Abstract
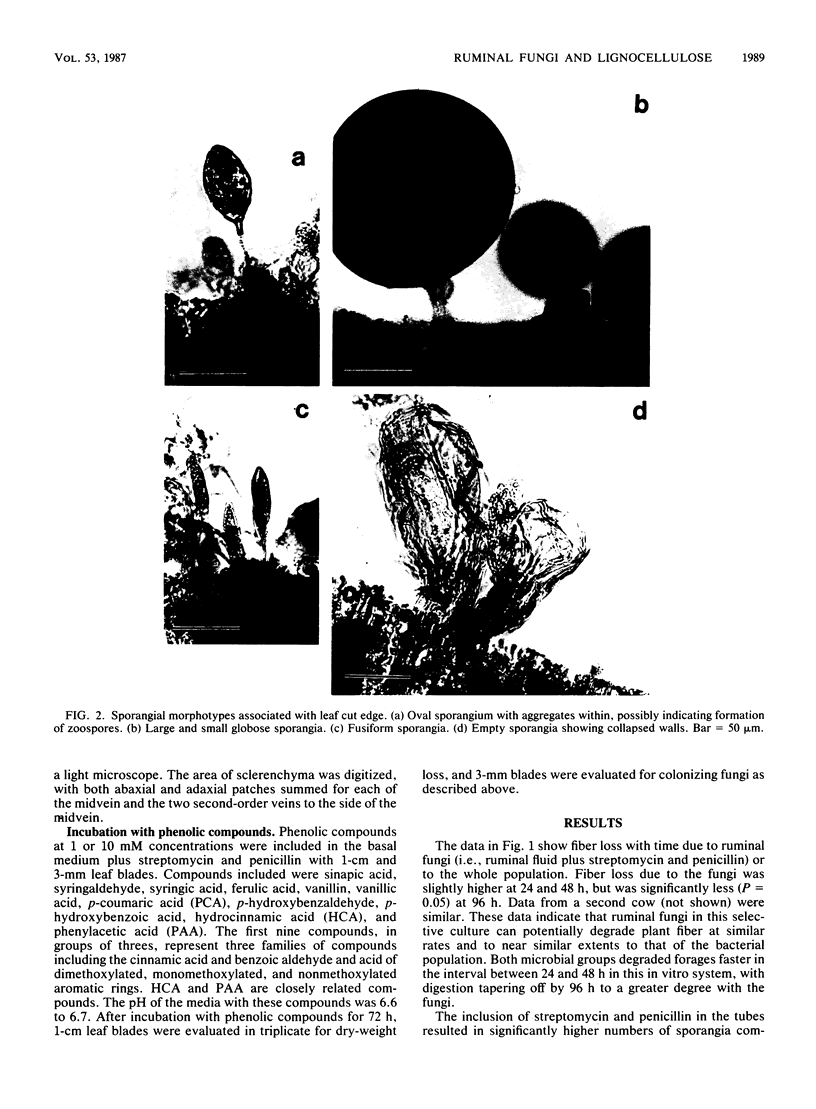
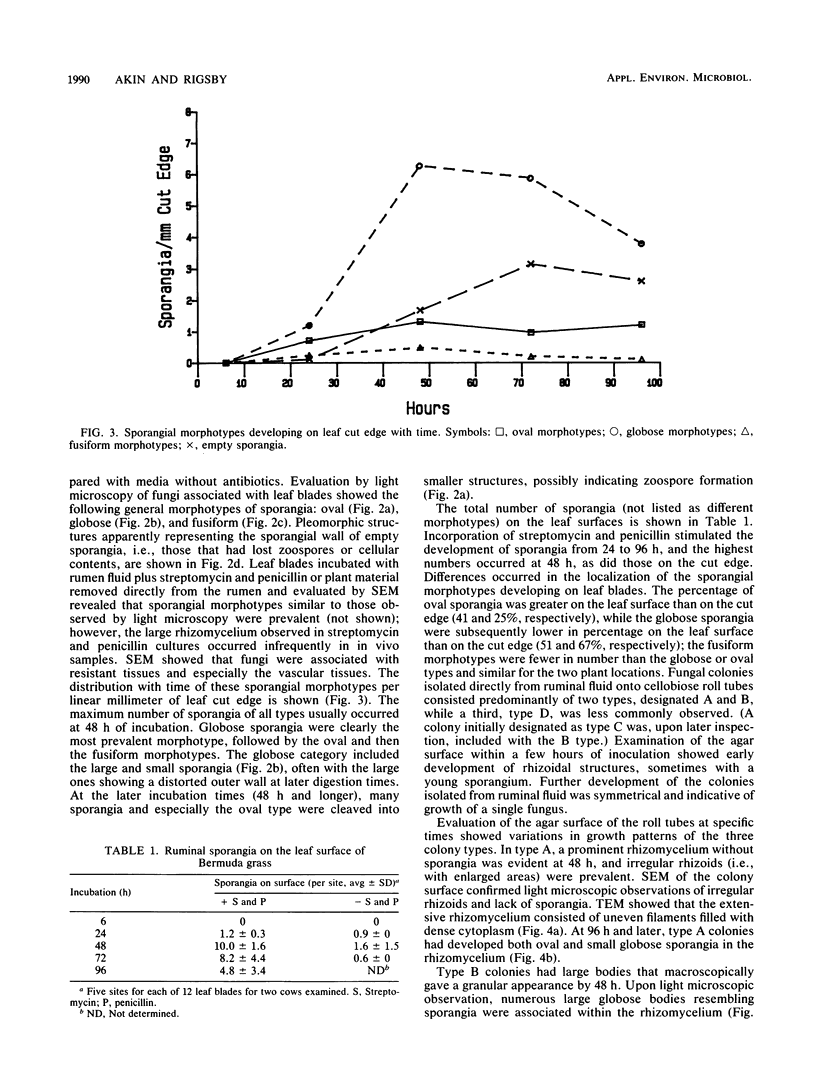
Anaerobic fungi in ruminal fluid from cows eating Bermuda grass hay plus a grain and minerals supplement were evaluated for diversity in sporangial morphotypes and colony growth patterns and for the degradation of various lignocelluloses. In selective cultures containing streptomycin and penicillin, an active population of ruminal fungi colonized leaf blades and degraded fiber at rates and extents almost equal to that of the total ruminal population. Three major sporangial morphotypes were consistently observed on leaf blades: oval, globose, and fusiform. Fungal colonies representing three distinct growth types consistently developed in anaerobic roll tubes inoculated with strained ruminal fluid. Sporangial morphotypes could not be matched to colony types due to multiple sporangial forms within a colony. Under identical growth conditions, one type exhibited a monocentric growth pattern, while two types exhibited polycentric growth patterns previously unreported in ruminal fungi. Mixed ruminal fungi in selective cultures or in digesta taken directly from the rumen produced a massive clearing of the sclerenchyma. Quantitation of tissue areas in cross sections by light microscopic techniques showed that fungal incubations resulted in significant (P = 0.05) increases in sclerenchyma degradation compared to whole ruminal fluid incubations. The mestome cell wall was at times penetrated and partially degraded by fungi; the colonization was less frequent and to a lesser degree than with the sclerenchyma. Conversely, ruminal bacteria were not observed to degrade the mestome sheath. Phenolic monomers at 1 mM concentrations did not stimulate to a significant (P = 0.05) extent the dry weight loss or fungal colonization of leaf blades; at 10 mM concentrations cinnamic and benzoic acids were toxic to ruminal fungi.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akin D. E. Evaluation by electron microscopy and anaerobic culture of types of rumen bacteria associated with digestion of forage cell walls. Appl Environ Microbiol. 1980 Jan;39(1):242–252. doi: 10.1128/aem.39.1.242-252.