Abstract
The control of anthocyanin accumulation in maize by the cooperation of the basic helix–loop–helix (bHLH) protein R with the MYB transcription factor C1 provides one of the best examples of plant combinatorial transcriptional control. Establishing the function of the bHLH domain of R has remained elusive, and so far no proteins that interact with this conserved domain have been identified. We show here that the bHLH domain of R is dispensable for the activation of transiently expressed genes yet is essential for the activation of the endogenous genes in their normal chromatin environment. The activation of A1, one of the anthocyanin biosynthetic genes, is associated with increased acetylation of histone 3 (H3) at K9/K14 in the promoter region to which the C1/R complex binds. We identified R-interacting factor 1 (RIF1) as a nuclear, AGENET domain-containing EMSY-like protein that specifically interacts with the bHLH region of R. Knockdown experiments show that RIF1 is necessary for the activation of the endogenous promoters but not of transiently expressed genes. ChIP experiments established that RIF1 is tethered to the regulatory region of the A1 promoter by the C1/R complex. Together, these findings describe a function for the bHLH domain of R in linking transcriptional regulation with chromatin functions by the recruitment of an EMSY-related factor.
Keywords: anthocyanin, BRCA2, chromatin
The evolution of multicellular organisms was accompanied by an increase in the complexity of gene regulatory mechanisms, reflected in the dramatic expansion of transcription factor families and in the intricate interactions between regulatory proteins and cis-regulatory elements in what is commonly known as combinatorial transcriptional control. Superimposed on this complexity is the understanding that histone modifications and chromatin structure are intimately linked to the regulatory activity of many transcription factors (1). Establishing the interactions between combinatorial gene regulation and histone functions thus poses a problem of significant biological importance.
The basic helix–loop–helix (bHLH) family of transcription factors is among the largest in animals and plants (2). bHLH domains are characterized by the presence of an ≈18-residue hydrophilic basic helix followed by two amphipathic α-helices separated by a loop (3, 4). When present, basic regions contribute to the binding of bHLH factors to DNA, through cis-regulatory elements, termed E-boxes, with the CANNTG consensus, whereas HLH motifs participate in homodimer or heterodimer formation (4). Maize R was the first plant bHLH transcription factor described (5). R belongs to a small gene family, which includes B, and R/B specify anthocyanin pigmentation in different plant tissues (6). They participate in the transcriptional regulation of the anthocyanin pathway genes through the cooperation with the R2R3-MYB transcription factor C1 or its paralog, PL1 (7). C1 and R/B physically interact through the MYB domain of C1 and the N-terminal region of R (which does not contain the bHLH motif) (8, 9), and C1 makes direct DNA contacts with specific cis-regulatory elements, which, in the case of the A1 gene (encoding dihydroflavonol reductase, DFR), correspond to the high- and low-affinity P1 binding sites (haPBS and laPBS, respectively) (10, 11). R/B belong to the group IIIf of plant bHLH factors (12), a subfamily that is shared with similar anthocyanin regulators in various plants as well as with the Arabidopsis GL3/EGL3 regulators of epidermal cell patterning (13). All of these factors function by interacting with R2R3-MYB proteins, recognizing particular signature motifs in the corresponding MYB DNA-binding domains (9, 14). In addition, members of this group of bHLH proteins contain a conserved ACT-like domain at the C termini, which participates in homodimer formation (15). Despite the extensive knowledge implicating the cooperation of MYB and bHLH factors in a number of important plant functions (16), the mechanism by which the bHLH region contributes to protein function has remained elusive.
Here we describe a function for the bHLH region of R in recruiting an EMSY-like factor to flavonoid biosynthetic gene promoters participating in chromatin functions. We show that the deletion of the bHLH region of R has minor consequences on the transient expression of reporter constructs but is essential for the activation of flavonoid genes in their normal chromatin environment. Highlighting a role of histone modification in the regulation of flavonoid gene expression, we show that H3K9/14 acetylation is intimately associated with the recruitment of R to DNA. We identified R-interacting factor 1 (RIF1) as a nuclear maize factor with homology to the BRCA2-interacting EMSY N-terminal region (the ENT domain), which specifically interacts with the bHLH region of R. EMSY associates with “Royal Family” domain proteins (HP1 and BS69), and it relocalizes to sites of DNA damage, consistent with the role of chromatin remodeling in DNA repair (17). RIF1 is an example of how plants combined the ENT domain and a royal family domain (i.e., AGENET) into one protein. Mutations that abolish the R–RIF1 interaction significantly decrease pigment formation, with a similar effect observed when RIF1 expression is knocked down. Together, our findings reveal a role of bHLH domains in tethering non-HLH proteins to DNA and modulating gene expression by histone modifications.
Results
An Essential Function for the bHLH Domain of R.
