Abstract
Bilateral, high-frequency stimulation (HFS) of the subthalamic nucleus (STN) is the surgical therapy of choice for movement disability in advanced Parkinson's disease (PD), but this procedure evokes debilitating psychiatric effects, including depressed mood, of unknown neural origin. Here, we report the unexpected finding that HFS of the STN inhibits midbrain 5-hydroxytryptamine (5-HT) neurons to evoke depression-related behavioral changes. We found that bilateral HFS of the STN consistently inhibited (40–50%) the firing rate of 5-HT neurons in the dorsal raphe nucleus of the rat, but not neighboring non-5-HT neurons. This effect was apparent at clinically relevant stimulation parameters (≥100 Hz, ≥30 μA), was not elicited by HFS of either neighboring or remote structures to the STN, and was still present in rat models of PD. We also found that bilateral HFS of the STN evoked clear-cut, depressive-like behavior in a widely used experimental paradigm of depression (forced swim test), and this effect was also observed in a PD model. Importantly, the depressive-like behavior elicited by HFS of the STN was reversed by a selective 5-HT-enhancing antidepressant, thereby linking the behavioral change to decreased 5-HT neuronal activity. Overall, these findings link reduced 5-HT function to the psychiatric effects of HFS of the STN observed in PD patients and provide a rational basis for their clinical management. More generally, the powerful interaction between the STN and 5-HT system uncovered here offers insights into the high level of comorbidity of basal ganglia disease and mood disorder.
Keywords: Parkinson's disease, mood disorder, deep brain stimulation
The use of stimulation electrodes implanted in the brain to control severely disabling neurological and psychiatric conditions is an exciting and fast emerging area of clinical neuroscience (1). As an example, bilateral, high-frequency stimulation (HFS) of the subthalamic nucleus (STN) has become the surgical therapy of choice for advanced Parkinson's disease (PD), and to date >30,000 PD patients worldwide have benefited from this procedure (2, 3). The relief of movement disability by HFS of the STN is predictable because this nucleus is a critical part of the basal ganglia motor circuitry that is dysfunctional in PD patients (4, 5) and in animal models of PD (6–8).
Despite having important beneficial motor effects, in up to 40% of PD patients, bilateral STN HFS is associated with the occurrence of unpleasant and debilitating psychiatric effects, including cognitive alterations, low mood, aggression, and impulsive acts and thoughts that are linked to suicide (9–12). These psychiatric effects can be a major burden to patients and their families and often mitigate the positive effects on motor symptoms. In animals, HFS and other manipulations of the STN also produce a range of nonmotor behavioral changes, including increased impulsivity and altered cognitive responses that appear to correlate with the adverse effects experienced by PD patients (12). Collectively, these findings associate the STN with the generation of mood disorder and related symptoms, not only in STN-stimulated PD patients, but also in basal ganglia disease patients more generally, for whom there is a high level of mood disorder comorbidity (13).
Currently there is no rational basis for the clinical management of the nonmotor effects of STN HFS because the neural origin of these effects is unknown. Candidate neural substrates include altered functioning of the connections between the STN and limbic circuits in the forebrain (12, 14), but direct evidence is lacking. Moreover, it seems unlikely that the diversity of psychiatric effects of STN stimulation can be explained by specific STN-limbic connections, and a key role for a neuromodulatory system seems more plausible. In this respect, it is notable that many of the psychiatric effects of STN HFS, and especially low mood, aggression, increased impulsivity, and suicidal ideation, have long been associated with the reduced functioning of 5-hydroxytryptamine (5-HT; serotonin) neurons in the midbrain (15–17), which are the source of an extensive 5-HT innervation to the limbic forebrain (18, 19). Here, we tested the hypothesis that bilateral STN HFS inhibits the activity of midbrain 5-HT neurons to evoke depression-related behavioral outputs.
Results
Neuron Firing Properties.
