Age-related macular degeneration (AMD) is an etiologically complex human disease of late life. Although environmental risk factors have been studied extensively (1), underlying genetic risk factors stubbornly resisted attempts at discovery until 2005. Since then, several genetic variations affecting risk have been identified, but some of these data have been contradictory, and the resolutions have remained elusive. The paper by Kanda et al. (2) in a recent issue of PNAS substantially clarifies one of the major genetic controversies by examining in detail the role of two proposed genes on chromosome 10q36.
AMD affects the central region of the retina (macula) that is responsible for central visual acuity. AMD is the most common cause of irreversible blindness in older adults and affects at least 15 million people in the United States. AMD is defined by an abnormal number of intermediate or large drusen (yellow-colored deposits) in the macula, along with other variable changes in macula, including extensive cell death (dry AMD) and abnormal blood vessel growth (wet AMD). Treatment is limited primarily to wet AMD and is focused on preventing further loss of vision. Thus, there is a tremendous amount of interest in understanding the pathophysiology of the disease.
Overwhelming data suggest that AMD has a complex etiology resulting from the interplay of multiple risk factors, both genetic and environmental. A genetic contribution is supported by epidemiologic studies (3, 4) and twin studies (5, 6). Given the late age at onset of AMD, standard family-based approaches toward gene discovery, including genetic linkage analysis, are hampered in their ability to localize these genes. Thus, the numerous genetic linkage studies performed over the last 10 years (reviewed in ref. 7) were not able to generate sufficient support and localization to identify any single AMD-related gene. However, consensus emerged that strongly implicated regions on several chromosomes, two of which (chromosomes 1q32 and 10q26) have been the focus of further efforts (7). Detailed investigations of the 1q32 region identified a common variation (Y402H) within the complement factor H (CFH/ARMS1) gene using multiple different approaches (8–10), including one of the first pilot genome-wide association studies (11). Further investigation demonstrated that the genetic role of CFH is complicated because the risk effect of Y402H is not seen in Asian populations (12, 13), and there are additional protective effects of haplotypes that do not include the risk allele of Y402H, including at least one containing a significant deletion of two closely related genes (14, 15).
The identification of variation in CFH as a major risk factor implicated the alternative complement inflammatory pathway in the etiology of AMD. Testing genes coding for other proteins in this pathway has revealed additional protective effects of variants in the complement component 2/complement factor B (C2/CFB) gene complex on chromosome 6 (16, 17) and a risk effect for variation in the complement component 3 (C3) gene on chromosome 19 (18). Thus, the complement system clearly plays a critical role in the development of AMD.
The region on chromosome 10q26 has also been subjected to intense scrutiny and has elicited substantially more controversy. The linkage signal was quickly localized through allelic association analysis to a small chromosomal region containing three genes, PLEKHA1, HTRA1(PRSS11), and a DNA sequence (LOC387715) coding for a hypothetical protein of unknown function (19). Two additional studies then proposed that the primary effect lay within the putative LOC387715 gene (20, 21) and specifically the SNP rs10490924, which encodes a putative protein-coding change (A69S). Soon thereafter, two studies (22, 23) suggested that a different SNP (rs11200638) within a regulatory element for HTRA1 was the primary location for the effect, that this variant significantly impacted expression of HTRA1, and that the LOC387715 gene was nonexistent.
Kanda et al. (2) now describe several convincing experiments that provide a resolution to these contradictory data. First, using a large and independent data set, they tested a comprehensive panel of SNPs across the proposed region of association and show that many of them have significant association with AMD. Using conditional statistical analysis, however, they demonstrate that the primary association can be explained by a single SNP (rs10490924), the original SNP identified in the earlier studies (19–21), and that the strong association signals for the other SNPs, including rs11200638, can be explained by their statistical correlation to rs10490924. These data conclusively support LOC387715 and not HTRA1 as the chromosome 10q26 AMD gene.
Second, they attempted to reproduce the proposed effect of rs11200638 on the expression of HTRA1 using multiple different expression constructs and did not find a significant difference in expression between the two alleles, with the trend in expression levels being the opposite of that proposed previously (22, 23).