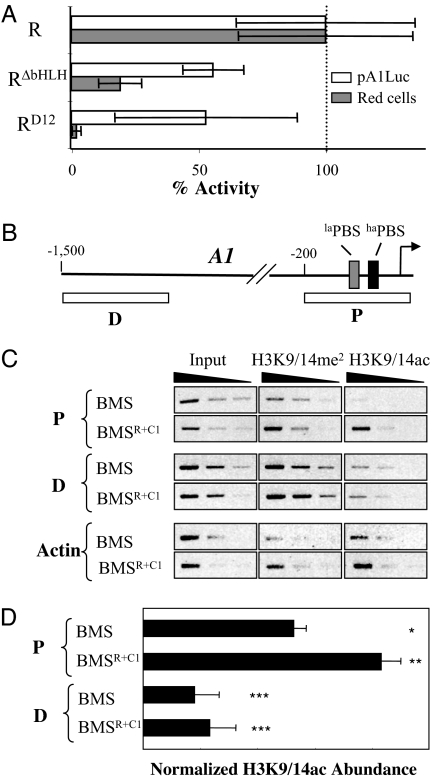
The bHLH region of R/B is very conserved, yet in transient expression experiments it appears to be dispensable for the activation of reporter constructs containing promoters from the anthocyanin pathway (8, 18, 19). In agreement with these studies, the deletion of the bHLH of R has only a moderate effect on the activation of the pA1Luc reporter (≈40% reduction) when cobombarded with C1 into Black Mexican Sweet (BMS) maize cells (Fig. 1A, RΔbHLH). The previously characterized RD12 mutant, containing an insertion of three amino acids at the C termini of the second helix of the bHLH motif, was originally identified as a Ds transposition-derived mutation that significantly reduces anthocyanin accumulation and flavonoid gene expression (18). RD12 shows a similarly moderate reduction on the normalized expression of the pA1Luc reporter (Fig. 1A, RD12). However, both RΔbHLH and RD12 show a dramatic reduction in the number of cells accumulating anthocyanins (≈80% reduction for RΔbHLH and 98% reduction for RD12) (Fig. 1A) when these mutants of R were cobombarded with C1 (p35SC1) into BMS cells. These results are in agreement with previous in vivo findings showing that the RD12 mutation has a 97% reduction in anthocyanin accumulation in maize aleurones (18) and demonstrate that the bHLH region of R is necessary for the expression of endogenous flavonoid gene promoters, but not for the transient expression of reporter constructs. The reproducible difference in the activity of RΔbHLH and RD12 (Fig. 1A) might reflect different stability of these proteins in the transient expression system. Alternatively, the bHLH motif may have both positive and negative regulatory activities; the D12 mutation perhaps abolishes only the positive regulatory activity whereas the deletion of the bHLH present in RΔbHLH might abolish both, resulting in increased activation of the anthocyanin genes when compared with RD12.
Fig. 1.
The bHLH region of R and chromatin functions. (A) Activation of the A1 promoter and red cells by mutants in the bHLH region of R. Shown are results of transient expression experiments after cobombardment of cultured maize BMS cells with R and bHLH mutants of R driven by the CaMV 35S promoter (p35S) together with p35SC1 and pA1Luc. A construct expressing GUS from pUbi (pUbiGUS) was included in all bombardments as a normalization control. Each treatment was done in triplicate, and the number of red cells and the activity of luciferase were normalized for GUS activity. The fold activation corresponds to the ratio of each particular treatment and the treatment with pA1Luc without activator. The scale for the normalized red cells is in arbitrary units of red cells/GUS. The average values are shown, and the error bars indicate the standard deviation of the samples. (B) Structure of the A1 gene promoter indicating the proximal (P) and distal (D) fragments analyzed by ChIP. The arrow corresponds to the transcription start site at position +1, and numbers in the promoter are in reference to this. The laPBS and haPBS recognized by C1 (11) are indicated. (C and D) Enrichment of H3K9/14ac in the P but not in the D region of the A1 promoter in BMSR+C1 cells compared with BMS, determined by semiquantitative PCR using three 4-fold serial dilutions of chromatin immunoprecipitated DNA (C) and by quantitative PCR (D). The normalization of the H3K9/14ac abundance is described in Materials and Methods. Different asterisk numbers indicate sample groups that are statistically significantly different (P < 0.05).
One way to explain the difference in the activation of the endogenous genes and the transiently expressed constructs is that epigenetic modifications, such as chromatin modifications, are different for the endogenous and transiently expressed promoters. Micrococcal nuclease protection experiments, showing a significantly higher sensitivity of the transiently expressed pA1Luc construct compared with the endogenous A1 promoter [see supporting information (SI) Fig. 5], may suggest that, at most, a small fraction of the pA1Luc plasmid is nucleosome-associated in BMS cells under the conditions tested. This is consistent with earlier studies in animal cells that have shown that transiently introduced and stably integrated genes can have different chromatin structures and hence can be transcribed differently (20, 21).
To investigate whether histone modifications are associated with the activation of the flavonoid genes by C1 and R, we analyzed previously described (22) transgenic BMS cells constitutively expressing R and C1 from the 35S promoter (BMSR+C1). ChIP experiments were performed in parallel on BMS and BMSR+C1 cells with commercial antibodies that recognize several different modifications of H3, including H3K9/14ac, H3K9/14me2, H3K4me2, H3K4me3, and H3K27me2. The most striking and reproducible histone modification difference in the A1 gene promoter observed between BMS and BMSR+C1 is in the acetylation of Lys-9 and Lys-14 in H3 (H3K9/14ac), particularly in the proximal region (Fig. 1B, P) immediately upstream of the transcription start site, a promoter fragment necessary and sufficient for the regulation by C1 and R (10, 19) (Fig. 1C). H3K9/14ac is significantly enriched in histones associated with the A1 promoter in BMSR+C1 cells compared with BMS cells that do not express the pathway regulators (Fig. 1D). In contrast, the levels of H3K9/14ac are similar in a distal region (probe D; Fig. 1B) positioned 1.5 kb upstream of the transcription start site (Fig. 1D). Similarly, no difference between BMS and BMSR+C1 cells was observed in H3K9/14ac in a gene unrelated to the flavonoid pathway (Fig. 1C, actin).