Extracellular single-unit recordings were taken from 64 putative 5-HT neurons and 10 putative non-5-HT neurons, in the dorsal raphe nucleus (DRN) of treatment naïve, anesthetized rats. The former neurons had electrophysiological properties characteristic of DRN 5-HT neurons, i.e., slow (0.7 ± 0.1 Hz) and regular (coefficient of variation, 0.32 ± 0.01) firing pattern, and a triphasic waveform of wide spike duration (2.33 ± 0.05 ms). In comparison, the other neurons were faster firing (4.2 ± 1.48 Hz), less regular (coefficient of variation, 0.85 ± 0.11), and had a shorter spike duration (1.9 ± 0.12 ms), characteristics typical of DRN non-5-HT neurons (20).
Effect of STN HFS on the Firing of DRN Neurons.
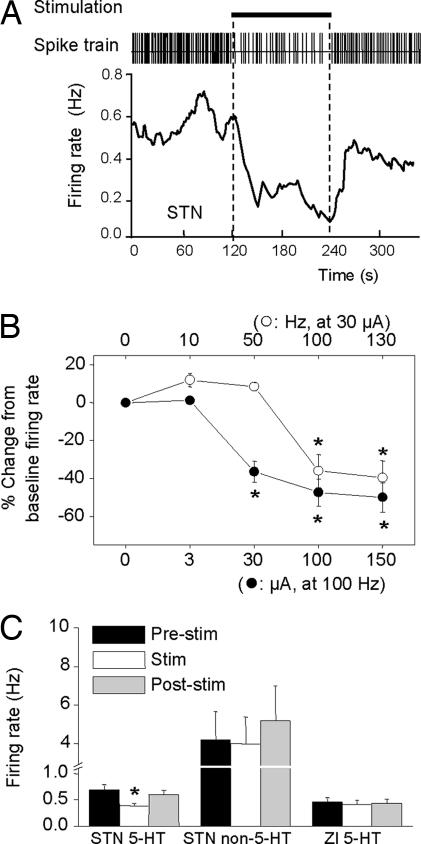
Bilateral STN HFS (100 or 130 Hz) inhibited the firing rate of the vast majority of 5-HT neurons tested (53 of 58, F (1, 52) = 31.81, P < 0.05, −45.1 ± 3.6% of baseline firing rate). This effect had a rapid onset and quickly returned to baseline on cessation of stimulation (Fig. 1A). Analysis of frequency– and current–response relationships (Fig. 1B) revealed that the inhibition occurred at high frequencies (≥100 Hz) and low currents (≥30 μA), but not at lower frequencies (≤50 Hz), thus closely resembling STN stimulation parameters used clinically. STN HFS did not influence the firing rate of non-5-HT neurons in the DRN (Fig. 1C).
Fig. 1.
Effect of HFS of the STN on 5-HT neuronal firing. (A) Spike train and instantaneous firing rate of a 5-HT neuron in response to STN HFS. Horizontal bar indicates stimulation period (100 Hz, 100 μA, 2 min). Note inhibition of single-unit activity during stimulation. (B) Effect of stimulation frequency and current on 5-HT neuron firing rate. Each point is a mean ± SEM value (n = 6). (C) Firing rate of 5-HT neurons (Left) and non-5-HT neurons (Center) before, during, and after STN stimulation (100 Hz, 100 μA). Also, firing rate of 5-HT neurons (Right) before, during, and after stimulation of the zona incerta (ZI). *, P < 0.05 versus relevant prestimulus baseline control values.
Region Specificity of Effect of HFS.
