatched antisense oligo had no effect on MMP-9 activity (Fig. 1D). These results suggest that MMP-9 secreted from macrophages is a key mediator of the wound-driven angiogenic response of NO in this ex vivo model and support a role of MMP-9 in promoting epithelial cell migration in another wound model (23).
Fig. 1.
NO-mediated cell migration in an ex vivo model of wound-driven angiogenic response is MMP-9-dependent. (A) Conditioned media of untreated cells or cells treated with 10 μM Sper/NO were applied to an ex vivo model of wound-driven angiogenesis, demonstrating increased vascular cell migration as indicated by the outgrowth of vascular cells (arrow) away from the perimeter of explanted tissue. (B) Quantitation of vascular cell migration in explanted tissue exposed to conditioned media of control and Sper/NO-treated ANA-1 cells shown in A, as well as deactivated Sper/NO control media and 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ)-treated cells with or without Sper/NO. (C) Enhanced vascular cell migration is suppressed in conditioned media from MMP-9 knockdown cells treated with Sper/NO. (D) Knock down of MMP-9 translation by using MMP-9 antisense morpholino completely inhibits MMP-9 translation, as demonstrated by gel zymography. In contrast, the scrambled control oligo does not affect MMP-9 translation. Symbols indicate statistical significance when compared with conditioned media of untreated control: *, P < 0.001; **, P < 0.05.
MMPs are primarily regulated at the levels of transcription and posttranslation (29). Low NO activates sGC to generate cGMP, which regulates the expression of many genes, including MMPs and their endogenous TIMP inhibitors (2). The ability of NO to regulate MMP activity secreted from ANA-1 cells exposed to Sper/NO (1–1,000 μM) for 4 h was examined. These donor concentrations yielded steady-state NO levels shown in Fig. 2A. When compared with conditioned media of untreated control cells, exogenous NO mediated a biphasic increase in total MMP activity that peaked at 10 μM Sper/NO (≈50 nM steady-state NO) but declined with increasing donor concentration (Fig. 2B). Cells treated with 10 μM 8-bromo-cGMP demonstrated ≈2-fold increase in MMP activity when compared with conditioned media from untreated control cells, suggesting sGC-dependent MMP regulation by NO. This biphasic regulation of MMP by NO is similar to that shown in airway epithelial cells treated with the NO donor DETA/NO (23). The effects of NO on MMP-9, as analyzed by gelatin zymography, are shown in Fig. 2C. The consistent signal intensities observed in the conditioned media of control and 1 and 10 μM Sper/NO-treated cells suggest posttranslational regulation with no change in protein accumulation. Moreover, the reduced signal intensity shown in 1,000 μM Sper/NO media is supportive of a posttranslational modification causing zymogen inactivation. Real-time PCR was also performed but showed no significant effect of Sper/NO on the levels of MMP-9 mRNA (data not shown). Also, in the absence of exogenous NO, NOS inhibition by NG-nitro-l-arginine methyl ester suppressed constitutive MMP activity (Fig. 2D). Together, these results suggest that basal NO/cGMP regulates constitutive MMP activity secreted from ANA-1 macrophages, which is elevated in a cGMP-dependent manner as steady-state NO approaches 50 nM. Further increases in steady-state NO suppress MMP activity that is sGC-independent.
Fig. 2.
MMP regulation is NO/sGC-dependent and sGC-independent. (A) Steady-state nanomolar NO as a function of Sper/NO concentration in 10 ml of serum-free, phenol-red-free RPMI medium 1640 (pH 7.4; n = 3). (B) Exposure of resting macrophages to increasing concentrations of Sper/NO for 4 h results in biphasic and dose-dependent activation/inactivation of total MMP activity in the cell-conditioned media. sGC dependence is indicated by the ability of 10 μM 8-bromo-cGMP to increase MMP activity ≈2-fold beyond basal levels. The results are representative of at least five independent experiments. (C) Gel zymography showing MMP-9 in conditioned media of ANA-1 cells treated with Sper/NO. Suppressed signals in media samples from 100 to 1,000 μM Sper/NO suggest enzyme inactivation by high flux of NO/RNS. Sample loading was normalized per milligram of protein. (D) Basal MMP activity levels are suppressed by reducing constitutive NO levels in the presence of NG-nitro-l-arginine methyl ester (L-NAME) (n = 3). Symbols indicate statistical significance when compared with conditioned media of untreated control at P < 0.001 (*) and P < 0.01 (**) or when compared with 10 μM Sper/NO at P < 0.001 (†).
