Abstract
Depending on environmental demands, a decision based on a sensory evaluation may be either immediately reported or postponed for later report. If postponed, the decision must be held in memory. But what exactly is stored by the underlying memory circuits, the final decision itself or the sensory information that led to it? Here, we report that, during a postponed decision report period, the activity of medial premotor cortex neurons encodes both the result of the sensory evaluation that corresponds to the monkey's possible choices and past sensory information on which the decision is based. These responses could switch back and forth with remarkable flexibility across the postponed decision report period. Moreover, these responses covaried with the animal's decision report. We propose that maintaining in working memory the original stimulus information on which the decision is based could serve to continuously update the postponed decision report in this task.
Keywords: medial premotor cortex, monkeys, sensory discrimination, working memory
Studies in behaving monkeys that combine psychophysical and neurophysiological experiments have provided new insights into how a neural representation of a sensory stimulus relates to perception (1–8), memory (9–12), and decision making (13–19). In particular, there has been important progress regarding the neural codes associated with these cognitive functions in the visual and somatic modality (20, 21). The basic philosophy of this approach has been to investigate these cognitive functions by using highly simplified stimuli, so that diverse subcortical and cortical areas can be studied during the same behavior.
In the task we previously used, monkeys report whether the second stimulus frequency (f2) is higher or lower than the first stimulus frequency (f1) (22). This cognitive operation requires that subjects compare information of f1 temporally stored in working memory to the current information of f2 to form a decision of whether f2 > f1 or f2 < f1, and to immediately report the outcome by pressing one of two push buttons. We found that the activity of the recorded neurons of several cortical areas encodes f1 in a monotonic firing rate code beginning in the primary somatosensory cortex (5–7), continuing in the secondary somatosensory cortex (5), the ventral premotor cortex (19), the prefrontal cortex (10), and the medial premotor cortex (MPc) (18). Except for the primary somatosensory cortex, these cortical areas encode information of f1 during the delay period between f1 and f2 (5, 10, 11, 17–19). During presentation of f2, some neurons of all these cortical areas respond to f2, but some other neurons reflect past information of f1, or of the difference between f2 and f1, and generate a differential response consistent with the decision motor report (17–19). In this chain of neural processes, the primary motor cortex becomes engaged only during the motor report period (19, 21). These results showed that the stimulus parameters of f1 and f2 and their interactions can be decoded from the neuronal activity of several cortical areas during this task. The conclusion we reached from these studies is that the primary somatosensory cortex drives higher cortical areas where past and current sensory information are combined, such that a comparison of the two evolves into a behavioral decision.
The vibrotactile discrimination task used in these studies simulates a behavioral condition in which a perceptual decision based on a sensory evaluation is immediately reported through a voluntary action (22). However, depending on the behavioral demands, a perceptual decision can be postponed for later report. If postponed, it must be stored in working memory. But what is stored in the memory circuits, the final decision itself or the sensory information on which the decision is based? We investigated this question by recording from single neurons in MPc (the presupplementary motor area and the supplementary motor area proper), an area involved in decision making and motor choice (18, 23–25), while trained monkeys discriminated the difference in frequency between consecutive vibrotactile stimuli, f1 and f2. Crucially, monkeys were asked to report discrimination after a fixed delay period between the end of f2 and a cue that triggered the beginning of the motor report. This delay period thus separates the comparison between the two stimuli from the motor response. Notice that the postponed decision report in this task is different than the processes involved in purely working memory tasks, in which the relevant process is holding the sensory cue until a cue triggers the behavioral report (9, 26, 27). In our task, monkeys must hold f1 in working memory and must compare the current sensory input f2 to the memory trace of f1, and must postpone the decision until a sensory cue triggers the motor report. Here, we report that, during the postdiscrimination delay period, MPc neurons encode not only the differences between stimuli that correspond to the monkey's two possible choices but also past information on which the decision is based. These responses could switch back and forth with remarkable flexibility across the postdiscrimination period, from encoding the original information on which the decision is based, to encoding the monkey's two possible choices. Moreover, MPc responses appear to participate directly in the monkey's decision-making process, as quantified by high choice probability indices obtained during the postdiscrimination report period. We propose that maintaining in working memory the original stimulus information on which the decision is based could serve to continuously update the postponed decision report in this task.
Results
Two monkeys (Macaca mulatta) were trained to discriminate the difference in frequency between two consecutive mechanical vibrations delivered to one fingertip [refs. 3, 6, 10, 22; supporting information (SI)]. Monkeys were asked to report discrimination after a fixed delay period of 3 s between the end of f2 and the cue that triggered the beginning of the motor report (Fig. 1A, PU). We recorded from 907 (77%; n = 1,183) MPc neurons that had average firing during the vibrotactile discrimination task that were significantly different from those in a pretrial control period immediately before the probe moved down (PD; P < 0.01, Wilcoxon's rank-sum test) (28). All these neurons were recorded by using the stimulus set of Fig. 1B, which had large differences between the first (f1) and second (f2) stimulus frequencies. In this set, trials can be divided into two types: those in which f2 = f1 + 8 Hz (Fig. 2, black) and those in which f2 = f1 − 8 Hz (Fig. 2, gray), which correspond to the two possible behavioral choices that the monkeys have. Note also that three comparison frequencies (18, 22, and 26 Hz) can be judged as either higher or lower than f1; for these, the monkeys must rely on the stored f1 percept to discriminate consistently (10, 19, 22).