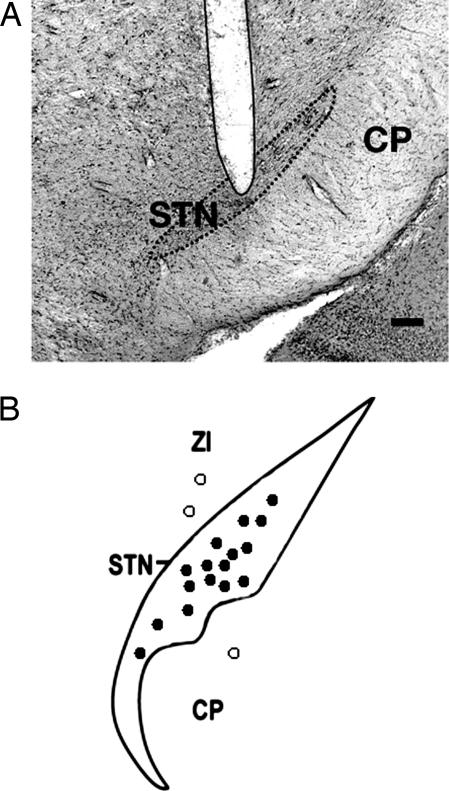
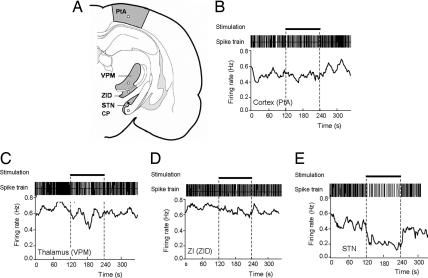
Histological analysis revealed electrode tip locations in the STN in cases where 5-HT neuron firing rate decreased (53 neurons, 16 rats) and locations immediately above or below the STN (zona incerta and cerebral peduncle, respectively) when the firing rate did not change (five neurons, three rats) (Figs. 1C and 2). To further investigate region-specificity of STN HFS, electrodes were stimulated at points during the descent to the STN while recording from the same DRN 5-HT neuron (six neurons, four rats). In this case 5-HT neurons were inhibited by HFS of the STN (F (1, 5) = 17.81, P < 0.05) but not neighboring structures (zona incerta; P > 0.05) or remote structures (parietal cortex, ventroposteromedial nucleus of thalamus, P > 0.05) (Fig. 3).
Fig. 2.
Histological evaluation of electrode localizations. (A) Illustrative coronal section showing the histological verification of the electrode location in the STN. (Scale bar, 200 μm.) (B) Locations of stimulation sites in or close to the STN, as verified by post hoc histology. Filled circles, stimulation sites that produced consistent inhibition of 5-HT cell firing in the DRN; open circles, stimulation sites that produced no significant change in 5-HT cell firing.
Fig. 3.
Effect of HFS at various sites during descent to the STN while recording the same 5-HT neuron in the DRN. (A) Illustration of stimulation sites during electrode descent to the STN. PtA, parietal association cortex; VPM, ventroposteromedial thalamus; ZID, dorsal zona incerta. (B–E) Typical effects of electrical stimulation at the level of the cortex (B), thalamus (C), zona incerta (D), and STN (E) on the firing rate of the same 5-HT neuron in the DRN. Horizontal bars indicate stimulation periods (100 Hz, 100 μA, 2 min).
Effect of HFS in PD Models.
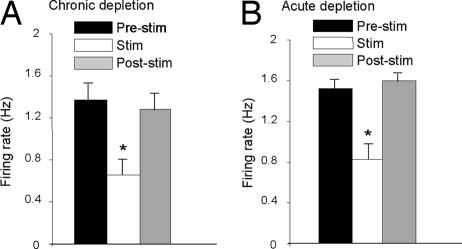
We tested the effect of STN HFS on 5-HT cell firing in two animal models of PD. In rats with chronic dopamine neuron lesions (i.c.v. 6-hydroxy-dopamine) (21), STN HFS inhibited 5-HT cell firing by a magnitude (−52.4 ± 4.3% of baseline; 17 neurons, five rats; F (1, 16) = 39.78, P < 0.05) not different from sham controls (−42.7 ± 7.8%; eight neurons, three rats; F (1, 7) = 10.76, P < 0.05) (Fig. 4A). Similarly, in rats administered reserpine and α-methyl-p-tyrosine to induce an acute dopamine depletion (22), STN HFS markedly inhibited 5-HT cell firing (−49.4 ± 8.3% of baseline; six neurons, six rats; F (1, 5) = 20.13, P < 0.05), (Fig. 4B). Notably, although the effects of STN HFS on 5-HT cell firing remained after dopamine depletion, basal firing was increased by both acute and chronic dopamine depletion. Because STN function is well known to be modulated by dopamine depletion, it is tempting to speculate that the latter finding is further support for a STN–5-HT link.
Fig. 4.