TIMP-1 inhibits MMP-9 activity with high efficiency by stoichiometrically binding its catalytic site (29). To examine an involvement of TIMP-1 in NO/sGC regulation of MMP activity, TIMP-1 was immunoprecipitated from the conditioned media of Sper/NO-treated cells and examined by Western blotting. Compared with control, TIMP-1 protein levels were similarly suppressed by low-dose Sper/NO and 8-bromo-cGMP (Fig. 3A, B, D, and E). In contrast, the inhibitor of NO and sGC signaling, thrombospondin-1 (TSP-1) (28, 30), abolished NO-induced TIMP-1 suppression (Fig. 3 C and F), as well as NO-increased MMP activity [supporting information (SI) Fig. 6]. When plotted together, Fig. 3G demonstrates inverse modulation of MMP activity and TIMP-1 levels by NO, suggesting that at low concentrations, NO/cGMP regulates MMP activity by suppressing TIMP-1 protein secreted from ANA-1 macrophages.
Fig. 3.
NO suppression of endogenous TIMP-1 inhibitor is sGC-dependent. (A) Exogenous NO suppresses TIMP-1 levels in conditioned media from Sper/NO-treated ANA-1 cells. (B) Dose-dependent TIMP-1 suppression by 8-bromo-cGMP suggests that low-dose NO effects are sGC-dependent. (C) Inhibition of NO and sGC signaling by thrombospondin-1 (TSP-1) abolished NO-mediated TIMP-1 suppression. All results are representative of n = 3 blots. (D–F) Quantitation of results shown in A–C, respectively. (G) Inverse modulation of MMP activity and TIMP-1 protein levels in the conditioned media of Sper/NO-treated cells suggests perturbation of MMP-9/TIMP-1 balance by NO. Symbols indicate statistical significance when compared with untreated control: *, P < 0.05; **, P < 0.01.
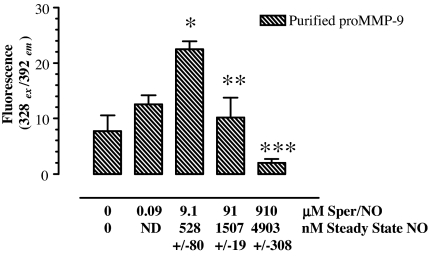
Earlier reports have demonstrated the direct activation of MMPs by oxidants and RNS (19–22, 24, 31). We examined RNS-mediated activation of purified MMP-9 zymogen. Pro-MMP-9 protein (20 ng) was treated with Sper/NO as described in Materials and Methods section. Because the rate of NO release by the donor is dictated by vessel size, head space, temperature, and agitation, the steady-state NO levels specific to these conditions were measured. Steady-state NO levels are provided on the x axis of Fig. 4, which demonstrates biphasic activation and inactivation of MMP-9 protein by transient RNS generated by NO. This regulatory trend is similar to the results shown with conditioned media of Sper/NO-treated cells (Fig. 2B). However, because of the differences in specific conditions stated above regarding cell culture versus purified protein experiments, the steady-state nanomolar concentration of NO required for peak activation of the purified enzyme was ≈10× that of the conditioned media from cells in culture, whereas inactivation occurred at ≈2- to 3-fold higher NO levels (Fig. 2A). Importantly, whereas the cell culture experiments shown in Figs. 2 and 3 suggest a cGMP-dependent involvement of TIMP-1 suppression in the biological regulation of MMPs by low steady-state NO, the results in Fig. 4 suggest a distinct mechanism involving NO flux-dependent chemical activation of pro-MMP-9 (Fig. 4) and inactivation of the mature enzyme by RNS generated at higher levels of NO (Figs. 2 B and C and 4).
Fig. 4.
NO/RNS regulation of MMP-9 activity. Biphasic and dose-dependent activation/inactivation of pro-MMP-9 by exogenous NO released from Sper/NO. The x axis shows both Sper/NO concentration and nanomolar steady-state NO measured by chemiluminescence. The results represent the mean of n = 3 measurements and are representative of two independent experiments. Symbols indicate statistical significance when compared with untreated control (*, P < 0.01) or when compared with 10 μM Sper/NO (**, P < 0.05; ***, P < 0.001). ND, not done.