Fig. 1.
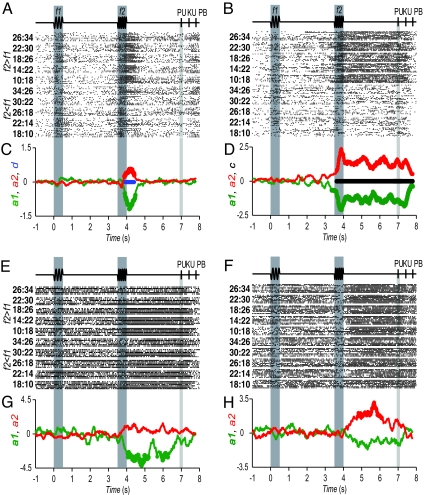
Discrimination task. (A) Sequence of events during discrimination trials. The mechanical probe is lowered, indenting the glabrous skin of one digit of the restrained hand (PD); the monkey places its free hand on an immovable key (KD); the probe oscillates vertically, at the base stimulus frequency (f1); after a delay, a second mechanical vibration is delivered at the comparison frequency (f2); after another delay between the end of f2 and probe up (PU), the monkey releases the key (KU) and presses either a lateral or a medial push button (PB) to indicate whether the comparison frequency was higher or lower than the base, respectively. (B) Stimulus set used during recordings. Each box indicates a base/comparison frequency stimulus pair. The number inside the box indicates overall percentage of correct trials for that (f1, f2) pair. (C) Top view of the MPc. MPc was subdivided by a line passing from the midline to the posterior edge of the arcuate sulcus (AS); rostral to this line is the presupplementary motor cortex (pre-SMA), and posterior to this line is the SMA proper. (D) Symbols in the insets indicate microelectrode penetrations and the types of the neuronal responses: vibration frequency f1 (green), f2 (red), and sign of the difference f2 − f1 (d, partial differential response; blue), or strictly f2 − f1 (c, full differential response; black). The filled circles indicate 1–8 neurons, the open triangles indicate 9–16 neurons, and the plus signs indicate 17–24 neurons recorded at the shown locations.
Fig. 2.
Responses of four MPc neurons during the postponed decision report. (A) Raster plot of a neuron that responded immediately after f2 for trials f2 > f1. Each row of ticks is a trial, and each tick is an action potential. Trials were delivered in random order (10 trials per stimulus pair). Labels at the left indicate f1:f2 stimulus pairs. (B) Raster plot of a neuron that responded at the end of f2 and continued responding during the entire delay period between f2 and PU for pairs of trials f2 > f1. (C and D) Resulting coefficient values for f1 (a1, green) and f2 (a2, red) for neurons in A and B, as functions of time. During the postponed decision report period, these coefficients had significantly different magnitudes in A (blue trace in C) and statistically equal magnitudes and opposite signs in B (black trace in D). (E) Raster plot of a neuron that encoded f1 during the postponed decision report period. Its firing increased for low f1 frequencies for both f2 > f1 and f2 < f1 pairs. (F) Raster plot of a neuron that encoded f2 during the postponed decision report. Its firing increased for high f2 frequencies for both f2 > f1 and f2 < f1 pairs. (G and H) Values of a1 (green) and a2 (red) coefficients for the neurons in E and F. The filled circles indicate significant values.
Responses of MPc Neurons During the Postponed Decision Report Period.
Here, we study the neuronal representations observed during the postponed decision report. The neuronal responses across trials can be analyzed as functions of f1, f2, f2 − f1, or as functions of the two possible motor choices. However, in principle, once the comparison between frequencies is carried out, the monkeys only need to remember the sign of the difference (f2 > f1 or f2 < f1). If this is indeed the case, MPc neurons should reflect only the outcome of the comparison between f2 and f1, providing simply a categorical signal consistent with the decision motor report.
Interestingly, however, many MPc neurons responded in an entirely different manner. Fig. 2 illustrates the firing of four example neurons during the postponed decision report. The neurons of Fig. 2 A and B were differentially responsive: they fired at higher rates for stimulus pairs f2 > f1 than for stimulus pairs f2 < f1. These differential responses could be interpreted as encoding the motor choice, because discrimination of f2 > f1 trials and f2 < f1 trials is reported by pressing the lateral and medial push buttons, respectively, and similar responses were also recorded in other MPc neurons for f2 < f1 trials. However, this simple interpretation does not hold for some other types of responses observed during the postdiscrimination delay. For example, the neurons in Fig. 2 E and F did not respond differentially; their firing rates did vary strongly across trials, but this happened within both f2 > f1 and f2 < f1 stimulus pairs. Many other neurons had similar dynamics during the postponed decision report (Table 1), so we investigated these dependencies further.
