DEVELOPMENTAL BIOLOGY. For the article “Proteasomal selection of multiprotein complexes recruited by LIM homeodomain transcription factors,” by Cenap Güngör, Naoko Taniguchi-Ishigaki, Hong Ma, Alexander Drung, Baris Tursun, Heather P. Ostendorff, Michael Bossenz, Catherina G. Becker, Thomas Becker and Ingolf Bach, which appeared in issue 38, September 18, 2007, of Proc Natl Acad Sci USA (104:15000–15005; first published September 11, 2007; 10.1073/pnas.0703738104), the authors note that, due to a printer's error, Fig. 4 appeared incorrectly. The corrected figure and its legend appear below. This error does not affect the conclusions of the article.
Fig. 4.
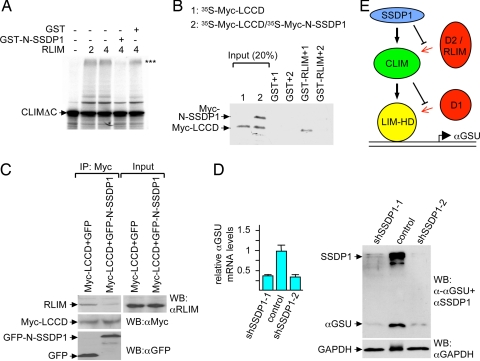
SSDP1 inhibits binding of RLIM to CLIM and regulates Lhx3 target gene expression. (A) In vitro ubiquitination experiment using 35S-labeled CLIMΔC, RLIM as E3, and UbcH5 as E2 enzyme in the presence of GST-N-SSDP1 or GST alone. Arrows point at unmodified CLIM proteins. Polyubiquitinated proteins are indicated by three asterisks (***). Note the partial inhibition of CLIMΔC ubiquitination by N-SSDP1. (B) N-SSDP1 inhibits binding of RLIM to the LCCD. Shown is the GST pull-down using GST-RLIM and 35S-Myc-LCCD (1) or Myc-LCCD cotranslated with Myc-N-SSDP1 (35S-Myc-LCCD/35S-Myc-N-SSDP1) (2). (C) Co-IP of endogenous RLIM in cells cotransfected with Myc-LCCD and GFP-N-SSDP1. Note the decreased RLIM precipitation in the presence of N-SSDP1. (D) Down-regulation of SSDP1 results in lower levels of αGSU mRNA and protein. (Left) SSDP1 levels were knocked down in αT3 cells via retroviral infection of mouse shRNAs (shSSDP1–1, -2) or the empty retroviral vector. mRNA encoding αGSU was measured by qRT-PCR (n = 3; values are mean ± SE). (Right) Western blot of the same shRNA-treated cells. Note that knocking down endogenous SSDP1 levels leads to a significant decrease in endogenous αGSU levels at the mRNA and protein levels. (E) Shown is a model of a cascade of protein interactions that protects LIM-HDs from proteasomal degradation. Via binding to LIM domains, a destabilizing enzyme (D1, red) targets LIM-HDs (yellow) for degradation. In the presence of CLIM (green), the binding of D1 to LIM domains is inhibited, resulting in stabilization of LIM-HDs. CLIM is targeted by another destabilizing enzyme(s) (D2/RLIM) for ubiquitination/degradation. The presence of SSDP1 (blue) prevents binding of D2/RLIM to CLIM, thereby protecting the LIM-HD/CLIM protein complex.