Effect of HFS of the STN on 5-HT neuronal firing after chronic and acute dopamine depletions. (A) Firing rate of 5-HT neurons before, during, and after STN stimulation (100 Hz, 100 μA) in animals chronically depleted of dopamine by i.c.v. injection of 6-hydroxy-dopamine. (B) Firing rate of 5-HT neurons before, during, and after STN stimulation (100 Hz, 100 μA) in animals acutely depleted of dopamine after coadministration of reserpine and α-methyl-p-tyrosine. *, P < 0.05 versus relevant prestimulus baseline control values.
Effect of Intra-STN Muscimol.
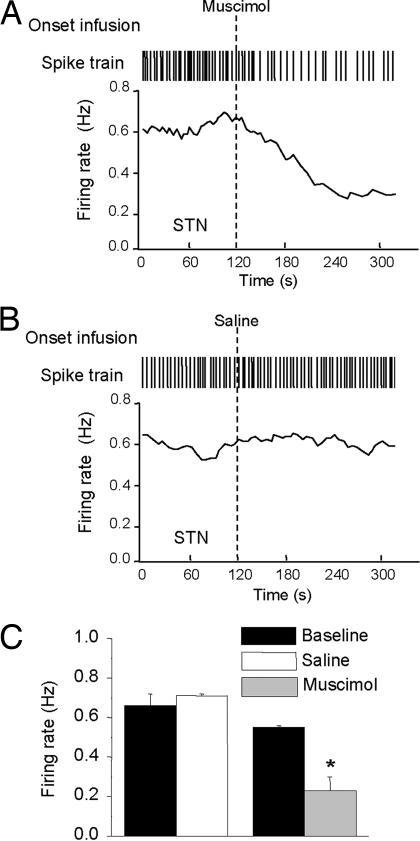
Previous studies have shown that intra-STN infusion of muscimol, a GABAA receptor agonist, recapitulates the effects of STN HFS in animal models of PD (23–25). We used muscimol to test whether neuronal inhibition in the STN might underlie the decrease in 5-HT cell firing induced by HFS. Bilateral infusion of muscimol (0.1 μg), but not vehicle, inhibited the firing of DRN 5-HT neurons (five neurons, five rats; F (1, 4) = 8.36, P < 0.05; Fig. 5), suggesting that STN HFS also inhibits DRN 5-HT neurons by reducing STN output. A reduction in STN output is commonly perceived to underlie the beneficial motor effects of STN HFS (26–28).
Fig. 5.
Effect of muscimol and saline vehicle infusion into STN on 5-HT neuronal firing. (A) Spike train and instantaneous firing rate of a 5-HT neuron in response to bilateral muscimol (0.1 μg per side) infusion into STN. (B). Spike train and instantaneous firing rate of a 5-HT neuron in response to saline infusion into STN. (C) Grouped data for all 5-HT neurons tested with bilateral intra-STN infusion of muscimol or saline. Data are presented as mean ± SEM values. *, P < 0.05 versus relevant prestimulus or predrug baseline control values.
Effect of STN HFS on Depressive-Like Behavior.
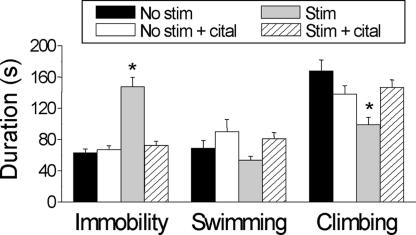
We predicted that the marked inhibitory effect of STN HFS on the firing of 5-HT neurons would also evoke 5-HT-dependent, mood-related behavioral outputs. To test this, we examined the behavioral effects of STN HFS in the forced swim test (FST), a widely used and validated animal model of depression that is sensitive to decreases in 5-HT (29). Bilateral STN HFS (130 Hz, 150 μA) caused a striking increase in immobility and decrease in climbing time of stimulated rats compared with nonstimulated controls (F values >11.37, P < 0.05; Fig. 6), thereby indicating the induction of behavioral “despair” (29). This effect was replicated in animals with chronic dopamine neuron lesions (Fig. 7A). Importantly, the effects of STN HFS in the FST were completely prevented by a prior course of treatment with the selective 5-HT reuptake inhibitor citalopram, at a dose (10 mg/kg s.c., once daily for 14 days) that by itself had no significant effect in nonstimulated controls (Fig. 6). The latter findings are analogous to recent data showing that chronic treatment with citalopram reversed the immobility in the FST induced by stress but did not change immobility by itself (30).