Table 1.
Database of MPc
| Task component |
|||||
|---|---|---|---|---|---|
| f1 | Delay f1 − f2 | f2 | Delay f2 − PU | KU − PB | |
| Tuned to f1 | 35 + (4%) | 130 + (14%) | 19 + (2%) | 146 + (16%) | 17 + (2%) |
| 22 − (2%) | 165 − (18%) | 56 − (6%) | 219 − (24%) | 25 − (3%) | |
| Tuned to f2 | 59 + (7%) | 205 + (23%) | 24 + (3%) | ||
| 36 − (4%) | 190 − (21%) | 27 − (3%) | |||
| d | 61 (7%) | 117 (13%) | 6 (1%) | ||
| c | 17 (2%) | 26 (3%) | 6 (1%) | ||
Recorded, n = 1183. Responsive, n = 907 (77%). f1, first stimulus; f2, second stimulus; PU, probe up; KU, key up; PB, push button; tuned, encoding stimulus frequency with positive (+) or negative (−) slopes; d, tuned to stimuli and differential for f2 > f1 or f2 < f1; c, differential activity to f2 > f1 or f2 < f1.
Dynamics of the Postponed Decision Report Process in MPc.
To quantify the different possible encoding schemes, we modeled the firing rates during the task as arbitrary linear functions of both f1 and f2, such that for each cell, firing rate (t) = a1(t) f1 + a2(t) f2 + a3*(t) (see refs. 17–19 and 29 for further details of the analysis). In this formulation, t represents time, and the coefficients a1 and a2 serve as direct measurements of firing rate dependence on f1 and f2, respectively. Because the constant associated with coefficient a3 can be an arbitrary value, for each neuron we set it to the mean firing rate calculated in the sample period studied. These measures were calculated in sliding windows of 200 ms moving in steps of 100 ms. To illustrate this analysis, the resulting coefficients a1 and a2 for the four neurons of Fig. 2 are plotted in C, D, G, and H as functions of time. The magnitude and sign of the coefficients reveals the tuning properties of the neurons (i.e., their selectivity) during the postponed decision report. For example, Fig. 2B illustrates a response that turns out to be close to the ideal expected decision motor report. This neuron responded strongly throughout the entire postponed decision period for f2 > f1 trials; furthermore, the analysis shows significant coefficients a1 (green trace) and a2 (red trace) of opposite signs and similar magnitudes, thus confirming a differential or categorical response (Fig. 2D). The neuron in Fig. 2A displayed some important deviations from the ideal expected decision motor report. It responded briskly approximately during the first 700 ms of the postdecision delay for f2 > f1 trials, and also had significant coefficients a1 (green trace) and a2 (red trace) of opposite signs (Fig. 2C). But in this case a1 was about twice as large in magnitude as a2, indicating a sensory component superimposed on the differential response. The neurons in Fig. 2 E and F deviated even further from the ideal, and did not exhibit differential activity at all. The coefficients for the neuron in Fig. 2E revealed that, during the postponed delay period, this unit carried only information about f1, because only a1 was significantly different from zero (Fig. 2G, green trace). Similarly, the neuron of Fig. 2F only carried information about f2 (Fig. 2H, red trace).
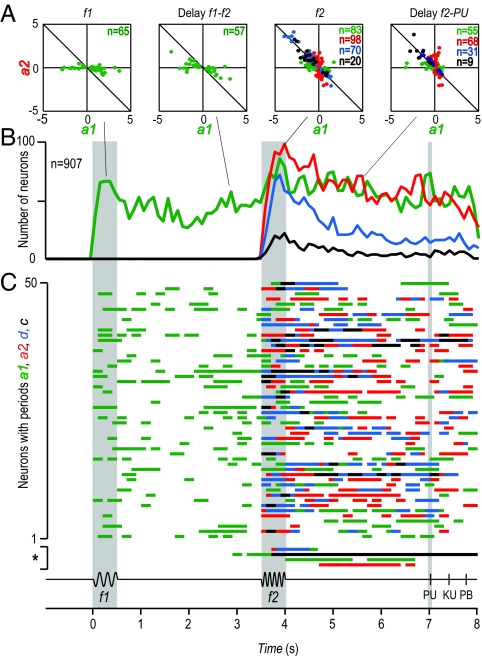
We also plotted the values of a1 and a2 against each other to compare the responses at different points during the task (Fig. 3A). Three lines are relevant in these plots: points that fall on the a2 = 0 line represent responses that depend only on f1 (green dots); points that fall on the a1 = 0 line represent responses that depend only on f2 (red dots); and points that fall near the a2 = −a1 line represent responses that depend on sign f2 − f1 (black dots). This last consideration is of particular importance, because the sign of the difference between f1 and f2 determines correct task performance. These three are not the only possibilities, however. For example, suppose, hypothetically, that a neuron is modulated by the vibrotactile stimuli such that its firing rate varies as a function of f1 + f2. The corresponding point in Fig. 3A would fall close to the a1 = a2 line, meaning that the memory of f1 was added to the f2 representation. This result was very rarely observed, although. In fact, most points in Fig. 3A occupy an area of the plane representing those conditions where both coefficients are significantly different from zero but are significantly different from each other (a1 ≠ 0 and a2 ≠ 0, |a1| ≠ |a2|; green, red, and blue dots). This means that, typically, each neuron responds more to one of the two frequencies. In other words, the canonical differential response (a1 ≠ 0, a2 ≠ 0, a1 = −a2; black dots in Fig. 3A) is relatively rare.