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akin D. E., Gordon G. L., Hogan J. P. Rumen bacterial and fungal degradation of Digitaria pentzii grown with or without sulfur. Appl Environ Microbiol. 1983 Sep;46(3):738–748. doi: 10.1128/aem.46.3.738-748.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauchop T., Mountfort D. O. Cellulose fermentation by a rumen anaerobic fungus in both the absence and the presence of rumen methanogens. Appl Environ Microbiol. 1981 Dec;42(6):1103–1110. doi: 10.1128/aem.42.6.1103-1110.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauchop T. Rumen anaerobic fungi of cattle and sheep. Appl Environ Microbiol. 1979 Jul;38(1):148–158. doi: 10.1128/aem.38.1.148-158.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borneman W. S., Akin D. E., VanEseltine W. P. Effect of phenolic monomers on ruminal bacteria. Appl Environ Microbiol. 1986 Dec;52(6):1331–1339. doi: 10.1128/aem.52.6.1331-1339.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell D. R., Bryant M. P. Medium without rumen fluid for nonselective enumeration and isolation of rumen bacteria. Appl Microbiol. 1966 Sep;14(5):794–801. doi: 10.1128/am.14.5.794-801.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorak P. M., Westlake D. W. Fungal Metabolism of n-Alkylbenzenes. Appl Environ Microbiol. 1986 Feb;51(2):435–437. doi: 10.1128/aem.51.2.435-437.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard B. D., Richards G. N. Presence of soluble lignin-carbohydrate complexes in the bovine rumen. Carbohydr Res. 1975 Jun;42(1):135–145. doi: 10.1016/s0008-6215(00)84106-3. [DOI] [PubMed] [Google Scholar]
- Latham M. J., Brooker B. E., Pettipher G. L., Harris P. J. Adhesion of Bacteroides succinogenes in pure culture and in the presence of Ruminococcus flavefaciens to cell walls in leaves of perennial ryegrass (Lolium perenne). Appl Environ Microbiol. 1978 Jun;35(6):1166–1173. doi: 10.1128/aem.35.6.1166-1173.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison I. M. Structural invesiigations on the lignin-carbohydrate complexes of Lolium perenne. Biochem J. 1974 Apr;139(1):197–204. doi: 10.1042/bj1390197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountfort D. O., Asher R. A. Production and regulation of cellulase by two strains of the rumen anaerobic fungus Neocallimastix frontalis. Appl Environ Microbiol. 1985 May;49(5):1314–1322. doi: 10.1128/aem.49.5.1314-1322.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munn E. A., Orpin C. G., Hall F. J. Ultrastructural studies of the free zoospore of the rumen phycomycete Neocallimastix frontalis. J Gen Microbiol. 1981 Aug;125(2):311–323. doi: 10.1099/00221287-125-2-311. [DOI] [PubMed] [Google Scholar]