Fig. 6.
Effect of HFS of the STN on different behavioral measures in the FST. Four groups of rats (six per group) were implanted with STN electrodes and tested as follows: (i) no STN stimulation during test (No stim), (ii) pretreatment with citalopram, followed by no STN stimulation during test (No stim + cital), (iii) STN stimulation during test (Stim), and (iv) pretreatment with citalopram, followed by STN stimulation during test (Stim + cital). Stimulation parameters were 130 Hz and 150 μA for the duration of the test. Citalopram pretreatment comprised 10 mg/kg s.c. for 14 days. Bars represent mean ± SEM values (n = 6). The effect of STN stimulation was significantly different from other groups with respect to immobility and climbing: F values >11.4, *, P < 0.05 (two-way ANOVA with repeated measures, post hoc Duncan's test).
Fig. 7.
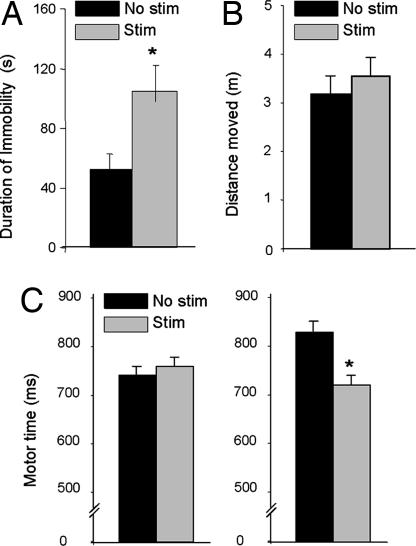
Effect of STN HFS on behaviors in the rat. (A) Immobility time of 6-hydroxydopamine-lesioned rats in the FST. (B) Locomotor activity in the open field of nonlesioned rats. Note that stimulation had no effect. (C) Motor time in nonlesioned (Left) and 6-hydroxydopamine-lesioned (Right) rats in a serial choice reaction time task (data redrawn from previous studies; refs. 31 and 32). Note that effects of stimulation were present only in lesioned animals. STN stimulation parameters were 130 Hz and 30–150 μA for the duration of the tasks. Bars represent mean ± SEM values (n = 6). *, P < 0.05, post hoc analyses revealed that STN stimulation induced a significant effect.
We tested whether STN HFS might alter motor activity and thereby confound the results of the FST. In an open-field paradigm, STN HFS had no effect on motor activity (Fig. 7B). In contrast, we found that STN HFS (at the same stimulation parameters) reversed motor-time deficits in rats with chronic dopamine neuron lesions (Fig. 7C), as observed previously (31, 32).
Discussion
Bilateral STN HFS has important therapeutic value for the relief of movement disability in advanced PD, but this beneficial effect is often offset by disabling psychiatric effects, including depression and suicide ideation, which have no identified cause. The current experiments provide evidence from animal models that adverse psychiatric effects of STN HFS may be underpinned by a previously unknown interaction between the STN and midbrain 5-HT neurons.
In particular, our experiments show that bilateral STN HFS caused a clear-cut inhibition of the firing of DRN 5-HT neurons. This effect was observed in animal PD models and elicited at stimulation parameters (≥100 Hz, ≥30 μA) that produce both the beneficial motor effects of STN HFS (33, 34) and the unwanted psychiatric effects (12, 35) in PD patients. Moreover, this effect reversed upon cessation of the stimulus and was highly specific; STN HFS did not alter the firing rate of non-5-HT DRN neurons, and the firing rate of 5-HT DRN neurons was not inhibited by HFS of either neighboring or remote structures to the STN. It is notable that STN HFS inhibited the vast majority of 5-HT neurons tested (>90%). Because the DRN is the principal source of the 5-HT innervation to the forebrain (36), the latter finding predicts that STN HFS would inhibit transmission throughout much of the ascending 5-HT system and impact on the many functions that this system subserves, including mood regulation (37).