A physiologically relevant environment consistent with wound response involves cytokine stimulation of macrophages. Under these conditions, iNOS and MMP expression is enhanced and has detrimental, as well as beneficial effects, depending on the activation state of the macrophage (26). MMP regulation by NO after cytokine stimulation (INF-γ/LPS) of macrophages was examined; these stimulatory conditions resulted in ≈150 nM steady-state levels of endogenously produced NO (32). Cytokine-stimulated cells (described in Materials and Methods) were incubated for 4 h with 1 mM l-arginine to facilitate NO production, 5 mM aminoguanidine (Ag) to inhibit iNOS generated NO, or Ag plus Sper/NO. Compared with untreated cells, conditioned media from INF-γ/LPS and l-arginine cells (Fig. 5A, lane 4, and B) showed nearly 2-fold increased MMP-9 activity. In contrast, iNOS inhibition yielded nearly 3-fold increased MMP-9 activity when compared with conditioned media of untreated cells (Fig. 5 A, lanes 3–5, and B). Moreover, the addition of Sper/NO to iNOS-inhibited cells (Fig. 5 A, lanes 6–9, and B) yielded MMP-9 biphasic regulation, consistent with results shown in Fig. 2. Because the level of NO production depends on the activation state of the macrophage (26), these results suggest that within the microenvironment of an activated macrophage, NO/RNS is capable of biphasic regulation of MMP-9.
Fig. 5.
iNOS inhibition by Ag augments activity levels of MMP-9 secreted from IFN-γ/LPS-stimulated ANA-1 cells. (A) Gel zymography demonstrating NO/RNS modulation of MMP-9 activity in the conditioned media of control untreated cells (lane 3), IFN-γ/LPS-stimulated cells supplemented with 1 mM l-arginine to promote generation of endogenous NO (lane 4), IFN-γ/LPS-stimulated cells supplemented with 5 mM Ag to inhibit iNOS generated NO (lane 5), or IFN-γ/LPS-stimulated cells supplemented with 5 mM Ag plus increasing concentrations of Sper/NO (lanes 6–9). Lanes 1 and 2 contain purified standard MMP-2 and MMP-9 proteins. (B) Quantitation of NO/RNS-mediated effects on MMP-9 activity measured by using internally quenched fluorescent MMP substrates (multiple enzyme multiple reagent assay) as described in Materials and Methods and ref. 43. MMP activity is expressed as the mean value determined from duplicate linear regression plots (fluorescence vs. time) (n = 18), as described in Materials and Methods. All sample linear regression correlation coefficients (r) were within 0.88–1.00 at P < 0.0001.
Compared with MMP-9, differential regulation of MMP-1 and MMP-13 activities was observed in cytokine-stimulated cells. Endogenous NO production dramatically increased both MMP-1 and MMP-13 activity, which was suppressed by iNOS inhibition (SI Fig. 7 A and B). Sper/NO treatment of iNOS-inhibited cells demonstrated an NO biphasic pattern of activation/inactivation for both MMP-1 and MMP-13 (SI Fig. 7 A and B). Similarly, Sper/NO treatment of resting macrophages demonstrated biphasic regulation of MMP-9, -13, and -1 (SI Fig. 7 C–E). Whereas 10 or 1,000 μM 8-bromo-cGMP yielded similar increases in active MMP-9 and MMP-13, 1,000 μM Sper/NO suppressed the activity of these MMPs, suggesting that NO/RNS at this donor concentration inactivates the mature zymogen. The differential regulation of these zymogens may be explained in part by structural differences. For example, the MMP-9 promoter responds to cytokines including TNF-α, IL1-β, and LPS and contains binding sites for several growth factors/hormones, as well as activator protein-1 and NF-κB, and resembles the promoter of MMP-1. Moreover, although a role of the hemopexin domain is less clear, it confers substrate specificity of collagenases (i.e., MMP-1, MMP-13). In contrast, the hemopexin domain of MMP-9 is important for TIMP binding (29). Because MMP-9 can be proteolytically cleaved and activated by MMP-1 and MMP-13 (29), these results suggest multiple mechanisms for NO regulation of MMPs that may be important during the transition of an inflammatory site to resolution of inflammation and wound healing (26).