Fig. 3.
Dynamics of MPc population responses during the vibrotactile discrimination task. (A) Values of a1 and a2 coefficients for all neurons selected times (200 ms) in B. For each point, at least one coefficient is significantly different from zero. Different plots are for various times in B; n = number of neurons. (B) Number of neurons with significant coefficients as a function of time. The green and red traces correspond to a1 and a2, respectively. The blue trace corresponds to neurons with both significant a1 and a2 coefficients of opposite signs, but significantly different magnitudes; these are partially differential (d) responses. The black trace corresponds to neurons with both significant a1 and a2 coefficients of opposite signs and statistically equal magnitude; these are fully differential (c) or categorical responses encoding f2 − f1. (C) Bar graphs of 50 randomly selected neurons from the 907 neurons that contributed to B. These bars indicate periods of responses encoding f1 (green bars), f2 (red bars), partially differential f2 − f1 (blue bars), and fully differential or categorical responses encoding f2 − f1 (black bars). Each line of bars represents the dynamics of the responses of one single neuron during the discrimination task. The dynamics of these coefficients was analyzed by using a sliding window of 200 ms duration moving in steps of 100 ms. Dynamics of coefficients for the four neurons of Fig. 2 A, B, E, and F are shown at the bottom of C (*). PU, probe up; KU, key up; PB, push button.
Interestingly, this effect becomes more pronounced late into the postdiscrimination delay. Fig. 3B shows the numbers of neurons with significant a1 or a2 coefficients as functions of time (SI). According to the graph, some MPc neurons encode only f1 (green trace) starting after the onset of the base stimulus (response latency, 187.26 ± 11.22 ms; mean ± SE). Subpopulations of MPc neurons continue to encode only f1 during the delay period between f1 and f2, during the comparison period (while the f2 stimulus is presented), and most remarkably, during the postponed decision report period until the monkeys indicate the motor choice. As expected, some MPc neurons respond as a functions of f2 only (red trace), starting after the onset of the comparison stimulus (response latency, 198.05 ± 11.64 ms), but as with f1, many units encode information about f2 during the postponed decision report period. In addition, other neurons combine information about f1 and f2 to generate differential responses (d, blue trace). For some of these cells, the response is categorical (c, black trace; response latency, 224.0 ± 11.58 ms after f2 onset), whereas for others one of the two vibration frequencies is represented more strongly (blue trace; response latency was 209.36 ± 9.39 ms after f2 onset). The plot in Fig. 3B hides some interesting dynamics of single neurons; for instance, we found that 26% and 28% of the neurons that initially encoded f1 and f2 during the stimulation periods, respectively, became partially differential (d) or categorical (c) during the postponed decision report, and could switch back and forth with remarkable flexibility across the postdiscrimination period (Fig. 3C). However, it does convey how strongly a quantity is represented by the MPc population at any moment. In particular, it shows that the number of differential cells decreases sharply as a function of time during the postdiscrimination delay. In fact, during this period, more MPc neurons directly reflect the frequencies of the two stimuli rather than the motor choice.
MPc Responses Correlate with the Postponed Decision Motor Report.
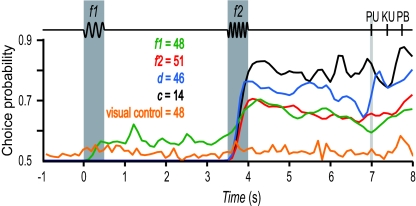
Next, we investigated whether these four types of responses predicted the motor choice of the monkeys observed after the postponed decision delay. For this, we sorted the responses into hits and errors and calculated a choice probability index (17–19, 30, 31). This quantified for each (f1, f2) pair whether responses during error trials were different from responses during correct trials. Choice probability indices were computed separately for neurons that encoded information about f1 only, about f2 only, that were partially differential (d) or fully differential (c). The result is shown in Fig. 4, which plots the four choice probability indices as functions of time. The four traces are significantly above 0.5, indicating that, during the postdiscrimination delay, there are significant differences in activity between trials that result in hits versus errors. These differences are maintained by neurons that contribute at different times during this period. The crucial point, however, is that even those neurons that encode only f1 or f2 have choice probability indices well above the 0.5 chance level. They show that all types of neurons are correlated with the animals' motor behavior. This means that their activity contributes to the observed variations in performance, even though, in principle, after the end of f2 only the categorical signals are needed for generating the postponed decision report.
Fig. 4.