We tested the prediction that STN HFS would elicit changes in mood-related outputs using the FST, which is a well validated animal model of depression that is sensitive to changes in 5-HT (29). This test is based on exposure to a learned inescapable stressor, and the measurement of immobility that is thought to reflect a failure of persistence in escape-directed behavior (29, 38, 39). Our experiments show that STN HFS significantly increased immobility (and decreased climbing time) in both naïve animals and PD models, without confounding motor effects.
The depressive-like behavior evoked by STN HFS is most likely mediated by decreased 5-HT cell firing. In support to this, depletion of 5-HT also induces depressive-like behavior (increases immobility) in the FST (40). Moreover, our data show that the behavioral effect of STN HFS was completely prevented by the selective 5-HT augmenting antidepressant citalopram. Interestingly, two clinical case reports found that depression due to STN stimulation was successfully treated with a selective serotonin reuptake inhibitor (SSRI), including citalopram (41, 42), very much in support of our observations. Citalopram probably does not prevent the decrease in STN stimulation-induced 5-HT firing but, rather, counteracts this effect through blockade of 5-HT reuptake and increasing extracellular 5-HT in nerve terminal regions (43, 44). It is plausible that other behavioral effects of STN HFS are also mediated by reduced 5-HT cell firing. Indeed, STN HFS is reported to result in increased impulsivity (45), a behavioral change that has long been associated with lowered 5-HT function (46).
In patients at risk of depression, depletion of tryptophan, the 5-HT precursor, increased depressive symptoms and induced impulsivity (19), and there is evidence that tryptophan depletion produces similar effects in PD patients (47). In a recent metaanalysis (9), STN HFS evoked disabling mood changes in 15% of PD patients. However, patients with a psychiatric history are usually excluded, and individual studies report an incidence as high as 40% (e.g., ref. 41), suggesting an underestimate of the problem. The fact that not all patients are influenced in this way suggests that affected patients have an underlying vulnerability. Determination of the psychological effects of acute tryptophan depletion before surgery may offer a means to detect those patients most at risk of developing 5-HT-related psychiatric side effects in response to STN stimulation.
Although there is no unified concept for the mechanism underlying the therapeutic effects of STN HFS in PD, studies across a range of species, including humans, consistently emphasize a role for reduced neural activity within the STN and consequent effects on basal ganglia output structures (26–28, 48–54). Reduced STN activity may also underpin the inhibition of 5-HT neurons (and associated depressive-like behavior) induced by STN HFS because bilateral infusion of the GABAA receptor agonist, muscimol, into the STN also inhibited 5-HT neurons. Intra-STN infusion of muscimol also elicits anti-PD effects in animal models (23–25), further emphasizing the commonality of mechanisms underlying the motor and nonmotor effects of STN manipulations.
The neural circuits through which STN HFS inhibits the firing of DRN 5-HT neurons likely involves polysynaptic connections because the STN does not directly project to the DRN (36). Accordingly, our detailed evaluation of peristimulus time histograms for STN-inhibited DRN 5-HT neurons revealed no evidence of antidromic activation or short-latency (<10 ms) orthodromic responses (data not shown). The lack of effect of dopamine lesions and reserpinization on the 5-HT response to STN stimulation, rules out an involvement of monoamine (dopamine, 5-HT, noradrenaline) transmitter pathways. Several brain regions providing important inputs to the DRN, including the substantia nigra pars reticulata, lateral habenula nucleus, and medial prefrontal cortex, are direct or indirect targets of STN outputs (55). These convergent pathways are attractive candidate substrates for the inhibitory effect of STN HFS on 5-HT cell firing and the associated behavioral changes.
Collectively, the current data show that STN HFS causes a striking inhibition of 5-HT neuronal activity and elicits a 5-HT-dependent, mood-related behavioral output. We propose that these changes contribute to the adverse psychiatric effects of STN HFS in PD patients and provide a rational basis for their clinical management and prevention by using 5-HT-targeted drug manipulations. More generally, this unsuspected link between the STN and 5-HT system supports the existence of a “motor–limbic interface” that may contribute to mood disturbances in other populations of psychiatric and neurological patients.
