Abstract
Wild chimpanzees produce acoustically distinct scream vocalizations depending on their social role during agonistic interactions with other group members. Here, we show that victims during such agonistic interactions alter the acoustic structure of their screams depending on the severity of aggression experienced, providing nearby listeners with important cues about the nature of the attack. However, we also found that victims of severe attacks produced screams that significantly exaggerated the true level of aggression experienced, but they did so only if there was at least one listener in the audience who matched or surpassed the aggressor in rank. Our results are consistent with the more general hypothesis that chimpanzees possess sophisticated understanding of third-party relationships, so-called triadic awareness, and that this knowledge influences their vocal production.
Keywords: audience effect, language evolution, Pan troglodytes, primate vocalizations, social intelligence
Agonistic interactions are a natural consequence of sociality. For comparative studies of cognition, social conflicts are particularly interesting because they often reveal most clearly the social skills and intelligence of the individuals involved. In nonhuman primates, a particular focus has been given to the various peacemaking behaviors that follow agonistic interactions. One such behavior is to engage in reconciliation, which typically involves the former opponents engaging in affiliatory behavior, using vocal or tactile signals (1, 2). However, reconciliation does not necessarily need to involve the former opponents, as close kin can sometimes reconcile on behalf of victims or aggressors (3–6). Bystanders often intervene to support the victim during a fight or to provide “consolation” afterward (1, 7, 8). In some species, high-ranking individuals engage in what has been termed “policing”; they intervene impartially to terminate aggression between two group members (9–11). A recent example comes from free-ranging chimpanzees in the Budongo Forest, Uganda, where high-ranking males have attempted to stop a group of resident females from committing coalitionary lethal attacks on the infants of recent immigrant mothers (12).
There is some controversy concerning the cognitive sophistication required to process these types of third-party interactions. In baboons, there is evidence that individuals understand the hierarchical organization of their group at two levels (individuals and matrilines) (13). In contrast, it has been suggested that juvenile sooty mangabeys may employ egocentric heuristics when soliciting third-party aid rather than use sophisticated knowledge of triadic relationships (14). In chimpanzees, an individual's social position does not depend on belonging to a particular matriline, as is the case for baboons, but the outcome of a network of relationships in which unrelated individuals change coalition partners in seemingly strategic ways (11). One prediction from this is that chimpanzees may require a higher degree of social intelligence and triadic awareness [i.e., a capacity to perceive social relationships between other individuals and to form varied triangular relationships (ref. 11, p. 182)] than other groups of primates if they are to successfully navigate in this type of social system.
We conducted a study on the vocal behavior of wild chimpanzees in the Budongo Forest, Uganda, during agonistic interactions. Primates often produce acoustic signals during conflicts, most likely to address an opponent, to alert nearby group members, and to recruit aid (15). Our first goal was to examine to what degree chimpanzee victim screams conveyed information about the nature of the conflict, thus providing valuable information for nearby receivers deciding whether or not to interfere. Previous research on macaques has revealed that callers produce acoustically distinct screams types that are meaningful to listeners (15). Analyses showed that these vocalizations contained cues to identity of the caller (16, 17) and that they were given in context-specific ways, with regards to the severity of the attack and the rank of the opponent (15, 18). In captive chimpanzees, victims of aggression also use screams, often in combination with gestural signals, to solicit support from bystanders (19). In doing so, victims of aggression tend to solicit support from group members that are more likely to support them than their opponent, an observation that suggests a considerable amount of triadic awareness (19). In the chimpanzees' low-visibility natural habitat, the dense tropical forests of Africa, gestural communication is less effective, and in the Budongo Forest, individuals' visual gesture rates tend to be substantially lower than vocal rates (K.E.S., personal observation). Thus, in their natural environment, we expect vocalizations to play a principal role in aid recruitment.
In previous work, we have shown that wild chimpanzees produce acoustically distinct scream vocalizations depending on their social role during agonistic interactions: victim screams and aggressor screams (20). We also observed a large amount of acoustic variation within the victim screams, and our goal therefore was to examine which social factors determined this acoustic variation. First, we tested whether victims adjusted their screams depending on the nature of the attack they experienced. Second, we investigated whether victims adjusted their screams to increase the likelihood of obtaining support from nearby listeners. The presence or absence of a conspecific audience can have effects on call production in primates, but these effects are typically in terms of call rates or latency to call (21–24). Some primates also seem sensitive to the size or composition of the audience, particularly the presence of kin or mates (25–27). There is little evidence, however, that nonhuman primates can alter the acoustic structure of their calls in systematic, meaningful ways in response to audience composition (25).
Results
Victim Screams Vary with Severity of Aggression.
Types of aggression.
We were able to objectively distinguish four types of aggression, based on the presence or absence of key behaviors in the aggressor (Table 1).
Table 1.