To ensure that the differences observed between BMS and BMSR+C1 cells were not a consequence of the cell culture selection process, we investigated the status of H3K9/14ac between B-I (fully pigmented) and b (no pigment) maize plants. As we established for the BMS cells (Fig. 1C), the expression of B-I correlated with increased accumulation of H3K9/14ac at the A1 gene promoter in the proximal, but not in the distal region (SI Fig. 6). Taken together, these results indicate that the bHLH region of R is essential for the activation of flavonoid genes in their normal chromatin environment and that the R/B and C1/PL1 pathway regulators influence histone modifications specifically associated with the proximal region of the A1 promoter.
Identification of RIF1 as an ENT Domain Protein That Specifically Interacts with the bHLH Domain of R.
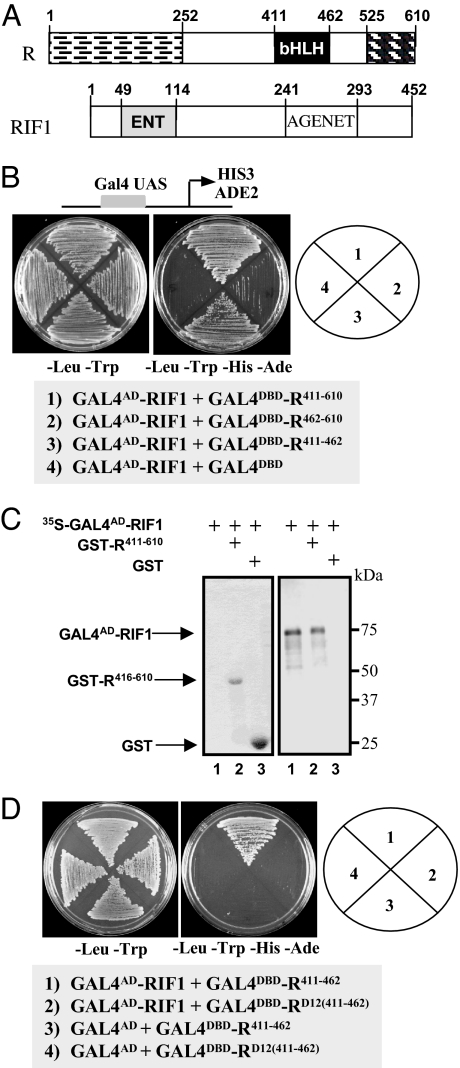
To determine the function of the bHLH region of R, we carried out yeast two-hybrid screens using the C-terminal region of R (residues 411–610) (Fig. 2A) fused to the GAL4 DNA-binding domain (GAL4DBD–R411–610) in the PJ69.4a strain (23). A total of ≈3.5 × 106 clones were screened, resulting in the identification of several positive clones, including two bHLH factors. Four different clones corresponding to RIF1 were identified, all of them containing the complete ORF for a 452-aa protein (Fig. 2A). The bHLH region of R (R411–462; Fig. 2B) is sufficient for the interaction of R with RIF1, and GST pull-down experiments using in vitro transcribed/translated RIF1 (35S-GAL4AD-RIF1) and Escherichia coli-expressed GST-R411–610 provided an independent biochemical confirmation for the interaction (Fig. 2C).
Fig. 2.
RIF1 corresponds to an EMSY-like protein that interacts with the bHLH domain of R. (A) Schematic representation of the structure of R and RIF1 indicating the position of the domains discussed in this study. (B) Yeast two-hybrid interaction of GAL4AD-RIF1 with the C-terminal region of R including the bHLH (R411–610), the C-terminal region of R excluding the bHLH (R462–610), and the R bHLH domain alone (R411–462). All R constructs were fused to the Gal4DBD at the N terminus in pBD-GAL4, which is the plasmid used in the empty vector control. (C) Autoradiogram (Right) and stained gel (Left) of an SDS/PAGE of a GST pull-down using GST-R411–610 (including the bHLH) as bait (lane 2) with an in vitro transcribed and translated GAL4AD-RIF1. GAL4AD-RIF1 was radiolabeled with [35S]methionine as shown in the input lane (lane 1). GST alone was used as a negative control (lane 3). (D) Yeast two-hybrid interaction of GAL4AD-RIF1 with the bHLH of R containing the D12 allele sequence [GAL4DBDRD12(411–462)]. GAL4DBDR411–462 was used as positive control, and the empty pAD-Gal4 plasmid was used as negative control. Yeast two-hybrid assays were done by using yeast strain PJ69.4a (23) containing the HIS3 and ADE2 genes under the control of Gal4-binding sites. Growth in the – Leu–Trp–His–Ade plate is indicative of activation.
We rationalized that, if RIF1 had anything to do with the R activities described in Fig. 1, then the D12 mutation should impair the R/RIF1 interaction. To determine whether this was the case, we fused the bHLH region of RD12 to the GAL4DBD [GAL4DBD-RD12(411–462)] and tested it for interaction with GAL4AD-RIF1 in yeast. No interaction was observed (Fig. 2D, compare 1 and 2), indicating that the D12 mutation in R abolishes the interaction with RIF1.