Given these results, the third set of experiments is highly informative. Kanda et al. (2) convincingly demonstrate that LOC387715 (which they now term ARMS2) in fact codes for a functional protein that is abundantly expressed in human placental tissue and moderately expressed in human retina. Furthermore, they localized this protein to the mitochondria. Given the high energy requirement of the retina, it is interesting to speculate that the retina may be especially sensitive to oxidative damage resulting from altered mitochondrial function. This may help explain the statistical gene–environment interaction between rs10490924 and a history of smoking (21, 24), a known cause of oxidative stress.
AMD is the most common cause of irreversible blindness in older adults.
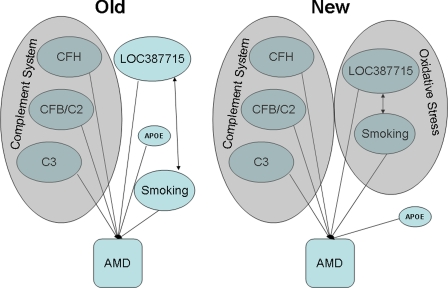
AMD has been a tremendous success story for the application of the latest tools of human genetics. Linkage analysis and locational cloning, genome-wide association, and pathway candidate gene approaches have all yielded genes involved in AMD. These latest findings, combined with other functional data (25), now implicate oxidative stress as a second major pathway, in addition to the complement system, that contributes to the pathogenesis of macular degeneration (Fig. 1).
Fig. 1.
Five genes with common variation that alter the risk of developing AMD have been identified, with three implicating the complement system (Left) in the genetic etiology of AMD. With the data presented by Kanda et al. (2), oxidative stress (Right) is a second pathway implicated in the genetic etiology of AMD.
Footnotes
The authors declare no conflict of interest.
See companion article on page 16227 in issue 41 of volume 104.
References
- 1.Smith W, Assink J, Klein R, Mitchell P, Klaver CC, Klein BE, Hofman A, Jensen S, Wang JJ, de Jong PT. Ophthalmology. 2001;108:697–704. doi: 10.1016/s0161-6420(00)00580-7. [DOI] [PubMed] [Google Scholar]
- 2.Kanda A, Chen W, Othman M, Branham KEH, Brooks M, Khanna R, He S, Lyons R, Abecasis GR, Swaroop A. Proc Natl Acad Sci USA. 2007;104:16227–16232. doi: 10.1073/pnas.0703933104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klaver CC, Wolfs RC, Assink JJ, van Duijn CM, Hofman A, de Jong PT. Arch Ophthalmol. 1998;116:1646–1651. doi: 10.1001/archopht.116.12.1646. [DOI] [PubMed] [Google Scholar]
- 4.Klein BE, Klein R, Lee KE, Moore EL, Danforth L. Am J Epidemiol. 2001;154:207–211. doi: 10.1093/aje/154.3.207. [DOI] [PubMed] [Google Scholar]
- 5.Meyers SM, Greene T, Gutman FA. Am J Ophthalmol. 1995;120:757–766. doi: 10.1016/s0002-9394(14)72729-1. [DOI] [PubMed] [Google Scholar]
- 6.Hammond CJ, Webster AR, Snieder H, Bird AC, Gilbert CE, Spector TD. Ophthalmology. 2002;109:730–736. doi: 10.1016/s0161-6420(01)01049-1. [DOI] [PubMed] [Google Scholar]
- 7.Fisher SA, Abecasis GR, Yashar BM, Zareparsi S, Swaroop A, Iyengar SK, Klein BE, Klein R, Lee KE, Majewski J, et al. Hum Mol Genet. 2005;14:2257–2264. doi: 10.1093/hmg/ddi230. [DOI] [PubMed] [Google Scholar]
- 8.Haines JL, Hauser MA, Schmidt S, Scott WK, Olson LM, Gallins P, Spencer KL, Kwan SY, Noureddine M, Gilbert JR, et al. Science. 2005;308:419–421. doi: 10.1126/science.1110359. [DOI] [PubMed] [Google Scholar]