Categorization of aggression based on key behaviors of the aggressor
| Aggression | Potential for harm | Key behaviors |
|---|---|---|
| Contact | High risk (severe) | Beating, stamping, thumping, slapping, kicking victim |
| Directed | High risk (severe) | Individual pursuit of victim but no physical contact |
| Nondirected | Low risk (mild) | Display charge but no pursuit of victim |
| Posture threat | Low risk (mild) | Pilo-erection, hunch walk, hunch sit |
The four categories were associated with different risks for the victim, ranging from high (direct physical contact with, or pursuit by, the aggressor) to low (aggressive displays or posture threat by the aggressor). We judged physical harm to be much more probable in categories 1 and 2 (severe aggression), either because of the aggressor's direct behavior or accidental self-injury by the victim, compared with categories 3 and 4 (mild aggression). We therefore distinguished between severe and mild aggression as a conservative measure of how victims might categorize aggressor behavior [see supporting information (SI) Table 3] and all further analyses were performed on these two basic categories.
Acoustic analysis.
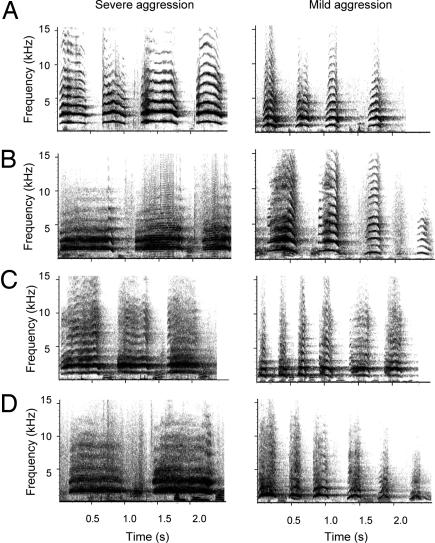
Analyses were conducted on 84 screaming bouts produced by 21 victims: Each individual contributed a total of four screaming bouts: two bouts in response to severe aggression and two in response to mild aggression. We took two calling bouts from each individual in each context to get a wide, representative sample. To avoid pseudoreplication, we either controlled statistically for multiple contributions from the same individuals, or we ran a between-subjects design with each individual contributing a single data point (average value of multiple calls or one randomly chosen call) to a single condition (see SI Table 4). Screams were measured along three temporal and six spectral parameters. In all contexts, screams consisted of tonal signals with a variable number of harmonic overtones (Fig. 1).
Fig. 1.
Time–frequency spectrograms of screams given by four individuals to severe and mild aggression. Screams were produced by BH, a subadult female (A); KT, a subadult male (B); FL, an adult female (C); and BB, an adult male (D). The darker the image, the higher was the amplitude.
Victims Modify Acoustic Structure of Screams as a Function of Aggression Experienced.
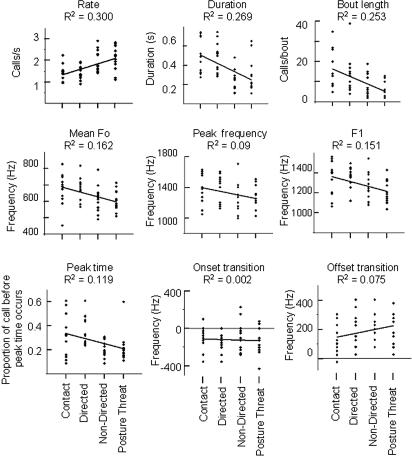
Inspection of spectrograms indicated the presence of subtle differences in the temporal and frequency domains of these calls as a function of the severity of aggression (Fig. 1). We ran a two-way ANOVA to establish whether the structure of calls given to severe and mild levels of aggression varied statistically. Type of aggression (severe/mild) was entered as a fixed factor and individual identity was entered as a random factor to account for repeated observations of the same subjects, thereby avoiding pseudoreplication. This analysis revealed that screams given to severe aggression were longer, contained higher frequencies, had most acoustic energy later in the call, and were given in longer, slower bouts, compared with the screams given to mild aggression [duration: F(1,20) = 24.83, P < 0.001; mean fundamental frequency: F(1,20) = 44.38, P < 0.001; peak frequency: F(1,20) = 32.32, P < 0.001; first formant frequency: F(1,20) = 34.07, P < 0.001; peak time: F(1,20) = 9.855, P = 0.005; rate: F(1,20) = 17.89, P < 0.001; bout length: F(1,20) = 28.29, P < 0.001]. These differences occurred in a graded manner across contexts (Fig. 2). The overall shape of the call, however, was unaffected by the severity of aggression encountered [transition onset: F(1,20) = 4.283, P = 0.052; transition offset: F(1,20) = 0.01, P = 0.993]. We then conducted a direct discriminant function analysis (DFA). The function derived from all nine acoustic variables explained a significant amount of the variation in the acoustic structure of screams given to severe and mild aggression [Wilks' lambda = 0.52, χ2 (df = 9) = 50.64, P < 0.001]. In a cross-validated analysis, the function correctly classified 81% (68/84) of the screams according to the level of aggression that caused the scream. Since the data were two-factorial (subject; type of aggression) and comprised of two calls per combination of the two factors, it has been argued that conventional DFA does not allow for a valid estimation of the overall significance of discriminability (28). To estimate the significance of the number of correctly classified calls (cross-validated), we thus used a permuted DFA (pDFA) (ref. 28; see SI Text), which indicated that the level of discrimination was highly significant (P = 0.001).