Discussion
MMP-9 plays several critical roles in wound repair and angiogenesis. MMP-9, secreted predominantly from macrophages, localizes to capillary branch points during neoangiogenesis in ischemic muscle tissue (33). Macrophages are also a major source of NO in the innate immune system (26). The activation state of macrophages directs their NO flux as well as the transition from mediating cell killing and matrix degradation to stimulating the cell proliferation and matrix generation necessary for resolution of inflammation and wound healing (26). Clearly, the roles of macrophages in controlling NO flux and MMP regulation during wound response are complex. Although our observations are limited to a cell culture model, the multiple pathways that we demonstrate for NO regulation of MMPs secreted by macrophages may account for how macrophages differentially respond to the high NO microenvironment at the inflammatory site versus the low NO levels present during resolution of inflammation and the initiation of wound healing (26).
We identified an NO/sGC-dependent perturbation of MMP-9/TIMP-1 balance that may be pertinent to the initiation of wound healing. TIMPs are generally known as inhibitors of MMP activities in vitro because under these conditions, proteolysis requires only a protease and a substrate (34). However, other factors may be important in vivo, including compartmentalization and/or anchoring of MMPs via accessory proteins, which provide an important regulatory mechanism for MMP regulation (34). Indeed, TIMP involvement in pro-MMP activation has been demonstrated in vivo (35, 36). Therefore, the identification of NO-mediated perturbation of MMP-9/TIMP-1 balance may be of significance in our explant model and during the resolution of inflammation and initiation of wound healing, when the endogenous generation of NO is suppressed (26).
Furthermore, we show that NO flux and the resultant RNS biphasically activate and inactivate purified pro-MMP-9 (Fig. 4). Under these in vitro conditions, MMP-9 activation required roughly 500 nM steady-state NO (Fig. 4) and was distinct from the perturbation of MMP/TIMP-1 balance, which occurred at 10-fold lower steady-state levels of NO (Figs. 2B and 3). In contrast, steady-state NO exceeding levels of 1 μM led to inactivation of the enzyme (Fig. 4). Although the physiologic relevance of higher NO concentrations remains a subject of debate, various studies have shown that both inflammatory and noninflammatory cell types provide factors that resolve inflammation and facilitate wound healing (26). In macrophages, several levels of NO can be achieved depending on the activation state and specific signaling pathways (26, 37). Comparison of the concentrations of NO-releasing agents with steady-state NO produced by activated macrophages in MCF-7/macrophage coculture experiments have shown that the NO flux generated from activated macrophages depends on macrophage density. A steady-state NO environment resembling that generated by low micromolar levels of NO donors can be achieved that modulates signaling in MCF-7 cells (37). The NO flux associated with chemical activation/inactivation of purified MMP-9 by RNS (Fig. 4) may be representative of the NO microenvironment within close proximity of activated macrophages. Indeed, Johnson et al. have recently demonstrated that MMP-9 is a key mediator of neoangiogenesis in ischemic muscle and that the predominant source of MMP-9-expressing cells at capillary branch points was macrophages (33).
Another continuing debate within the NO field pertains to NO-related toxicities, particularly at higher concentrations of NO that are consistent with an inflammatory microenvironment. Interestingly, this debate exists in the MMP literature as well, because MMP regulation and the proteolytic effects of mature enzymes are cell and tissue specific, dependent on the microenvironment, and the physiologic effects of active MMPs can be beneficial, as well as detrimental (4). Of relevance to inflammatory pathologies, low levels of macrophage-derived oxidants activate MMPs (31, 38), whereas higher oxidant levels cause MMP inactivation (31, 38–40) by cross-linking of critical amino acid residues within or near the catalytic domain that structurally blocks active site/substrate binding (39, 40). Later studies have shown that MMP inactivation by oxidants actually protects against extensive macrophage-mediated damage associated with the development of emphysema in a mouse model (41). Although the current study does not identify specific protein modifications associated with RNS-mediated activation/inactivation of MMP-9, the trend is the same as that shown with oxidants (31, 38). It is plausible that although NO/RNS-mediated MMP activation may be important in promoting wound response, RNS-mediated inactivation of MMPs may also protect under conditions of high NO flux and nitrosative stress. Indeed, the combination of H2O2/MPO/NO2− significantly reduced the activity levels of MMP-12 (41), suggesting a role of RNS in MMP inhibition.