Correlation between neuronal and behavioral responses. Choice probability indices as a function of time. Green trace, Neurons that encoded information about f1; red trace, neurons that carried information about f2; blue trace, partially differential neurons that carried information about f1 and f2 (d); black trace, fully differential neurons that carried information about f2 − f1 only (c). The orange trace corresponds to neurons that had large choice probability indices and were tested in a control task in which animals received the same stimulus pairs but had to follow a visual cue to produce the motor choice response.
One interpretation of this result is that the perceptual discrimination is not entirely consolidated once the second stimulus ends, but rather keeps brewing until the motor report is actually initiated. Another possibility is that the choice probabilities of MPc neurons simply reflect a purely motor signal. To investigate this, in addition to the standard tests, some of the neurons that carried information about f1, f2, or f2 − f1 were tested in a variant of the task in which the same vibrotactile stimuli were applied but the monkeys were instructed to press one of the push buttons according to a visual cue (see Methods). In this case, the somatosensory information could be ignored. Under this condition, the choice probability indices of MPc neurons dropped considerably (Fig. 4, orange trace). This suggests that at least part of the association between neuronal activity and behavior quantified by the high choice probabilities is due to sensory or perceptual processing.
Discussion
This result might be somewhat surprising in view of previous studies showing that a categorical decision consistent with the motor choice develops immediately after a sensory evaluation is completed (8, 13, 14). This could be explained by fundamental differences between these tasks. For example, decision reports in these tasks (8, 13, 14) are based on the evaluation of one single sensory quantity that varies from trial to trial, whereas, in our task, discrimination is based on the evaluation of two quantities that vary also from trial to trial: one being the current sensory input f2 and the other the memory trace of f1 (17–19). In our task, however, some of the partially and fully differential responses that develop around the offset of f2 are consistent with those findings, because these predicted the monkey's motor choice. The question is why, during the postponed decision report period, information about the stimuli on which the decision is based is still present. As mentioned above, it is possible that the perceptual decision is revised or updated as long as there is time for it to be reconsidered. Our results are consistent with this interpretation because most of the single neuron responses switched back and forth during the postdiscrimination period, from encoding the original information on which the decision is based, to encoding the two monkey's possible choices.
In our everyday life, decisions based on sensory information are often postponed until the last moment. For instance, some of us find it difficult to decide what to order for lunch until the waiter asks, even when we know perfectly well what is in the menu. Such postponed decision reports may sometimes be advantageous, particularly when they are uncertain, in which case using more time can increase the probability of a correct decision. In this study, control experiments using the same animals are consistent with this interpretation, and suggest that there is a critical postdiscrimination delay period for improving overall performance. When animals were asked to report their discrimination choices immediately after f2, their performance with the stimulus set of Fig. 1B was 92.5% correct. But when animals were asked to postpone the discrimination report for various delay durations, their overall percentage of correct trials with the same stimulus set was of 100% at 1 s, 98.6% at 1.5 s, 92.6% at 2 s, and 85.5% at 3 s. Thus, performance was best when the decision report was postponed for 1 and 1.5 s, compared with the overall performance when the decisions were either immediately reported or postponed for 3 s (permutation test, n = 1,000, P < 0.02).
These results also have implications regarding the organization of the recurrent neuronal circuits thought to underlie working memory (32–34). It is possible, for instance, that the neurons that store information about the stimuli during the postponed decision report period are linked to the neurons that compute the difference between stimulus frequencies and thus the motor choice (32, 35). Although a substantial number of the neurons that initially carried only sensory information later developed a differential signal that predicted the motor choice, such link is difficult to prove. Another point to consider is that the MPc may be part of a larger network subserving working memory (32–36). This is consistent with the fact that responses similar to those reported here have been recorded in the ventral premotor cortex and in the prefrontal cortex (R.R., L.L., A.H., R.L., and A.Z., unpublished results). Whatever the underlying cellular mechanisms, our results show that the neuronal correlate of a postponed decision report is composed not only of signals reflecting the possible motor choices, but also of other signals encoding the original sensory information. So, all of these must be maintained by working memory circuits until the perceptual decision is finally reported.
Our results also suggest that MPc circuits reorganize in accord to behavioral demands. For example, in a variant of this task in which monkeys were asked to report discrimination immediately after the offset of f2, MPc neurons encoded f1 during f1 presentation and during the delay period between f1 and f2, as observed here (18). However, the f1 and f2 signals dropped immediately after f2 presentation, when the comparison between f2 and f1 took place. A similar profile was observed for the resulting comparison between f2 and f1 (see figure 5F in ref. 18). This is not the case when monkeys postpone the decision motor report, as shown here. In this case, MPc neurons reflect the sensory signals on which the decision is based during the postponed decision period. Another observation to consider is that few MPc neurons reflect the comparison outcome, suggesting that in this task the MPc circuits are less associated to the decision motor report than encoding the sensory signals on which the decision is based. Our results suggest that the functional role of the MPc circuits are more closely associated to maintaining in working memory the sensory information on which the decision is based than encoding the decision motor reports. This is consistent with other observations made in MPc. For example, if MPc neurons are tested while trained monkeys execute some other complex behavioral tasks, such complex motor sequences retrieved from memory, the neuronal responses correlate with these processes and less associated with the specification of motor details (25, 37, 38).