Fig. 2.
Scatter-plots illustrating the relationship between the type of aggression experienced by the caller and nine acoustic measurements of the screams produced. The x axis of each scatter-plot shows the four types of aggression the victim experienced. Contact and directed aggression were classified as severe, whereas nondirected and posture threat aggression were classified as mild. All further analyses reported in the text are based on these two aggression categories (severe and mild). Each point is the median value from the first three calls in each bout of screaming (n = 84).
Victims Modify Acoustic Structure of Screams as a Function of the Audience.
We examined whether party composition affected the acoustic structure of screams produced by victims, both in mild and severe cases of aggression. In particular, we investigated whether victims changed the acoustic structure of their screams depending on whether individuals of equal or higher rank to the aggressor were in the audience. Such individuals can be valuable to a victim because they are more likely to provide aid to victims than lower-ranking individuals (see Responses of Bystanders), because these individuals are able to effectively challenge the aggressor.
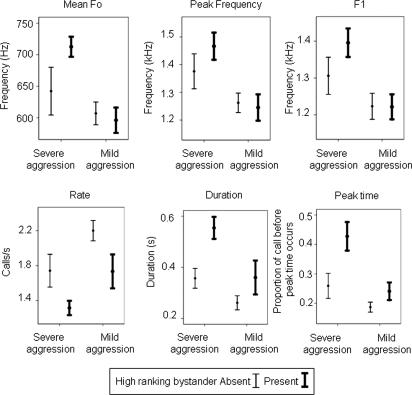
For mild cases of aggression, we found that the presence of individuals of equal or higher rank relative to the aggressor had virtually no effect on the acoustic structure of the screams [one-way between-subjects ANOVAs; high-ranking audience present (n = 10) or absent (n = 11); SI Table 5; Fig. 3]. The only variable to show a significant effect was the rate of calling, with slower calling bouts being produced when a high-ranking bystander was present [F(1,20) = 4.66, P = 0.044]. Crucially, a DFA, based on all nine acoustic variables, was unable to explain a significant amount of the variation in the acoustic structure of the screams to mild aggression as a function of audience composition at the time of the agonistic interaction [Wilks' lambda = 0.693, χ2 (df = 9) = 5.317, P = 0.806]. The function only correctly classified 38.1% of calls (8/21; cross-validated), a level below that expected by chance. In cases of mild aggression, there is no evidence that victims altered the acoustic structure of their screams as a function of the audience.
Fig. 3.
Changes in the vocal structure of victim screams as a function of the level of aggression experienced and whether or not an individual equal to or outranking the aggressor was in the audience. The mean and standard error values for these six variables are shown. Error bars represent one standard error. Sample sizes are as follows: for occurrences of severe aggression with a high-ranking bystander present, n = 12, and with a high-ranking bystander absent, n = 9; for occurrences of mild aggression with a high-ranking bystander present, n = 10, and with a high-ranking bystander absent, n = 11.
In cases of severe aggression, however, the acoustic structure of the screams differed significantly depending on whether or not an individual of equal or higher rank to the aggressor was in the audience. All of the seven acoustic parameters that varied significantly as severity of attack increased (Fig. 2) also changed in the same direction when an individual equal or higher ranking than the aggressor was present in the party. Despite a relatively small sample size (n = 21), three of the nine acoustic parameters were significantly affected by audience composition (see Table 2 and Fig. 3), and a further three variables approached significance (0.054 ≤ P ≤ 0.065). When a high-ranking bystander was present, victims significantly increased call duration and frequency change in the second half of the call, whereas peak acoustic energy was significantly shifted toward the end of the call. In addition, calling bouts tended to be slower, whereas the first formant and peak frequency tended to be greater. These changes in acoustic structure closely mirrored the ones that also occurred as the severity of aggression increased. Crucially, the discriminant function derived from all nine acoustic variables combined accounted for a significant amount of the variation in the acoustic structure of the screams given to severe aggression, depending on whether or not high-ranking bystanders were in the audience [Wilks' lambda = 0.257, χ2 (df = 8) = 20.39, P = 0.009]. The function correctly classified 76.2% of calls (16/21; cross-validated) according to whether high-ranking third parties were present or absent at the time of the agonistic interaction. To test whether 76.2% correct classification was significantly above chance, we conducted a binomial test, which confirmed that the function was classifying the calls at a level significantly above that expected by chance [binomial test (0.5) = 0.027 (two-tailed)].
Table 2.