In conclusion, the current report demonstrates that NO/RNS can regulate MMP-9 in a biphasic and flux-dependent manner. This process occurs, at least in part, at the protein level and may involve (i) inverse modulation of MMP-9 activity and TIMP-1 protein levels, (ii) NO/RNS-mediated protein modification, and/or (iii) proteolytic cleavage and activation of pro-MMP-9 by MMP-1 or -13. Although this study suggests that NO/RNS targets MMP-9 activation during wound/angiogenic responses, it also demonstrates a role for NO/RNS in MMP inactivation by high concentrations of NO, which may be protective during severe inflammatory conditions. Biphasic MMP regulation by oxidants and RNS may be important during the transition of macrophages from classically to alternatively activated states because it occurs in inflammation and wound resolution (26). Furthermore, because matrix remodeling is a critical component of inflammation, we believe that this work will extend our current mechanistic understanding of inflammatory pathologies and perhaps aid in our ability to design and evaluate the potential of therapeutic agents aimed at these targeted diseases.
Materials and Methods
Cell Culture and Cytokine Stimulation.
The ANA-1 macrophage cell line used in this study was established by immortalization of bone marrow macrophages from C57BL/6 mice with J2 recombinant retrovirus-expressing v-myc/v-raf oncogenes (25). Cells were routinely cultured in DMEM supplemented with 10% FBS and penicillin–streptomycin. For this study, cells were plated at a density of ≈106 per 100-mm tissue culture dish and grown overnight. For iNOS production of NO, cells were primed overnight with IFN-γ. The next day, the cells were stimulated with LPS for 4 h. After LPS stimulation, the medium was replaced with serum-free, phenol-red-free medium containing 1 mM l-arginine to promote NO production, 5 mM Ag to inhibit NO generation by iNOS, or Ag plus increasing concentrations of exogenous NONOate for 4 h. Under these conditions, NO production (≈150 nM) plateaus at 4 h (32). To accommodate this 4-h time period, Sper/NO was chosen because of its short half-life. Quiescent macrophages were also treated with Sper/NO to examine the effects of exogenous NO on MMP regulation in the absence of cytokine stimulation. The cells were plated and grown as described above and then washed with PBS and treated with increasing concentrations of Sper/NO in serum-free, phenol-red-free RPMI medium 1640 for 4 h. The medium was concentrated by centrifugation with Centricon filters (Mr 30,000 cutoff; Millipore, Billerica, MA). All concentrated medium samples were volume adjusted to equal volume by using the flow-through medium from the respective concentrated sample. Cells were scrape harvested and counted.
Steady-State NO Quantification.
Measurement of steady-state NO levels was accomplished by using a NO gas analyzer (Seivers, Boulder, CO). Measurements were representative of steady-state NO at 37°C in a 100-mm cell culture dish containing 10 ml of serum-free medium or a 1.8-ml sealed Eppendorf tube containing 200 μl of TCN buffer (50 mM Tris/10 mM CaCl2/150 mM NaCl; pH 7.5). Aliquots of medium or buffer (100–200 μl) were injected into a reaction chamber containing 10 mM NaOH to prevent decomposition of unreacted donor. The NO analyzer was continually purged with helium gas to prevent autoxidation of NO. Steady-state nanomolar concentrations of NO were calculated from the peak area(s) of absolute NO detected and compared with a nitrite (NO2−) standard curve.
Gel Zymography and Fluorogenic Peptide Evaluation of MMP Activity.
The effect of NO treatment on MMP-9 was qualitatively examined by gel zymography (42). Conditioned medium samples normalized according to cell number or protein content were prepared in 2× loading buffer without β-mercaptoethanol and electrophoresed on 10% gelatin zymogram gels. The gels were washed and incubated in renaturing and developing buffers according to the recommendation of the manufacturer and then stained. All reagents were purchased from Invitrogen (Carlsbad, CA).