Previous studies have shown that frontal lobe neurons encode complex motor sequences based on conditional sensory cues stored in working memory (39–41). Therefore, an important role of the frontal lobe, notably prefrontal and premotor cortices, is the readout of information in working memory at the service of complex behavior (39). In this respect, Miyashita and colleagues (39) made the remarkable finding that a remembered sequence of positional cues is reflected in the activity of dorsal premotor cortex neurons only when it is needed to determine a sequence of saccades in either the original or reverse order; otherwise, no trace of the remembered sequence is observed Furthermore, this study reported both sensory and motor signals stored in working memory, as found here. There exists, however, a fundamental difference between these two studies. In Miyashita's task, subjects are forced to remember the sensory information to generate saccades. In our task, once the decision is made the sensory information is not required to generate the motor report. However, during the postponed decision report period, MPc neurons reflected the past sensory information on which the decision was based. Another point to consider is that, during the postdiscrimination period, more MPc neurons carried sensory information than motor information. The presence of sensory information during the postdiscrimination period might be important to continuously update the decision motor report in this task. The final crucial step to consider in all these studies is showing what component(s) of the sensory information is encoded in the activity of the working memory neurons (10, 42–46). The stimuli in the vibrotactile discrimination task are highly simplified and our results show how the activity of frontal lobe neurons represents the sensory information during the stimulus presentation, working memory, the interactions between them, and how all of these processes contribute to perceptual decision reports.
To conclude, our results indicate that MPc is an important node in the readout of sensory information from working memory at the service of action selection, two important ingredients in decision making. In fact, MPc circuits are anatomically linked to sensory, memory, and motor circuits (47). Thus, MPc circuits appear critically suited to integrate and reorganize all of the elements associated with decision making in this task. Furthermore, they reflect the flexibility needed when a perceptual decision must be either immediately reported or postponed for later report.
Methods
Discrimination Task.
The sensory discrimination task used here has already been described (ref. 22; see also SI). Monkeys were handled according to the institutional standards of the National Institutes of Health and Society for Neuroscience.
Visual Instruction Task.
A simpler task, in which the same vibrotactile stimuli were delivered to the skin but the hand/arm movements were triggered by visual cues, was used as a control (for details, see SI).
Recordings.
Neuronal recordings were obtained with an array of seven independent microelectrodes (2–3 MΩ) (48) inserted into the MPc, contralateral and ipsilateral to stimulated hand. We used well established criteria to distinguish between the two subdivisions of the MPc (Fig. 1C) (49). The locations of the penetrations were confirmed through standard histological techniques for the two recorded monkeys. Recordings sites changed from session to session.
Data Analysis.
We considered a neuron's response as task-related if during any of the relevant periods (f1, delay between f1 and f2, f2, delay between f2 and PU, reaction time, or movement time) its mean firing rate was significantly different from that in a control period of equal duration but preceding the initial probe indentation at the beginning of each trial (Wilcoxon's test, P < 0.01) (28). By definition, f1 and f2 correspond to the base and comparison periods, respectively. The first delay was divided into consecutive intervals of 500 ms beginning at the end of f1 and up to the beginning of f2. Similar intervals were used for the second delay between f2 and PU. The reaction time was the period from the end of PU to the beginning of the key up (KU; Fig. 1A). The movement time was the period from the end of KU to the beginning of the push button press (PB; Fig. 1A).
The dependence on f1 and f2 was obtained through multivariate regression analysis (6). After finding the best-fit coefficients a1 and a2, differences between fitted and measured responses to the individual (f1, f2) stimulus pairs were calculated, resulting in a full 2D covariance matrix of errors (50). Coefficients were considered significantly different from (0, 0) if they were more than 2 standard deviations away. Neuronal responses were defined unambiguously as dependent on either f1 or f2 if the coefficients of the planar fit were within 2 standard deviations of either the a2 = 0 or the a1 = 0 line; responses were considered dependent on f2 − f1 if the coefficients were more than 2 standard deviations away from these two lines and within 2 standard deviations of the a2 = −a1 line. Responses not satisfying this criterion were classified as “mixed.” The dynamics of these coefficients was analyzed by using a sliding window of 200 ms duration moving in steps of 100 ms.
The choice probability index was calculated by using methods from signal detection theory (17–19, 30, 31). This quantity measures the overlap between two response distributions, in this case between hits and errors for each (f1, f2) pair. We restricted the analysis to those (f1, f2) pairs for which the animals had between 30 and 70% of errors. Notice that a value of 0.5 indicates full overlap and 1 indicates completely separate distributions. Thus, the choice probability index quantifies selectivity for one or the other outcome of the discrimination process. To compute it at different times, we used a sliding window of 200 ms duration moving in 100 ms steps, beginning 1,000 ms before f1 and ending 1,000 ms after the animal reported the comparison between f2 and f1. To establish the significance of the choice probability values, the neuronal responses in each time window were shuffled, such that hit and error trials were randomized, and new choice probability indices for the shuffled data were generated. By comparing the indices from the shuffled and unshuffled data and repeating the process 1,000 times, we estimated the probability of obtaining choice probability values as large or larger than those observed initially (with the unshuffled data) just by chance (Fig. 4).