Mean, standard deviation, and F values from one-way ANOVAs examining variation in the acoustic parameters describing screams given by 21 individuals to severe aggression as a function of audience composition
| High-ranking bystander present (n = 12) |
High-ranking bystander absent (n = 9) |
F value | P value | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| Rate | 1.28 | 0.25 | 1.65 | 0.56 | 4.234 | 0.054 |
| Bout length | 13.08 | 6.08 | 14.88 | 7.35 | 0.379 | 0.545 |
| Duration | 0.58 | 0.14 | 0.39 | 0.15 | 8.662 | 0.008 |
| Mean F0 | 702.02 | 44.38 | 646.88 | 101.29 | 2.864 | 0.107 |
| Peak time | 0.406 | 0.12 | 0.254 | 0.11 | 8.809 | 0.008 |
| Peak frequency | 1,491.29 | 130.76 | 1,351.11 | 186.64 | 4.114 | 0.057 |
| First formant | 1,390.21 | 92.81 | 1,289.78 | 142.06 | 3.847 | 0.065 |
| Transition onset | −179.04 | 112.20 | −147.67 | 114.87 | 0.394 | 0.538 |
| Transition offset | 200.29 | 93.03 | 101.39 | 96.38 | 5.639 | 0.028 |
Degrees of freedom: 1,20.
Responses of bystanders.
We recorded 16 cases of active third-party responses to victims of aggression (19% of 84 agonistic incidents). These comprised five cases of immediate aggression toward the aggressor, which terminated the fight; three cases of delayed aggression toward aggressor within 10 min after the fight; one case of following the victim to the aggressor with subsequent aggression toward the aggressor; five cases of following the victim with subsequent proximity; one case of embracing the victim (consolation) (5); and one case of delayed aggression toward the aggressor and embracing by a second individual. In the 84 agonistic interactions examined, we did not record any instances of third parties intervening and supporting the aggressor by directing aggression toward the victim. There was a trend for active responses to be given more often to victims of severe than mild aggression [12/16 cases; binomial (0.5) = 0.077, two-tailed]. Individuals providing such support were equal or higher ranking than the aggressor significantly more often than expected by chance [13/16 cases; 81.3%; P = 0.021, two-tailed; binomial (0.5)]. Finally, when callers facing severe aggression modified their calls in the presence of a high-ranking bystander, there was a trend toward them receiving more support (10/31 cases; 31%), compared with 2/11 cases (18%) when they did not modify their calls, however, this difference was not statistically significant (exact P = 0.635, two-tailed; Fisher's exact test).
Potential alternative explanations.
Our data are observational, and it is therefore important to address potentially more parsimonious alternative explanations. For example, it might have been the case that the acoustic differences in screams given to cases of mild or severe aggression simply reflected particular relationships between specific social dyads, rather than the severity of aggression experienced per se. To examine this possibility, we examined a subset of the calls, in which we compared the acoustic structure of screams given to severe and mild aggression, when the identity of the victim and aggressor dyad remained constant across the two levels of aggression. Despite the reduced power associated with a reduced sample size (n = 24, one mild and one severe screaming bout from 12 individuals), the pattern of acoustic change in the screams across different levels of aggression remained the same as in the main analysis: Screams given to severe aggression were longer in duration [F(1,23) = 11.918, P = 0.002], higher in fundamental frequency [F(1,23) = 9.989, P = 0.005], and produced in longer bouts [F(1,23) = 5.265, P = 0.032] and at a slower rate [F(1,23) = 8.231, P = 0.009] than screams to mild aggression. In a DFA, the function derived from all nine acoustic variables explained a significant amount of the variation in the acoustic structure of screams given to severe and mild aggression [Wilks' lambda = 0.317, χ2 (df = 9) = 20.196, P = 0.017]. In a cross-validated analysis, the function correctly classified 83.3% of the screams (20/24) according to the level of aggression that elicited the scream. A binomial test [(0.5) = 0.002 (two-tailed)] confirmed that this was at a level significantly above that expected by chance. The pattern of acoustic variation in the screams we observed, therefore, could not be attributed to special relationships between certain individuals, but rather it represented a general feature of chimpanzee communication: Screams vary systematically with the severity of the attack experienced by the victim.
An alternative way of interpreting the audience effect, reported in Fig. 3 and Table 2, is to argue that contact aggression (more severe) is generally more common than directed aggression (less severe) in the presence of high-ranking individuals (see Table 1), and that the acoustic variation we recorded within severe cases of aggression simply reflected this fact. However, inspection of the data revealed that the opposite was the case. We examined the 42 screaming bouts on which the audience effect analyses were based (see SI Text) and found that, of all screams given in the presence of a high-ranking audience, only 36% (n = 11) were in response to contact aggression, whereas 64% (n = 20) were in response to directed aggression. Conversely, in the presence of a low-ranking audience, 64% cases (n = 7) involved contact aggression compared with 36% cases (n = 4) involving directed aggression. Our data suggest that aggressors take the audience into account as well and are more willing to escalate during a conflict if individuals in the audience are lower ranking than the aggressor.