RIF1 encodes a plant protein harboring a unique domain architecture (Fig. 2A and SI Fig. 7). The ENT domain was first described as necessary and sufficient for the interaction of EMSY and BRCA2 (17). In humans, it is found only in EMSY. In plants, however, the ENT domain is present in a small plant protein family, but usually in association with an AGENET domain, a member of the royal family, which includes the Tudor domain. Proteins belonging to this family are often associated with chromatin functions (24). Whereas the ENT domain of RIF1 homodimerizes in yeast (SI Fig. 8), as is the case for the EMSY ENT domain (25), neither the ENT nor the AGENT domain of RIF1 is sufficient for the interaction with R, suggesting that multiple regions in RIF1 are involved (data not shown). Together, these results provide evidence for the interaction of a bHLH domain with an ENT/AGENET-containing protein.
RIF1 Displays Speckled Nuclear Localization and Is Necessary for the Activation of Maize Flavonoid Genes.
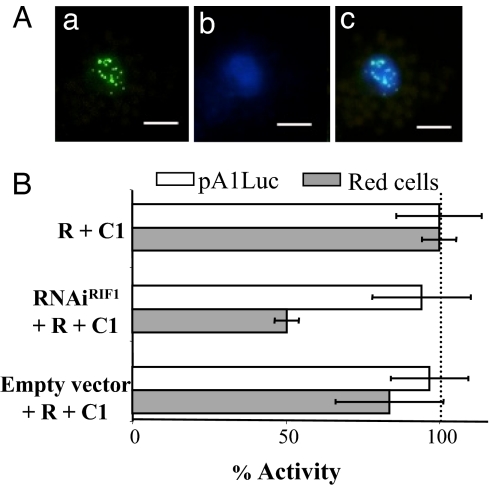
To determine whether RIF1 localizes to the nucleus, as would be expected for an R partner, we fused RIF1 to GFP and investigated the localization of the RIF1-GFP fusion in maize protoplasts (Fig. 3A) and in Nicotiana benthamiana leaf epidermal cells (SI Fig. 9). In both cases, RIF1-GFP was detected exclusively in the nucleus in a distinct speckled pattern. No difference in the RIF1-GFP localization pattern was observed when R (SI Fig. 9) or RΔbHLH (data not shown) were coexpressed, suggesting that the speckled nuclear localization of RIF1 is independent of the similar pattern observed for R-GFP (15).
Fig. 3.
RIF1 is nuclear and necessary for anthocyanin accumulation. (A) GFP fluorescence of maize protoplasts transiently transformed with RIF1-GFP. (a) GFP fluorescence. (b) DAPI stain. (c) Merged images. (Scale bars: 5 μm.) (B) Activation of the A1 promoter (open bars) and red cells (gray bars) by p35SR and p35SC1 in the absence (−) or presence of plasmid expressing a double-stranded fragment of RIF1 (RNAiRIF1) or with the corresponding empty vector control. All other experimental details are described in the legend of Fig. 1. p35SRenilla was used as a normalization control.
We next investigated whether RIF1 is necessary for the R regulatory activity. Several ESTs for RIF1 have been identified, including some from BMS cells (SI Table 1), indicating that this gene is ubiquitously expressed, consistent with the ability of maize to accumulate anthocyanins in almost every plant organ. Because no maize RIF1 mutants are currently available, we generated a construct that, when expressed, would generate a dsRNA that should target RIF1 for degradation (p35SRNAiRIF1). A 500-bp fragment of the RIF1 coding region (SI Fig. 7) in the forward and reverse orientations separated by the rice waxy-a intron was cloned in the pMCG161 vector (www.chromdb.org). We then investigated the effect of p35SRNAiRIF1 on the ability of R and C1 to activate anthocyanin biosynthesis. p35SRNAiRIF1 very significantly (P < 0.01) reduces the normalized number of red cells induced by R+C1 in BMS cells (Fig. 3B, RNAiRIF1), whereas no significant difference was observed when R+C1 were cobombarded with the pMCG161 empty vector (Fig. 3B, empty vector). These results confirm that RIF1 participates in the C1/R regulatory function. We reasoned that, if this activity of RIF1 is related to its role in chromatin functions, then the knocking-down of RIF1 should affect anthocyanin accumulation but not the expression of the pA1Luc reporter. When we assayed luciferase and normalized it for Renilla activity provided by the cobombarded p35SRen plasmid, we found that the expression levels of pA1Luc remained the same (Fig. 3B). Taken together, these results indicate that RIF1 is a nuclear protein that is specifically required for the regulation of the endogenous flavonoid genes by C1 and R.
R Recruits RIF1 to the A1 Gene Promoter.