Supplementary Material
Acknowledgments
R.R.'s research was partially supported by an International Research Scholars Award from the Howard Hughes Medical Institute and by grants from the Dirección del Personal Académico de la Universidad Nacional Autónoma de México and the Consejo Nacional de Ciencia y Tecnología of Mexico.
Abbreviation
- MPc
medial premotor cortex.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0707961104/DC1.
References
- 1.Newsome WT, Britten KH, Movshon JA. Nature. 1989;341:52–54. doi: 10.1038/341052a0. [DOI] [PubMed] [Google Scholar]
- 2.Salzman CD, Britten KH, Newsome WT. Nature. 1990;346:174–177. doi: 10.1038/346174a0. [DOI] [PubMed] [Google Scholar]
- 3.Romo R, Hernández A, Zainos A, Salinas E. Nature. 1998;392:387–390. doi: 10.1038/32891. [DOI] [PubMed] [Google Scholar]
- 4.Romo R, Hernández A, Zainos A, Brody CD, Lemus L. Neuron. 2000;26:273–278. doi: 10.1016/s0896-6273(00)81156-3. [DOI] [PubMed] [Google Scholar]
- 5.Salinas E, Hernández A, Zainos A, Romo R. J Neurosci. 2000;20:5503–5515. doi: 10.1523/JNEUROSCI.20-14-05503.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hernández A, Zainos A, Romo R. Proc Natl Acad Sci USA. 2000;97:6191–6196. doi: 10.1073/pnas.120018597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luna R, Hernández A, Brody CD, Romo R. Nat Neurosci. 2005;8:1210–1219. doi: 10.1038/nn1513. [DOI] [PubMed] [Google Scholar]
- 8.de Lafuente V, Romo R. Nat Neurosci. 2005;8:1698–1703. doi: 10.1038/nn1587. [DOI] [PubMed] [Google Scholar]
- 9.Funahashi S, Bruce CJ, Goldman-Rakic PS. J Neurophysiol. 1989;61:331–349. doi: 10.1152/jn.1989.61.2.331. [DOI] [PubMed] [Google Scholar]
- 10.Romo R, Brody CD, Hernández A, Lemus L. Nature. 1999;399:470–473. doi: 10.1038/20939. [DOI] [PubMed] [Google Scholar]
- 11.Brody CD, Hernández A, Zainos A, Romo R. Cereb Cortex. 2003;13:1196–1207. doi: 10.1093/cercor/bhg100. [DOI] [PubMed] [Google Scholar]
- 12.Nieder A, Miller EK. Neuron. 2003;37:149–157. doi: 10.1016/s0896-6273(02)01144-3. [DOI] [PubMed] [Google Scholar]
- 13.Shadlen MN, Newsome WT. Proc Natl Acad Sci USA. 1996;93:628–633. doi: 10.1073/pnas.93.2.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shadlen MN, Newsome WT. J Neurophysiol. 2001;86:1916–1936. doi: 10.1152/jn.2001.86.4.1916. [DOI] [PubMed] [Google Scholar]
- 15.Kim JN, Shadlen MN. Nat Neurosci. 1999;2:176–185. doi: 10.1038/5739. [DOI] [PubMed] [Google Scholar]
- 16.Schall JD. Nat Rev Neurosci. 2001;1:33–42. doi: 10.1038/35049054. [DOI] [PubMed] [Google Scholar]
- 17.Romo R, Hernández A, Zainos A, Lemus L, Brody CD. Nat Neurosci. 2002;5:1217–1225. doi: 10.1038/nn950. [DOI] [PubMed] [Google Scholar]
- 18.Hernández A, Zainos A, Romo R. Neuron. 2002;33:959–972. doi: 10.1016/s0896-6273(02)00613-x. [DOI] [PubMed] [Google Scholar]
- 19.Romo R, Hernández A, Zainos A. Neuron. 2004;41:165–173. doi: 10.1016/s0896-6273(03)00817-1. [DOI] [PubMed] [Google Scholar]
- 20.Parker AJ, Newsome WT. Annu Rev Neurosci. 1998;21:227–277. doi: 10.1146/annurev.neuro.21.1.227. [DOI] [PubMed] [Google Scholar]
- 21.Romo R, Salinas E. Nat Rev Neurosci. 2003;4:203–218. doi: 10.1038/nrn1058. [DOI] [PubMed] [Google Scholar]
- 22.Hernández A, Salinas E, García R, Romo R. J Neurosci. 1997;17:6391–6400. doi: 10.1523/JNEUROSCI.17-16-06391.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Romo R, Ruiz S, Crespo P, Zainos A, Merchant H. J Neurophysiol. 1993;70:2690–2694. doi: 10.1152/jn.1993.70.6.2690. [DOI] [PubMed] [Google Scholar]