- Orpin C. G. Invasion of plant tissue in the rumen by the flagellate Neocallimastix frontalis. J Gen Microbiol. 1977 Feb;98(2):423–430. doi: 10.1099/00221287-98-2-423. [DOI] [PubMed] [Google Scholar]
- Orpin C. G. Studies on the rumen flagellate Neocallimastix frontalis. J Gen Microbiol. 1975 Dec;91(2):249–262. doi: 10.1099/00221287-91-2-249. [DOI] [PubMed] [Google Scholar]
- Orpin C. G. Studies on the rumen flagellate Sphaeromonas communis. J Gen Microbiol. 1976 Jun;94(2):270–280. doi: 10.1099/00221287-94-2-270. [DOI] [PubMed] [Google Scholar]
- Orpin C. G. The rumen flagellate Piromonas communis: its life-history and invasion of plant material in the rumen. J Gen Microbiol. 1977 Mar;99(1):107–117. doi: 10.1099/00221287-99-1-107. [DOI] [PubMed] [Google Scholar]
- Pearce P. D., Bauchop T. Glycosidases of the rumen anaerobic fungus Neocallimastix frontalis grown on cellulosic substrates. Appl Environ Microbiol. 1985 May;49(5):1265–1269. doi: 10.1128/aem.49.5.1265-1269.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stack R. J., Hungate R. E. Effect of 3-Phenylpropanoic Acid on Capsule and Cellulases of Ruminococcus albus 8. Appl Environ Microbiol. 1984 Jul;48(1):218–223. doi: 10.1128/aem.48.1.218-223.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stack R. J., Hungate R. E., Opsahl W. P. Phenylacetic acid stimulation of cellulose digestion by Ruminococcus albus 8. Appl Environ Microbiol. 1983 Sep;46(3):539–544. doi: 10.1128/aem.46.3.539-544.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafford H. A. Histochemical & Biochemical Differences Between Lignin-Like Materials in Phleum pratense L. Plant Physiol. 1962 Sep;37(5):643–649. doi: 10.1104/pp.37.5.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windham W. R., Akin D. E. Rumen fungi and forage fiber degradation. Appl Environ Microbiol. 1984 Sep;48(3):473–476. doi: 10.1128/aem.48.3.473-476.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]