- 9.Edwards AO, Ritter R, III, Abel KJ, Manning A, Panhuysen C, Farrer LA. Science. 2005;308:421–424. doi: 10.1126/science.1110189. [DOI] [PubMed] [Google Scholar]
- 10.Hageman GS, Anderson DH, Johnson LV, Hancox LS, Taiber AJ, Hardisty LI, Hageman JL, Stockman HA, Borchardt JD, Gehrs KM, et al. Proc Natl Acad Sci USA. 2005;102:7227–7232. doi: 10.1073/pnas.0501536102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klein RJ, Zeiss C, Chew EY, Tsai JY, Sackler RS, Haynes C, Henning AK, SanGiovanni JP, Mane SM, Mayne ST, et al. Science. 2005;308:385–389. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li M, Atmaca-Sonmez P, Othman M, Branham KE, Khanna R, Wade MS, Li Y, Liang L, Zareparsi S, Swaroop A, et al. Nat Genet. 2006;38:1049–1054. doi: 10.1038/ng1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Okamoto H, Umeda S, Obazawa M, Minami M, Noda T, Mizota A, Honda M, Tanaka M, Koyama R, Takagi I, et al. Mol Vis. 2006;12:156–158. [PubMed] [Google Scholar]
- 14.Hughes AE, Orr N, Esfandiary H, az-Torres M, Goodship T, Chakravarthy U. Nat Genet. 2006;38:1173–1177. doi: 10.1038/ng1890. [DOI] [PubMed] [Google Scholar]
- 15.Hageman GS, Hancox LS, Taiber AJ, Gehrs KM, Anderson DH, Johnson LV, Radeke MJ, Kavanagh D, Richards A, Atkinson J, et al. Ann Med. 2006;38:592–604. doi: 10.1080/07853890601097030. [DOI] [PubMed] [Google Scholar]
- 16.Gold B, Merriam JE, Zernant J, Hancox LS, Taiber AJ, Gehrs K, Cramer K, Neel J, Bergeron J, Barile GR, et al. Nat Genet. 2006;38:458–462. doi: 10.1038/ng1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spencer KL, Hauser MA, Olson LM, Schmidt S, Scott WK, Gallins P, Agarwal A, Postel EA, Pericak-Vance MA, Haines JL. Hum Mol Genet. 2007;16:1986–1992. doi: 10.1093/hmg/ddm146. [DOI] [PubMed] [Google Scholar]
- 18.Yates JR, Sepp T, Matharu BK, Khan JC, Thurlby DA, Shahid H, Clayton DG, Hayward C, Morgan J, Wright AF, et al. N Engl J Med. 2007;357:553–561. doi: 10.1056/NEJMoa072618. [DOI] [PubMed] [Google Scholar]
- 19.Jakobsdottir J, Conley YP, Weeks DE, Mah TS, Ferrell RE, Gorin MB. Am J Hum Genet. 2005;77:389–407. doi: 10.1086/444437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rivera A, Fisher SA, Fritsche LG, Keilhauer CN, Lichtner P, Meitinger T, Weber BH. Hum Mol Genet. 2005;14:3227–3236. doi: 10.1093/hmg/ddi353. [DOI] [PubMed] [Google Scholar]
- 21.Schmidt S, Hauser MA, Scott WK, Postel EA, Agarwal A, Gallins P, Wong F, Chen YS, Spencer K, Schnetz-Boutaud N, et al. Am J Hum Genet. 2006;78:852–864. doi: 10.1086/503822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang Z, Camp NJ, Sun H, Tong Z, Gibbs D, Cameron DJ, Chen H, Zhao Y, Pearson E, Li X, et al. Science. 2006;314:992–993. doi: 10.1126/science.1133811. [DOI] [PubMed] [Google Scholar]
- 23.Dewan A, Liu M, Hartman S, Zhang SS, Liu DT, Zhao C, Tam PO, Chan WM, Lam DS, Snyder M, et al. Science. 2006;314:989–992. doi: 10.1126/science.1133807. [DOI] [PubMed] [Google Scholar]
- 24.Schaumberg DA, Hankinson SE, Guo Q, Rimm E, Hunter DJ. Arch Ophthalmol. 2007;125:55–62. doi: 10.1001/archopht.125.1.55. [DOI] [PubMed] [Google Scholar]
- 25.Winkler BS, Boulton ME, Gottsch JD, Sternberg P. Mol Vis. 1999;5:32. [PMC free article] [PubMed] [Google Scholar]