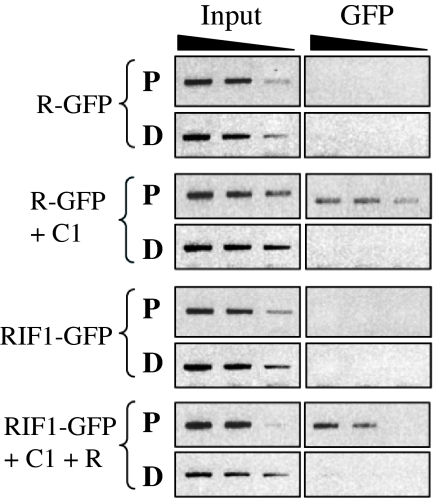
To determine whether RIF1 would be recruited to the A1 promoter in a C1/R-dependent fashion, we adopted a maize protoplast transient expression system (26, 27), which provides significantly higher transformation efficiency than the bombardment of BMS cells. Previously, we determined that p35SR-GFP is equally active as p35SR in promoting anthocyanin accumulation (15). When protoplasts were electroporated with p35SR-GFP (R-GFP) and ChIP experiments were performed with GFP-specific antibodies and probed on the P and D regions of the endogenous A1 promoter (Fig. 1B), no recruitment of R to DNA was observed (Fig. 4, R-GFP), as expected based on current models on how C1 and R function (28). However, when p35SC1 (C1) was cotransformed, a very significant recruitment of R-GFP to DNA was observed, yet only to the P region of the A1 promoter (Fig. 4, R-GFP + C1). No significant DNA recruitment of RIF1-GFP was observed in the absence of R and C1 (Fig. 4, RIF1-GFP). However, the presence of R and C1 was sufficient to tether RIF1 to the proximal region, yet not to the distal region, of the A1 promoter (Fig. 4, RIF1-GFP + C1 + R). Taken together, these results show that RIF1 is part of the C1/R regulatory complex and provide evidence that, in vivo, C1 is essential for the assembly of the C1/R/RIF1 complex on A1.
Fig. 4.
The recruitment of RIF1 to the A1 promoter depends on the presence of C1 and R. Plasmids expressing R-GFP, C1, RIF1, and RIF1-GFP were transformed into maize protoplast, and ChIP experiments were performed by using antibodies against GFP. The semiquantitative PCR of chromatin immunoprecipitated material was performed as described in Fig. 1C.
Discussion
Our results provide a function for the bHLH region of the maize transcription factor R in linking transcriptional activation of flavonoid biosynthetic genes with chromatin functions. The finding that the R bHLH region is essential for the regulation of the endogenous genes in maize cells yet is dispensable for the activation of transiently expressed reporters reconciles a number of conflictive reports (8, 18, 19) while providing evidence that it may play a role in chromatin functions. The identification of RIF1 as a partner for the bHLH domain of R further supports the link between R function and chromatin structure. The presence in RIF1 of an AGENET motif, which is closely related to Tudor domains (24), further highlights its likely participation in chromatin functions. In addition to the AGENET, RIF1 also contains an ENT domain, located toward the N terminus of the protein (Fig. 2). Whereas humans contain a single ENT domain protein, the BRCA2-interacting EMSY factor (17), plant genomes exhibit several genes that code for ENT domain-containing proteins [nine in Arabidopsis and six in rice (J.M.H. and E.G., unpublished data, and ref. 17)]. ENT domains in plants are almost always accompanied by AGENET domains, as we found for RIF1. The function of the ENT domain remains unknown; however, a conserved sequence adjacently located to the ENT domain mediates the interaction of EMSY with HP1β and BS69 (29). The structure of the EMSY ENT domain was recently solved, and it revealed that EMSY forms homodimers through its ENT domain (25). As expected, given the high sequence identity, the RIF1 ENT domain mediates RIF1 homodimer formation (SI Fig. 8).
RIF1 accumulates in the nucleus of maize and Nicotiana cells in discrete speckles, yet the presence or absence of R does not influence the nuclear patterning of RIF1 (Fig. 3A and SI Fig. 9). Interestingly, EMSY is also exclusively nuclear and forms speckles that colocalize with γ-H2AX after DNA damage (29). LHP1, one of the Arabidopsis HP1-like factors, also shows a speckled pattern (30). Many plant nuclear proteins localize to heterogenous nuclear foci (31), yet the presence of royal family domains in both LHP1 (chromodomain) and RIF1 (AGENET), and the interaction of EMSY with HP1 (17), prompted us to investigate whether LHP1 and RIF1 might associate. Yeast two-hybrid experiments failed to detect an interaction between LHP1 and RIF1, which is consistent with the absence of the EMSY HP1-interacting region in RIF1 (data not shown). Given that both the chromodomain in HP1 and the AGENET domain of RIF1 belong to the royal family, it is possible that the functions contributed by HP1 to the EMSY complex in metazoans are fulfilled by the RIF1 AGENET domain (similar to the chromodomain in HP1) in the C1/R/RIF1 complex.
EMSY interacts with BRCA2 and colocalizes with γ-H2AX, which strongly suggests that EMSY is part of the BRCA2-containing DNA repair complex. Coordinating the recruitment of chromatin-remodeling proteins by EMSY strengthens the link between BRCA2 and chromatin repair (17, 32). This is not surprising given that DNA repair, like transcription, is a process that is challenged by chromatin structure. In addition to its role in DNA repair, EMSY regulates the transcriptional activity of BRCA2. But, unlike what our studies suggest with regard to the function of RIF1, EMSY is a transcriptional repressor that interacts with the activation domain of BRCA2 (17), possibly by association with chromatin-remodeling proteins.