Quantitative evaluation of total MMP proteolytic activity was measured by using the internally quenched synthetic MCa peptide [7-methoxycoumarin-4-acetyl-Pro-Leu-Gly-Leu-β-(2,4-dinitrophenylamino)Ala-Ala-Arg-NH2] (Sigma-Aldrich, St. Louis, MO), which fluoresces at 328 nm (excitation) and 392 nm (emission) upon cleavage of the quenching moiety. Conditioned media were normalized according to cell number or protein content and then mixed 1:4 with TCNB buffer [50 mM Tris·HCl (pH 7.5)/0.2 M NaCl/10 mM CaCl2/0.05% Brij35]. MCa peptide was added to a final concentration of 2 μM, and the solutions were incubated for 20 min at 37°C. Fluorescence was measured at 328 nm (excitation) and 392 nm (emission) on a Luminescence LS50B Spectrometer equipped with FL WinLab software (Perkin-Elmer, Wellesley, MA).
An additional method was used for the quantitation of individual MMP activities (43). Briefly, internally quenched fluorescent substrates with various MMP selectivity profiles were identified that were preferentially cleaved by single MMPs. These substrates were combined and cleaved with individual recombinant MMPs in separate reactions. The combined substrates were also incubated with samples containing unknown amounts of MMP activities and were incubated under the same conditions. Fluorescence from each sample reaction was measured every 30 minutes for 4h in duplicate (n = 18) to generate time vs. fluorescence curves as a measure of proteolytic cleavage of substrate peptides; all sample linear regression correlation coefficients (r) were within 0.88–1.00 at P ≤ 0.0002. By using the multiple enzyme multiple reagent assay equations to solve for multiple unknowns, the relative activity of individual MMPs was calculated (43).
NO Treatment of Purified Latent MMP-9 Zymogen.
Purified pro-MMP-9 zymogen was resuspended in TCNB buffer [50 mM Tris/10 mM CaCl2/150 mM NaCl/0.05% Brij; pH 7.5] at a concentration of 100 ng/ml. Twenty nanograms of protein were allocated in 200-μl volumes into 1.8-ml Eppendorf tubes, and then 2 μl of Sper/NO was added to yield mole:mole ratios (donor:enzyme) ranging from 100:1 to 106:1 (31). The tubes were capped, and the samples were incubated at 37°C for 1 h while shaking.
Suppression of MMP-9 Translation.
Silencing of MMP-9 protein translation was accomplished by using an antisense 22-mer oligo (Gene Tools, Philomath, OR) designed specifically to block the AUG translational start site of mouse MMP-9 (GenBank accession no. NM_013599: sequence, 5′-GCTGCCAGGGACTCATGGTGAG). This oligo complements the sequence from −6 to + 16 relative to the initiation codon. Briefly, cells (≈50% confluence) were incubated with 10 μM MMP-9 antisense or a mismatched antisense control oligo and 6 μl of Endoporter peptide (Gene Tools) per milliliter of growth medium for 72 h. Suppression of secreted MMP-9 protein levels were then verified by using gel zymography.
Muscle Explant Assay.
Pectoralis major muscle biopsies were harvested from 8- to 10-week-old C57BL6 mice. The fascia was excised, and the muscle was cut into 1.5-mm fragments and explanted in 24-well culture plates (Nunc, Rochester, NY) on polymerized type I collagen as described (27, 28). Explants were incubated in EGM media containing FBS, as well as conditioned media from ANA-1 macrophages (1:1), for 7 days. Migration of vascular cells through the extracellular matrix was quantified as described (28). The results are presented as means ± SD (n ≥ 3).
Statistical Analysis.
The results are reported as means ± SD. Statistical comparisons were made by using one-way ANOVA and Bonferroni multiple comparison posttest.
Supplementary Material
Acknowledgments
We thank Drs. Jay Heinecke and Bill Parks for critical review of the manuscript. This research was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, and Center for Cancer Research.
Abbreviations
- Ag
aminoguanidine
- iNOS
inducible NO synthase
- MMP
matrix metalloproteinase
- sGC
soluble guanylyl cyclase
- Sper/NO
spermine NONOate
- TIMP-1
tissue inhibitor of MMP-1.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0702761104/DC1.