We also tested whether the observed audience effect was mediated by party size, since larger parties were more likely to contain high-ranking individuals. We found no evidence that victims adjusted their calling behavior according to relative party size during the severe aggression cases. One-way analyses of variance on all nine acoustic parameters between small (2–10 members; n = 10) and large (>10 members; n = 11) parties revealed no significant differences.
We then tested if the chimpanzees were simply responding to the presence of high-ranking individuals per se, rather than processing the rank relations between the aggressor and the individuals in the audience, as predicted by the triadic awareness hypothesis. We found that in 41 of 42 severe aggression events there were individuals in the audience that outranked the victim, ruling out explanations based on egocentric heuristics in which callers simply altered the acoustic structure of their calls if an individual dominant to themselves was in the party (e.g., ref. 14).
Finally, it could be argued that victims simply modified their calls if a high-ranking male was in the audience, regardless of the relationship between this individual and the aggressor. We scanned our data set for cases in which a high-ranking male was in the audience (defined as belonging to the top two rank groups; 2003/4: n = 7 individuals; 2006: n = 5 individuals) during agonistic interactions. For 20 individuals, we had recordings of victim screams when there was at least one of these high-ranking males in the party. For 12/20 cases the high-ranking male was equal or higher ranking than the aggressor. In 8/20 cases, however, the high-ranking male was lower ranking than the aggressor. If victims were simply responding to the presence of a high-ranking male, regardless of their relationship with the aggressor, acoustic variation across the two conditions should be purely random. However, we found no evidence to support this hypothesis. Instead we continued to find the same pattern of acoustic change in all acoustic variables as a function of the presence (n = 12) or absence (n = 8) of an individual who was equal or higher ranking than the aggressor, despite the reduced sample size. A DFA accounted for a significant amount of variation in the screams according to whether the high-ranking individuals present were equal/higher or lower ranking than the aggressor [Wilks' lambda = 0.136, χ2 (df = 9) = 26.89, P = 0.001]. The function correctly classified 90.0% of cases (18/20; cross-validated) according to whether the scream was given in the presence or absence of an individual equal or higher ranking than the aggressor. A binomial test [(0.5) = 0.001 (two-tailed)] confirmed that this was at a level significantly above that expected by chance. Our additional analyses indicated that the audience effect was not a simple product of high-ranking individuals being present; victims took into account the relationship between the aggressor and the bystander.
Discussion
In this study, we have demonstrated that the victim screams of free-ranging chimpanzees varied acoustically according to the severity of aggression experienced by the caller. Compared with screams given in response to mild aggression, screams produced in response to severe aggression were longer, were higher in frequency, and given in longer and slower bouts, and the peak acoustic energy occurred later in the call. Information about the severity of the attack thus seems to be encoded in the temporal and absolute frequency aspects of the calls. This finding is in contrast to information about the social role of the caller in a conflict (victim or aggressor) that is mainly conveyed in the overall shape of the call (20). Other work has shown that chimpanzee screams are individually distinct (29), demonstrating the rich array of social information mapped onto this graded call system. It is likely that listening individuals can obtain complex information about an ongoing interaction from the calls alone, but playback experiments are needed to confirm this.
Our data support the hypothesis that one function of chimpanzee screams is to recruit aid during conflict. Victims modified the acoustic structure of their calls to increase the chances of eliciting help from high-ranking individuals when they most needed it, that is, during severe cases of aggression. If high-ranking individuals were present, victims facing severe aggression gave longer, higher-pitched screams, with peak energy later in the call, at a slower rate than when they were absent. These screams were acoustically consistent with screams given in cases of very severe aggression. This is despite the trend for the actual level of aggression to be less severe (less contact aggression) when high-ranking individuals were in the audience. If faced with mild aggression, however, chimpanzees did not modify their calls in the same way. The presence of high-ranking individuals alone, in other words, did not explain the acoustic shifts. In humans, it has been shown that the mere presence of other individuals increases levels of arousal, which in turn can influence the behavior of individuals (30). Although it seems reasonable to assume that chimpanzees were more aroused if high-ranking group members were nearby, our analyses demonstrated that this variable alone did not explain the observed differences in chimpanzee calling behavior. Similarly, relative party size did not affect call structure, further challenging the idea that simple differences in arousal levels drove the effects.
The selective modification of calls shown by these chimpanzees is a further example of this species' ability to engage in functional, or tactical, deception (31). Tactical deception has been defined as “acts from the normal repertoire of the agent deployed such that another individual is likely to misinterpret what the acts signify, to the advantage of the caller” (32). Our data provide evidence for this ability, and to our knowledge, this is the first systematic empirical evidence to show that nonhuman primates are able to exaggerate distress to manipulate other group members. Unfortunately, however, our study does not allow us to make conclusive statements about the mental processes that underlie this behavior, and instead our results are consistent with a number of hypotheses. First, one could argue that our results are an example of sophisticated context-specific vocal production. Receiving severe or mild aggression may be perceived by a chimpanzee as two different social contexts that warrant different types of vocal behavior. By analogy, callers may perceive the presence of a high-ranking bystander during severe aggression as a third type of context that warrants specific vocal behavior. Second, the callers may experience a change in confidence or emotional state in the presence of high-ranking individuals who may be capable of helping them, and this may cause the change in vocal structure. Last, callers may know about the presence of particular individuals in the audience and intend to communicate with them directly. In this context, callers may exaggerate the level of aggression to manipulate the receiver's perception or understanding of the attack, thereby increasing the chances of obtaining aid. Because of the visually dense habitat, individuals may be able to engage in such behavior, as there is only a small risk of being identified as unreliable signalers or experiencing other types of negative feedback.