The recruitment of RIF1 to the C1/R enhanceosome is mediated by the bHLH region of R. bHLH domains are typically expected to interact with other bHLH proteins, and the finding that the corresponding region of R is essential for the interaction with RIF1 (Fig. 2) opens the possibility for additional protein–protein interactions mediated by similar domains in other plant or animal bHLH proteins. Given the high identity in the bHLH region between R and several other plant proteins (12, 33–35) it is very likely that RIF1 is shared by a number of regulatory complexes. Until maize RIF1 loss-of-function mutants become available, the identity of those regulatory complexes will remain unknown. Interestingly, however, mutations in the most related gene of Arabidopsis (At5g13020) display a number of developmental defects (J.M.H. and E.G., unpublished data).
Our results also provide in vivo evidence that the assembly of the C1/R enhanceosome on the proximal region of the A1 promoter requires C1 and that R, despite the presence of the bHLH region, is unable to be recruited to the cis-regulatory regions important for A1 regulation in the absence of C1 (Fig. 4). Such a model had been predicted from extensive transient expression experiments and mutational analyses of the A1 promoter (9, 11, 19, 28), but a direct in vivo tethering of C1 to the A1 promoter was never shown before. Most significant from a mechanistic perspective, however, our results support a model in which C1 is primarily responsible for specifying the promoters to which R needs to be recruited, while R furnishes a docking platform for the recruitment of additional factors to the complex, including RIF1, as shown in this study. As is the case for some of the other factors recruited by R [e.g., the WD-40 factor PAC1 (36) or the R dimerization (15)], the specific role that RIF1 plays in the complex remains to be fully determined. However, our results strongly suggest that the recruitment of the C1/R/RIF1 complex to the proximal region of the A1 promoter is required for chromatin functions that include the acetylation of H3K9/14 and ultimately results in the expression of A1. H3K9/14 acetylation in gene promoter regions has been extensively associated with a transcriptional activatory function (37). In conclusion, our study uncovered the recruitment of an EMSY-related protein to the regulatory region of the A1 gene as a function for a plant bHLH domain. This finding provides a link between gene transcriptional regulatory mechanisms and chromatin functions in one of the best-described plant regulatory systems to date and highlights a previously unknown function of bHLH domains.
Materials and Methods
Supporting Information.
For additional details, see SI Text, SI Table 1, and SI Figs. 5–9.
Plant Materials.
The generation and analysis of the BMS cells expressing p35SC1 and p35SR were previously described (22). B-I Pl seeds were kindly provided by Vicki Chandler (University of Arizona, Tucson, AZ), and the M142X stock (b Pl1 R1-g) was obtained from the Maize Genetics Cooperation Stock Center (http://maizecoop.cropsci.uiuc.edu).
Protoplast Isolation and Electroporation.
Protoplasts from 9- to 12-day-old etiolated maize seedlings were obtained essentially as previously described (38), with the modifications described in SI Text. Electroporation was carried out on ≈105 protoplasts with 30 μg of DNA using 100 V/cm, 10 msec, and one pulse with a BTX Electro-Square-Porator T820. After electroporation, protoplasts were incubated for 12–16 h in the dark at room temperature. The fluorescence furnished by p35SGFP was used to calculate the transformation efficiency, which was usually in the 30–50% range.
Plant Transformation and Confocal Microscopy.
RIF1-GFP was transformed into Agrobacterium tumefaciens strain GV3101, and infiltration was performed as described (15). Localization of GFP was determined by confocal laser scanning microscopy on a Nikon Eclipse E600 microscope.
Protein–Protein Interaction Analyses.
Yeast two-hybrid library screens were performed by using a bait containing the C-terminal 200 aa of R fused to the GAL4 DNA-binding domain in the pBD-Gal4 plasmid (Stratagene) and two maize cDNA libraries in the pAD-Gal4 vector (Stratagene) obtained from RNA extracted from immature B73 tassels (provided by Robert Schmidt, University of California, San Diego, CA) or from young maize seedlings (provided by Marja Timmermans, Cold Spring Harbor Laboratory, Cold Spring Harbor, NY). The screen was performed in the PJ69.4a yeast strain (23), and positives were selected in synthetic media lacking leucine and tryptophan (for selection of the prey and bait plasmids, respectively) and histidine and adenine (for the selection of interacting partners). Plasmid DNA was isolated from putative positives, transformed into E. coli DH5α cells (Invitrogen), and, after plasmid purification, retransformed into PJ69.4a with the bait. A combined total of ≈3.5 × 106 transformants was screened. For GST pull-down experiments, the RIF1 cDNA (GenBank accession no. EF647588) was cloned into the vector pGEX-KG (39) and expressed in the E. coli BL21(DE3) PlyS. Induction, purification, and GST pull-down experiments were performed as previously described (15).
ChIP Analyses.
Approximately 60 mg of tissue (or ≈104 protoplasts) were used for each immunoprecipitation. BMS cells and maize tissues were immersed in buffer A (0.4 M sucrose/10 mM Tris·HCl, pH 8.0/1 mM EDTA/1 mM PMSF), and protoplasts were resuspended in ES buffer (0.6 M mannitol/5 mM Mes, pH 5.7/10 mM KCl) containing 1% formaldehyde and incubated under vacuum for 20 min. Glycine was added to 0.1 M, and incubation was continued for an additional 10 min. ChIP experiments were carried out essentially as described (40) with the modifications indicated in SI Text. Quantitative PCR was performed by using standard PCR conditions with 1 μCi of [α-32P]dCTP, 0.2 mM each of dATP, dTTP, and dGTP, 0.02 mM of dCTP, and 1 unit of TaqDNA polymerase (Gene Script) for 24–30 cycles. Amplified products were immobilized on filter paper, TCA-precipitated, and counted on a scintillation counter. Normalization was performed by calculating the ratio to the signal of the reference fragment after scaling by the signal provided by the input.