References
- 1.Wink DA, Mitchell JB. Free Radic Biol Med. 1998;25:434–456. doi: 10.1016/s0891-5849(98)00092-6. [DOI] [PubMed] [Google Scholar]
- 2.Pilz RB, Broderick KE. Front Biosc. 2005;10:1239–1268. doi: 10.2741/1616. [DOI] [PubMed] [Google Scholar]
- 3.Ridnour LA, Thomas DD, Mancardi D, Palocci N, Espey MG, Miranda K, Feelisch M, Fukuto J, Wink DA. Biol Chem. 2003;358:1–10. doi: 10.1515/BC.2004.001. [DOI] [PubMed] [Google Scholar]
- 4.Sternlicht Werb Z. Ann Rev Cell Devel Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang C, Werb Z. Trends Cell Biol. 2001;11:S37–S43. doi: 10.1016/s0962-8924(01)02122-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parks WC. Wound Repair Regen. 1999;7:423–432. doi: 10.1046/j.1524-475x.1999.00423.x. [DOI] [PubMed] [Google Scholar]
- 7.Xue M, Le NTV, Jackson CJ. Expert Opin Ther Targets. 2006;10:143–155. doi: 10.1517/14728222.10.1.143. [DOI] [PubMed] [Google Scholar]
- 8.Coussens LM, Werb Z. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Egeblad M, Werb Z. Nat Rev Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 10.Brinckerhoff CE, Rutter JL, Benbow U. Clin Cancer Res. 2000;6:4823–4830. [PubMed] [Google Scholar]
- 11.Vihinen P, Kaharia VM. Int J Cancer. 2002;99:157–166. doi: 10.1002/ijc.10329. [DOI] [PubMed] [Google Scholar]
- 12.Woessner JF, Nagase H. Matrix Metalloproteinases and TIMPs. New York: Oxford Univ Press; 2000. [Google Scholar]
- 13.Holz RC, Salowe SP, Smith CK, Cuca GC, Que L. J Am Chem Soc. 1992;114:9611–9614. [Google Scholar]
- 14.Becker JW, Marcy AI, Rokosz LL, Axel MG, Burbaum JJ, Fitzgerald PM, Cameron PM, Esser CK, Hagmann WK, Hermes JD. Protein Sci. 1995;4:1966–1976. doi: 10.1002/pro.5560041002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Wart HE, Birkedal-Hanson H. Proc Natl Acad Sci USA. 1990;87:5578–5582. doi: 10.1073/pnas.87.14.5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Springman EB, Angleton EL, Birkedal-Hansen H, Van Wart HE. Proc Natl Acad Sci USA. 1990;87:364–368. doi: 10.1073/pnas.87.1.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kroncke KD. FASEB J. 2001;15:2503–2507. doi: 10.1096/fj.01-0240hyp. [DOI] [PubMed] [Google Scholar]
- 18.Wink DA, Nims RW, Darbyshire JF, Christodoulou D, Hanbauer I, Cox GW, Laval F, Laval J, Cook JA, Krishna MC, et al. Chem Res Toxicol. 1994;7:519–525. doi: 10.1021/tx00040a007. [DOI] [PubMed] [Google Scholar]
- 19.Okamoto T, Akaike T, Nagano T, Miyajima S, Suga M, Ando M, Ichimori K, Maeda H. Arch Biochem Biophys. 1997;342:261–274. doi: 10.1006/abbi.1997.0127. [DOI] [PubMed] [Google Scholar]
- 20.Maeda H, Okamoto T, Akaike T. Biol Chem. 1998;379:193–200. doi: 10.1515/bchm.1998.379.2.193. [DOI] [PubMed] [Google Scholar]
- 21.Okamoto T, Akaike T, Sawa T, Miyamoto Y, van der Vliet A, Maeda H. J Biol Chem. 2001;276:29596–29602. doi: 10.1074/jbc.M102417200. [DOI] [PubMed] [Google Scholar]
- 22.Gu Z, Kaul M, Yan B, Kridel SJ, Cui J, Strongin A, Smith JW, Liddington RC, Lipton SA. Science. 2002;297:1186–1190. doi: 10.1126/science.1073634. [DOI] [PubMed] [Google Scholar]
- 23.Bove PF, Wesley UV, Greul AK, Hristova M, Dostmann WR, van der Vliet A. Am J Respir Cell Mol Biol. 2007;36:138–146. doi: 10.1165/rcmb.2006-0253SM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang HJ, Weiling Z, Venkataraman S, Robbins MEC, Buettner GR, Kregel KC, Oberley LW. J Biol Chem. 2002;276:20919–20926. doi: 10.1074/jbc.M109801200. [DOI] [PubMed] [Google Scholar]