Although our data are not able to elucidate the underlying mental processes involved, they nevertheless indicate that chimpanzees have an intricate understanding of social relationships between third parties. Chimpanzees only modified the structure of their screams in the presence of an audience that included individuals that were equal or higher ranking to the aggressor. In direct contrast to juvenile sooty mangabeys (14), simple heuristics, such as the mere presence of socially dominant males or other individuals that are higher ranking than the caller, did not explain the effects observed. In conclusion, our study documents how the acoustic structure of chimpanzee screams varies reliably with the severity of the aggression a victim experiences, potentially providing third-party individuals with rich information about the nature of ongoing agonistic interactions. We have also shown that these vocalizations are flexibly modified in the presence of individuals who could effectively aid them. In doing so, chimpanzees demonstrate an intricate social knowledge of third-party relationships, i.e., of exactly who is able to effectively challenge whom, and such triadic awareness appears to mediate their vocal production.
Materials and Methods
Study Site and Animals.
Data were collected by K.E.S. on the Sonso chimpanzee community of Budongo Forest, Uganda (33), between September 2003 and March 2004 and between January 2006 and March 2006. Budongo Forest covers an area of 428 km2 of moist, semideciduous tropical forest, between 1°35′ and 1°55′N and between 31°08′ and 31°42′E (34). The study site is located at an altitude of 1,100 m and has an annual rainfall of about 1,600 mm. There is a dry season between December and February in between two rainy seasons (35). Habituation of the Sonso community to humans began in 1990 and provisioning has never been used. At the end of the data-collection period in 2006, the community consisted of 72 individuals: 8 adult males, 21 adult females, 8 subadult males, 5 subadult females, 18 juveniles, and 12 infants.
Sampling Procedures.
Recording of vocalizations and other variables started whenever two chimpanzees engaged in an aggressive interaction. Due to the rarity of agonistic encounters during the data-collection period, all-occurrence sampling (36) was used. A total of 31 different individuals gave victim screams in response to aggression, 290 screams in total. Ten of these individuals were not entered into any of the analyses because we did not have enough recordings of them that were of sufficiently high quality. Analyses were conducted on victim screams, recorded from a total of 21 individuals: 4 adult males, 9 adult females, 6 subadult males, and 2 subadult females. Each of the 21 individuals contributed a total of four screaming bouts: two bouts in response to severe aggression (contact or directed aggression, see Table 1) and two bouts in response to mild aggression (nondirected aggression or posture threat; see Table 1). For this analysis, we had 228 screaming bouts available (range of 2–15 per individual for severe or mild aggression, respectively). To ensure an unbiased selection, we selected the first two bouts in the database with three measurable calls for each type of aggression. This resulted in a sample of 84 screaming bouts (21 individuals × 4 bouts).
Sound Recordings and Acoustic Analyses.
Vocalizations were recorded with a SENNHEISER K6/ME67 directional microphone and Sony portable DAT recorders (TCD-D8, TCD-D100). Recordings were transferred digitally onto a Toshiba laptop computer (Celeron 1.8 GHz), by using Cool Edit Pro LE (sampling rate of 44.1 kHz, 16 bits accuracy). Quantitative analysis of calls was carried out using Praat software version 4.3.17 (www.praat.org). The following settings were used. Pitch settings: range 50–1,000 Hz, optimized for voice analysis; spectrogram settings: window length = 0.05 s, dynamic range = 70 dB; formant settings: max formant = 5,500 Hz, no formants = 5, window length = 0.025 s, dynamic range = 30 dB. Formant analysis was performed by using a script written by M. Owren (personal communication).
To describe the overall acoustic structure of the screams, we measured the following temporal parameters: (i) bout length, number of calls given successively and separated from other bouts by at least 30 s of silence; (ii) duration of call (s); and (iii) rate, number of calls per second within the first 4 s of a bout.
We also measured six spectral parameters: (i) mean fundamental frequency, the average value of the fundamental frequency (F0) across the entire call (Hz); (ii) peak frequency, location in the frequency domain where maximum acoustic energy occurred in the F0, at the middle of the call (Hz); (iii) peak time, location in the temporal domain where maximum acoustic energy occurs (proportion of the call duration); (iv) first formant frequency, mean frequency of the first formant across the call (Hz); (v) transition onset, frequency of maximum energy in the F0 at call onset minus frequency of maximum energy in the F0 at call middle (Hz); (vi) transition offset, frequency of maximum energy in the F0 at call middle minus frequency of maximum energy in the F0 at call offset (Hz). Measurements of the frequencies at which maximum acoustic energy was present were obtained from creating spectral slices (amplitude plotted against frequency).