Transient Expression Experiments in Maize Cells.
Microprojectile bombardment of maize BMS suspension cells and transient expression assays for luciferase and β-glucuronidase (GUS) were performed as previously described (9). For transient expression assays of firefly luciferase and Renilla luciferase (Renilla), the Dual-Luciferase Reporter Assay System (Promega) was used. For each microprojectile preparation, the mass of DNA was adjusted to 10 μg with p35SBAR (22) to equalize the amount of 35S promoter in each bombardment. One microgram of each regulator and 3 μg of reporter plasmid (pA1Luc) were used in each bombardment. To normalize the number of red cells (counted 36–48 h after bombardment) or the luciferase activity to the Renilla (Fig. 3B) or GUS (Fig. 1A) activity, 3 μg of p35SRen (41) or pUbiGUS (9) was included in each bombardment. Each treatment was done in triplicate, and entire experiments were repeated at least twice.
Supplementary Material
Acknowledgments
We thank Robert Schmidt and Marja Timmermans for the maize yeast two-hybrid libraries; Valerie Gaudin (Institut National de la Recherche Agronomique, Versailles, France) for the LHP1 construct; Sue Wessler (University of Wisconsin, Madison, WI) and Jerry Kermicle (University of Georgia, Athens, GA) for the RD12 constructs and seeds, respectively; Paula Elooma and Teemu Teeri (both of University of Helsinki, Helsinki, Finland) for the p35SRen construct; David Spector and Yuda Fang (both of Cold Spring Harbor Laboratory, Cold Spring Harbor, NY) for assistance with interpreting the nuclear speckle organization of RIF1; and J. C. Jang and laboratory members for help with standardizing the maize protoplast transient expression system. This research was supported by National Research Initiative Grant 2007-35318-17805 from the U.S. Department of Agriculture Cooperative State Research, Education, and Extension Service (to E.G.).
Abbreviations
- bHLH
basic helix–loop–helix
- BMS
Black Mexican Sweet
- RIF1
R-interacting factor 1
- GUS
β-glucuronidase.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. S.H. is a guest editor invited by the Editorial Board.
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. EF647588).
This article contains supporting information online at www.pnas.org/cgi/content/full/0705629104/DC1.
References
- 1.Kouzarides T. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 2.Riechmann JL, Heard J, Martin G, Reuber L, Jiang C, Keddie J, Adam L, Pineda O, Ratcliffe OJ, Samaha RR, et al. Science. 2000;290:2105–2110. doi: 10.1126/science.290.5499.2105. [DOI] [PubMed] [Google Scholar]
- 3.Murre C, McCaw PS, Baltimore D. Cell. 1989;56:777–783. doi: 10.1016/0092-8674(89)90682-x. [DOI] [PubMed] [Google Scholar]
- 4.Murre C, Baltimore D. In: Transcriptional Regulation. McKnight SL, Yamamoto KR, editors. Cold Spring Harbor, NY: Cold Spring Harbor Lab Press; 1992. [Google Scholar]
- 5.Ludwig R, Habera LF, Dellaporta SL, Wessler SR. Proc Natl Acad Sci USA. 1989;86:7092–7096. doi: 10.1073/pnas.86.18.7092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ludwig SE, Wessler SR. Cell. 1990;62:849–851. doi: 10.1016/0092-8674(90)90259-h. [DOI] [PubMed] [Google Scholar]
- 7.Cone KC, Cocciolone SM, Burr FA, Burr B. Plant Cell. 1993;5:1795–1805. doi: 10.1105/tpc.5.12.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goff SA, Cone KC, Chandler VL. Genes Dev. 1992;6:864–875. doi: 10.1101/gad.6.5.864. [DOI] [PubMed] [Google Scholar]
- 9.Grotewold E, Sainz MB, Tagliani L, Hernandez JM, Bowen B, Chandler VL. Proc Natl Acad Sci USA. 2000;97:13579–13584. doi: 10.1073/pnas.250379897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grotewold E, Drummond BJ, Bowen B, Peterson T. Cell. 1994;76:543–553. doi: 10.1016/0092-8674(94)90117-1. [DOI] [PubMed] [Google Scholar]
- 11.Sainz MB, Grotewold E, Chandler VL. Plant Cell. 1997;9:611–625. doi: 10.1105/tpc.9.4.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heim MA, Jakoby M, Werber M, Martin C, Weisshaar B, Bailey PC. Mol Biol Evol. 2003;20:735–747. doi: 10.1093/molbev/msg088. [DOI] [PubMed] [Google Scholar]
- 13.Bernhardt C, Zhao M, Gonzalez A, Lloyd A, Schiefelbein J. Development (Cambridge, UK) 2005;132:291–298. doi: 10.1242/dev.01565. [DOI] [PubMed] [Google Scholar]