- 25.Cox GW, Mathieson BJ, Gandino L, Blasi E, Radzioch D, Varesio L. J Natl Cancer Inst. 1989;81:1492–1499. doi: 10.1093/jnci/81.19.1492. [DOI] [PubMed] [Google Scholar]
- 26.Duffield JS. Clin Sci. 2003;104:27–38. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- 27.Isenberg JS, Calzada MJ, Zhou L, Guo N, Lawler J, Wang XQ, Frazier WA, Roberts DD. Matrix Biol. 2005;24:110–123. doi: 10.1016/j.matbio.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 28.Isenberg JS, Ridnour LA, Perruccio EM, Espey MG, Wink DA, Roberts DD. Proc Natl Acad Sci USA. 2005;102:13141–13146. doi: 10.1073/pnas.0502977102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van den Steen PE, Dubois B, Nelissen I, Rudd PM, Dwek RA, Opdenakker G. Crit Rev Biochem Mol Biol. 2002;37:375–536. doi: 10.1080/10409230290771546. [DOI] [PubMed] [Google Scholar]
- 30.Ridnour LA, Isenberg JS, Espey MG, Thomas DD, Roberts DD, Wink DA. Proc Natl Acad Sci USA. 2005;102:13147–13152. doi: 10.1073/pnas.0502979102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fu X, Kassim SY, Parks WC, Heinecke JW. J Biol Chem. 2001;276:41279–41287. doi: 10.1074/jbc.M106958200. [DOI] [PubMed] [Google Scholar]
- 32.Espey MG, Miranda KM, Pluta RM, Wink DA. J Biol Chem. 2000;275:11341–11347. doi: 10.1074/jbc.275.15.11341. [DOI] [PubMed] [Google Scholar]
- 33.Johnson C, Hak-Joon S, Lessner SM, Fini ME, Galis ZS. Circ Res. 2007;94:262–268. doi: 10.1161/01.RES.0000111527.42357.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parks WC, Wilson CL, Lopez-Boado S. Nat Immunol. 2004;4:617–629. doi: 10.1038/nri1418. [DOI] [PubMed] [Google Scholar]
- 35.Wang Z, Juttermann R, Soloway PD. J Biol Chem. 2000;275:26411–26415. doi: 10.1074/jbc.M001270200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Caterina JJ, Yamada S, Caterina NC, Longenecker G, Holmback K, Shi J, Yermovsky AE, Engler JA, Birkedal-Hansen H. J Biol Chem. 2000;275:26416–26422. doi: 10.1074/jbc.M001271200. [DOI] [PubMed] [Google Scholar]
- 37.Thomas DD, Espey MG, Ridnour LA, Hofseth LJ, Mancardi D, Harris CC, Wink DA. Proc Natl Acad Sci USA. 2004;101:8894–8899. doi: 10.1073/pnas.0400453101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peppin GJ, Weiss SJ. Proc Natl Acad Sci USA. 1986;83:4322–4326. doi: 10.1073/pnas.83.12.4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fu X, Kassim SY, Parks WC, Heinecke JW. J Biol Chem. 2003;278:28403–28409. doi: 10.1074/jbc.M304739200. [DOI] [PubMed] [Google Scholar]
- 40.Fu X, Kao JL, Bergt C, Kassim SY, Huq NP, d'Avignon A, Parks WC, Mecham RP, Heinecke JW. J Biol Chem. 2004;279:6209–6212. doi: 10.1074/jbc.C300506200. [DOI] [PubMed] [Google Scholar]
- 41.Kassim SY, Fu X, Liles WC, Shapiro SD, Parks WC, Heinecke JW. J Biol Chem. 2005;280:30201–30205. doi: 10.1074/jbc.M503292200. [DOI] [PubMed] [Google Scholar]
- 42.Kleiner DE, Stetler-Stevenson WG. Anal Biochem. 1994;218:325–329. doi: 10.1006/abio.1994.1186. [DOI] [PubMed] [Google Scholar]
- 43.Rasmussen FH, Yeung N, Kiefer L, Murphy G, Lopez-Otin C, Vitek MP, Moss ML. Biochem. 2004;43:2987–2995. doi: 10.1021/bi036063m. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.