We performed checks for colinearity amongst the nine parameters and found that all had acceptable variance inflation factors (VIF < 7.0; range, 1.2–6.1), indicating that our parameters did not suffer from colinearity. To get a good estimate of the typical acoustic structure of an individual's screams we measured the first three recorded calls per bout sequence and calculated the median values for each of the nine acoustic parameters. All statistical analyses were then performed on the median values from each bout.
Severity of Aggression.
To understand the relation between call structure and aggressive events, we distinguished four types of aggressive interactions, based on the behavior of the aggressor (Table 1): Severe cases of aggression consisted of cases in which (i) the aggressor physically attacked the victim and (ii) cases in which the victims were pursued individually but no physical interaction took place. Mild cases of aggression consisted of (iii) nondirected aggression (e.g., aggressor charges through a party but does not deviate from trajectory to chase individuals) or (iv) postural threats. The aggressors' behaviors were recorded on check sheets, or, in some cases, spoken commentary was given and later transcribed. Spoken commentary was especially useful during encounters in which the aggressive behaviors experienced by an individual changed rapidly. This allowed us to accurately match each recorded victim scream with ongoing aggressive behaviors. Interobserver agreement between K.E.S. and the field assistant (Raimond Ogen, 2003–2004, or Geresomu Muhamuza, 2006) was a precondition for an interaction to be considered for analysis. If both observers could not confirm that the aggressor chimpanzee had performed one of the key behaviors listed in Table 1, vocalizations remained unclassified and were not used for further analysis.
Party Composition.
Free-ranging chimpanzees typically travel and forage in small parties usually consisting of fewer than a dozen individuals. The composition of parties is fluid with group members joining or leaving a party throughout the day. In line with previous studies at this site, a party was defined as consisting of all individuals that were within a 35-m radius of the focal individual (37, 38). We considered only adult and subadult individuals. Adults were defined as having had infants or being older than 15 years, and subadults were defined as being between 10 and 15 years old and were regularly seen without their mothers (33). We conducted regular 15-min scan samples to keep track of party composition. After each agonistic interaction, we conducted an additional scan, which typically coincided with the composition determined by the previous scan.
Relative Ranks of Party Members.
To determine the relative ranks of the Sonso community, we analyzed pant grunt data collected by both K.E.S. and the project field assistants during the study period, allowing us to determine a dominance hierarchy for the study period. On the basis of these data, we were able to assign dominance scores to the different individuals by calculating a “respect” index (38). Individuals with similar scores were grouped together to make six rank groups in 2003–2004 and five rank groups in 2006 (Newton Fisher, personal communication). For each conflict, we then determined whether or not there were any individuals in the party of the same or higher rank group relative to the aggressor.
Responses of Third-Party Chimpanzees to Agonistic Interactions.
Third parties responded to victims in a number of ways, including ignoring them or permitting their approach and subsequent proximity or grooming. Active responses were less common. These included displaying aggression toward the aggressor immediately or within 10 min of the encounter, approaching or following the victim in an affiliative manner during or immediately after the encounter and embracing the victim. All responses (passive or active) of third-party individuals were recorded on a check-sheet; however, in this study we analyzed only active responses.
Acknowledgments
We thank Nick Newton Fisher for his help with the rank data analysis and the Budongo Forest Project for facilitating this work, particularly V. Reynolds, F. Babweteera, R. Ogen, and G. Muhamuza. We thank Roger Mundry and Eric Bowman for help with statistical analyses. We are grateful to Roman Wittig, Marc Hauser, Richard Byrne, Lucy Bates, Jenny McClung, Bill McGrew, and Kate Arnold for discussion of ideas and comments on the paper. In Uganda, the Uganda Wildlife Authority, the Uganda National Council for Science and Technology, and the President's office gave permission to conduct this study. We acknowledge the Royal Zoological Society of Scotland for providing core funding for the Budongo Forest Project. This work was funded by the Biotechnology and Biological Sciences Research Council.
Abbreviation
- DFA
discriminant function analysis.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0706741104/DC1.