- 14.Zimmermann IM, Heim MA, Weisshaar B, Uhrig JF. Plant J. 2004;40:22–34. doi: 10.1111/j.1365-313X.2004.02183.x. [DOI] [PubMed] [Google Scholar]
- 15.Feller A, Hernandez JM, Grotewold E. J Biol Chem. 2006;281:28964–28974. doi: 10.1074/jbc.M603262200. [DOI] [PubMed] [Google Scholar]
- 16.Ramsay NA, Glover BJ. Trends Plants Sci. 2005;10:63–70. doi: 10.1016/j.tplants.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 17.Hughes-Davies L, Huntsman D, Ruas M, Fuks F, Bye J, Chin SF, Milner J, Brown LA, Hsu F, Gilks B, et al. Cell. 2003;115:523–535. doi: 10.1016/s0092-8674(03)00930-9. [DOI] [PubMed] [Google Scholar]
- 18.Liu Y, Wang L, Kermicle JL, Wessler SR. Genetics. 1998;150:1639–1648. doi: 10.1093/genetics/150.4.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tuerck JA, Fromm ME. Plant Cell. 1994;6:1655–1663. doi: 10.1105/tpc.6.11.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Archer TK, Lefebvre P, Wolford RG, Hager GL. Science. 1992;255:1573–1576. doi: 10.1126/science.1347958. [DOI] [PubMed] [Google Scholar]
- 21.Almouzni G, Wolffe AP. Genes Dev. 1993;7:2033–2047. doi: 10.1101/gad.7.10.2033. [DOI] [PubMed] [Google Scholar]
- 22.Grotewold E, Chamberlin M, Snook M, Siame B, Butler L, Swenson J, Maddock S, Clair GS, Bowen B. Plant Cell. 1998;10:721–740. [PMC free article] [PubMed] [Google Scholar]
- 23.James P, Halladay J, Craig EA. Genetics. 1996;144:1425–1436. doi: 10.1093/genetics/144.4.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maurer-Stroh S, Dickens NJ, Hughes-Davies L, Kouzarides T, Eisenhaber F, Ponting CP. Trends Biochem Sci. 2003;28:69–74. doi: 10.1016/S0968-0004(03)00004-5. [DOI] [PubMed] [Google Scholar]
- 25.Chavali GB, Ekblad CM, Basu BP, Brissett NC, Veprintsev D, Hughes-Davies L, Kouzarides T, Itzhaki LS, Doherty AJ. J Mol Biol. 2005;350:964–973. doi: 10.1016/j.jmb.2005.05.047. [DOI] [PubMed] [Google Scholar]
- 26.Sheen J. Plant Physiol. 2001;127:1466–1475. [PMC free article] [PubMed] [Google Scholar]
- 27.He P, Shan L, Sheen J. Methods Mol Biol. 2007;354:1–9. doi: 10.1385/1-59259-966-4:1. [DOI] [PubMed] [Google Scholar]
- 28.Hernandez J, Heine G, Irani NG, Feller A, Kim M-G, Matulnik T, Chandler VL, Grotewold E. J Biol Chem. 2004;279:48205–48213. doi: 10.1074/jbc.M407845200. [DOI] [PubMed] [Google Scholar]
- 29.Ekblad CM, Chavali GB, Basu BP, Freund SM, Veprintsev D, Hughes-Davies L, Kouzarides T, Doherty AJ, Itzhaki LS. EMBO Rep. 2005;6:675–680. doi: 10.1038/sj.embor.7400415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Libault M, Tessadori F, Germann S, Snijder B, Fransz P, Gaudin V. Planta. 2005;222:910–925. doi: 10.1007/s00425-005-0129-4. [DOI] [PubMed] [Google Scholar]
- 31.Moriguchi K, Suzuki T, Ito Y, Yamazaki Y, Niwa Y, Kurata N. Plant Cell. 2005;17:389–403. doi: 10.1105/tpc.104.028456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hakimi MA, Bochar DA, Chenoweth J, Lane WS, Mandel G, Shiekhattar R. Proc Natl Acad Sci USA. 2002;99:7420–7425. doi: 10.1073/pnas.112008599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Atchley WR, Wollenberg KR, Fitch WM, Terhalle W, Dress AW. Mol Biol Evol. 2000;17:164–178. doi: 10.1093/oxfordjournals.molbev.a026229. [DOI] [PubMed] [Google Scholar]
- 34.Buck MJ, Atchley WR. J Mol Evol. 2003;56:742–750. doi: 10.1007/s00239-002-2449-3. [DOI] [PubMed] [Google Scholar]
- 35.Toledo-Ortiz G, Huq E, Quail PH. Plant Cell. 2003;15:1749–1770. doi: 10.1105/tpc.013839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carey CC, Strahle JT, Selinger DA, Chandler VL. Plant Cell. 2004;16:450–464. doi: 10.1105/tpc.018796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berger SL. Nature. 2007;447:407–412. doi: 10.1038/nature05915. [DOI] [PubMed] [Google Scholar]
- 38.Sheen J. Plant Cell. 1991;3:225–245. doi: 10.1105/tpc.3.3.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guan KL, Dixon JE. Anal Biochem. 1991;192:262–267. doi: 10.1016/0003-2697(91)90534-z. [DOI] [PubMed] [Google Scholar]
- 40.Johnson C, Boden E, Arias J. Plant Cell. 2003;15:1846–1858. doi: 10.1105/tpc.012211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Elomaa P, Mehto M, Kotilainen M, Helariutta Y, Nevalainen L, Teeri TH. Plant J. 1998;16:93–99. doi: 10.1046/j.1365-313x.1998.00273.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.