- 24.Romo R, Merchant H, Zainos A, Hernández A. Cereb Cortex. 1997;7:317–326. doi: 10.1093/cercor/7.4.317. [DOI] [PubMed] [Google Scholar]
- 25.Hoshi E, Tanji J. J Neurophysiol. 2004;92:3482–3489. doi: 10.1152/jn.00547.2004. [DOI] [PubMed] [Google Scholar]
- 26.Fuster JM. J Neurophysiol. 1973;36:61–78. doi: 10.1152/jn.1973.36.1.61. [DOI] [PubMed] [Google Scholar]
- 27.Muhammad R, Wallis JD, Miller EK. J Cogn Neurosci. 2006;18:974–989. doi: 10.1162/jocn.2006.18.6.974. [DOI] [PubMed] [Google Scholar]
- 28.Siegel S, Castellan NJ. Nonparametric Statistics for Behavioral Sciences. New York: McGraw-Hill; 1988. [Google Scholar]
- 29.Draper N, Smith H. Applied Regression Analysis. 2nd Ed. New York: Wiley; 1966. [Google Scholar]
- 30.Green DM, Swets JA. Signal Detection Theory and Psychophysics. New York: Wiley; 1966. [Google Scholar]
- 31.Britten KH, Newsome WT, Shadlen MN, Celebrini S, Movshon JA. Vis Neurosci. 1996;13:87–100. doi: 10.1017/s095252380000715x. [DOI] [PubMed] [Google Scholar]
- 32.Goldman-Rakic PS. Neuron. 1995;14:477–485. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- 33.Miller P, Brody CD, Romo R, Wang X-J. Cereb Cortex. 2003;13:1208–1218. doi: 10.1093/cercor/bhg101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fuster J. Trends Neurosci. 1997;20:451–459. doi: 10.1016/s0166-2236(97)01128-4. [DOI] [PubMed] [Google Scholar]
- 35.Machens CK, Romo R, Brody CD. Science. 2005;307:1121–1224. doi: 10.1126/science.1104171. [DOI] [PubMed] [Google Scholar]
- 36.Cisek P. J Neurosci. 2006;26:9761–9770. doi: 10.1523/JNEUROSCI.5605-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shima K, Mushiake H, Saito N, Tanji J. Proc Natl Acad Sci USA. 1996;93:8694–8698. doi: 10.1073/pnas.93.16.8694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shima K, Tanji J. J Neurophysiol. 2000;84:2148–2160. doi: 10.1152/jn.2000.84.4.2148. [DOI] [PubMed] [Google Scholar]
- 39.Ohbayashi M, Ohki K, Miyashita Y. Science. 2003;301:233–236. doi: 10.1126/science.1084884. [DOI] [PubMed] [Google Scholar]
- 40.Mushiake H, Saito N, Sakamoto K, Itoyama Y, Tanji J. Neuron. 2006;50:631–641. doi: 10.1016/j.neuron.2006.03.045. [DOI] [PubMed] [Google Scholar]
- 41.Shima K, Isoda M, Mushiake H, Tanji J. Nature. 2007;455:315–318. doi: 10.1038/nature05470. [DOI] [PubMed] [Google Scholar]
- 42.Rainer G, Asaad WF, Miller EK. Nature. 1998;393:577–579. doi: 10.1038/31235. [DOI] [PubMed] [Google Scholar]
- 43.Wallis JD, Anderson WK, Miller EK. Nature. 2001;411:953–956. doi: 10.1038/35082081. [DOI] [PubMed] [Google Scholar]
- 44.Freedman DJ, Riesenhuber M, Poggio T, Miller EK. Science. 2001;291:312–316. doi: 10.1126/science.291.5502.312. [DOI] [PubMed] [Google Scholar]
- 45.Nieder A, Freedman DJ, Miller EK. Science. 2002;297:1708–1711. doi: 10.1126/science.1072493. [DOI] [PubMed] [Google Scholar]
- 46.Nacher V, Ojeda S, Cadarso-Suarez C, Roca-Pardinas J, Acuna C. Eur J Neurosci. 2006;24:925–936. doi: 10.1111/j.1460-9568.2006.04964.x. [DOI] [PubMed] [Google Scholar]
- 47.Rizzolatti G, Lupino G. Neuron. 2001;31:889–901. doi: 10.1016/s0896-6273(01)00423-8. [DOI] [PubMed] [Google Scholar]
- 48.Mountcastle VB, Steinmetz MA, Romo R. J Neurosci. 1990;10:3032–3044. doi: 10.1523/JNEUROSCI.10-09-03032.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Matsuzaka Y, Aizawa H, Tanji J. J Neurophysiol. 1997;77:1132–1154. [Google Scholar]
- 50.Press W, Teukolsky SA, Vettering WT, Flannery BP. Numerical Recipes in C. 2nd Ed. Cambridge, UK: Cambridge Univ Press; 1992. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.