References
- 1.de Waal FBM, van Roosmalen A. Behav Ecol Sociobiol. 1979;5:55–56. [Google Scholar]
- 2.de Waal FBM. Science. 2000;289:586–590. doi: 10.1126/science.289.5479.586. [DOI] [PubMed] [Google Scholar]
- 3.Cheney DL, Seyfarth RM. Behaviour. 1989;110:258–275. [Google Scholar]
- 4.Wittig RM, Crockford C, Wikberg E, Seyfarth RM, Cheney DL. Proc R Soc B. 2007;274:1109–1115. doi: 10.1098/rspb.2006.0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Judge PG, Mullen SH. Anim Behav. 2005;69:1345–1355. [Google Scholar]
- 6.Judge PG. Am J Primatol. 1991;23:225–237. doi: 10.1002/ajp.1350230403. [DOI] [PubMed] [Google Scholar]
- 7.de Waal FBM, Aureli F. In: Reaching into Thought: The Minds of the Great Apes. Russon AE, Bard KE, Parker ST, editors. Cambridge, UK: Cambridge Univ Press; 1996. pp. 80–110. [Google Scholar]
- 8.Call J, Aureli F, de Waal FBM. Anim Behav. 2002;63:209–216. [Google Scholar]
- 9.Flack JC, de Waal FBM, Krakauer DC. Am Nat. 2005;165:E126–E139. doi: 10.1086/429277. [DOI] [PubMed] [Google Scholar]
- 10.Watts D, Colmenares F, Arnold K. In: Natural Conflict Resolution. Aureli F, de Waal FBM, editors. Berkeley: Univ of California Press; 2000. pp. 281–301. [Google Scholar]
- 11.de Waal FBM. Chimpanzee Politics. New York: Harper; 1982. [Google Scholar]
- 12.Townsend SW, Slocombe KE, Emery-Thompson M, Zuberbühler K. Curr Biol. 2007;17:R355–R356. doi: 10.1016/j.cub.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 13.Bergman TJ, Beehner JC, Cheney DL, Seyfarth RM. Science. 2003;302:1234–1236. doi: 10.1126/science.1087513. [DOI] [PubMed] [Google Scholar]
- 14.Range F, Noë R. Anim Behav. 2005;69:445–452. [Google Scholar]
- 15.Gouzoules S, Gouzoules H, Marler P. Anim Behav. 1984;32:182–193. [Google Scholar]
- 16.Gouzoules H, Gouzoules S, Marler P. In: The Cayo Santiago Macaques. Rawlings RG, Kessler MJ, editors. Albany: State Univ New York Press; 1986. pp. 111–129. [Google Scholar]
- 17.Gouzoules H, Gouzoules S. Behaviour. 1990;115:327–347. [Google Scholar]
- 18.Gouzoules H, Gouzoules S. Anim Behav. 1989;32:182–193. [Google Scholar]
- 19.de Waal FBM, van Hooff JARAM. Behaviour. 1981;77:164–198. [Google Scholar]
- 20.Slocombe KE, Zuberbühler K. J Comp Psychol. 2005;119:67–77. doi: 10.1037/0735-7036.119.1.67. [DOI] [PubMed] [Google Scholar]
- 21.Wich SA, Sterck EHM. Am J Primatol. 2003;60:155–159. doi: 10.1002/ajp.10102. [DOI] [PubMed] [Google Scholar]
- 22.Di Bitetti MS. Anim Behav. 2005;69:911–919. doi: 10.1006/anbe.1996.0416. [DOI] [PubMed] [Google Scholar]
- 23.Brosnan SF, de Waal FBM. Evol Commun. 2003;4:211–224. [Google Scholar]
- 24.Caine NG, Addington RL, Windfelder TL. Anim Behav. 1995;50:53–60. [Google Scholar]
- 25.Pollick AS, Gouzoules H, de Waal FBM. Anim Behav. 2005;70:1273–1281. [Google Scholar]
- 26.Cheney DL, Seyfarth RM. Behaviour. 1985;94:739–751. [Google Scholar]
- 27.Hauser MD, Marler P. Behav Ecol. 1993;4:194–205. [Google Scholar]
- 28.Mundry R, Sommer C. Anim Behav. 2007 doi: 10.1016/j.anbehav.2006.12.028. [DOI] [Google Scholar]
- 29.Kojima S, Izumi A, Ceugniet M. Primates. 2003;44:225–230. doi: 10.1007/s10329-002-0014-8. [DOI] [PubMed] [Google Scholar]
- 30.Zajonc RB. Science. 1965;149:269–274. doi: 10.1126/science.149.3681.269. [DOI] [PubMed] [Google Scholar]
- 31.Byrne RW, Whiten A. Man. 1992;27:609–627. [Google Scholar]
- 32.Byrne RW, Whiten A. Behav Brain Sci. 1988;11:267–271. [Google Scholar]
- 33.Reynolds V. The Chimpanzees of Budongo Forest: Ecology, Behaviour and Conservation. Oxford: Oxford Univ Press; 2005. [Google Scholar]
- 34.Eggeling WJ. J Ecol. 1947;34:20–87. [Google Scholar]
- 35.Newton-Fisher NE. Afr J Ecol. 1999;37:344–354. [Google Scholar]
- 36.Altmann J. Behaviour. 1974;49:227–267. doi: 10.1163/156853974x00534. [DOI] [PubMed] [Google Scholar]
- 37.Bates L. St. Andrews, Scotland: Univ of St. Andrews; 2005. PhD thesis. [Google Scholar]
- 38.Newton-Fisher NE. Primates. 2004;45:81–87. doi: 10.1007/s10329-003-0064-6. [DOI] [PubMed] [Google Scholar]