Materials and Methods
Animals.
Male rats for electrophysiological (270–330 g; Sprague–Dawley Harlan Olac, Bicester, U.K.) and behavioral (270–330 g; Lewis, Maastricht University, The Netherlands) studies were housed in groups under conditions of constant temperature (21 ± 1°C) and humidity under a 24-h light/dark cycle (lights on 0800–2000 h, Oxford; lights on 1700–0500 h, Maastricht) with food and water freely available. Electrophysiological experiments were carried out in accordance with the U.K. Home Office Animals (Scientific Procedures) Act (1986), and behavioral experiments were carried out in accordance with the Animal Experiments and Ethics Committee of Maastricht University.
Electrophysiological Recording of DRN Neurons.
Rats were anesthetized with chloral hydrate (460 mg/kg i.p.) with additional doses as required, supplemented with saffan (1.2 mg/kg i.v.) during surgery. Extracellular single-unit recordings of putative 5-HT and non-5-HT neurons were made as described (56). Glass recording electrodes (filled with 2 M NaCl; 2% pontamine sky blue dye; 6–20 MΩ in vitro) were lowered into the DRN (coordinates from bregma: anteroposterior (AP) −7.5, mediolateral (ML) 0.0, and dorsoventral (DV) −4.5 to −5.5 mm) by using a hydraulic micromanipulator. Single-unit potentials were amplified and filtered (gain × 1,000, 0.5–5 kHz band pass; Neurolog system; Digitimer, Wellwyn, Garden City, U.K.), captured by using a 1401plus A-D converter, and analyzed offline by using Spike2 software (Cambridge Electronic Design, Cambridge, U.K.).
Putative 5-HT neurons satisfied at least three of the following electrophysiological and pharmacological criteria (20, 57): slow (0.5–2 Hz) and regular (coefficient of variation, 0.3–0.5) firing pattern, triphasic extracellular spike waveform with a wide duration (1.9–2.5 ms), and an inhibitory response to administration of the 5-HT1A receptor agonist 8-hydroxy-2-(di-n-propylamino)tetralin (10 μg/kg i.v.). In comparison, putative non-5-HT neurons were identified as having a fast and irregular firing and less-broad spike duration. After recording, the final position of the electrode was marked by iontophoretic dye injection.
Electrophysiology Protocol.
Stimulation electrodes were stereotaxically implanted bilaterally into the STN (AP −3.8, ML ± 2.5, DV −8.0) and typically left in place for the remainder of the experiment. After 3–5 min of baseline recording, STN stimulations were typically applied for 2–3 min, and recordings continued for 2–3 min before any further stimulations (maximum of six per neuron). The effect of STN stimulation was tested in several neurons per rat (typically five to six) because repeated periods of STN stimulation evoked reproducible effects on the firing of individual neurons. In some experiments, low-frequency stimulation was applied before high-frequency stimulations (see below) to enable construction of peristimulus-time histograms.
Stimulation Electrodes and Parameters.
Stimulations were applied bilaterally by using two gold-plated coaxial electrodes with an inner wire of a platinum–iridium combination (250-μm shaft diameter, 50-μm tip diameter, 75-μm exposed tip length, interpole distance 50 μm; Technomed, Beek, The Netherlands). Electrodes were connected to a stimulator (Accupulser A310; World Precision Instruments Berlin, Germany) via a stimulus isolator (WPI A360; World Precision Instruments). Stimulations were bipolar (inner electrode negative, outer electrode positive, 60-μs pulse width), and parameters were verified on-line by using a digital oscilloscope.
Three stimulation protocols were used: (i) HFS was applied while recording from a DRN 5-HT or non-5-HT neuron. In some experiments, different stimulation frequencies (10, 50, 100, and 130 Hz) and currents (3, 30, 100, and 150 μA) were evaluated in the same animal. (ii) HFS was applied to electrodes at different locations during a descent into the STN while continuously recording from the same DRN 5-HT neuron. Here, electrodes were slowly advanced, and 2- to 3-min stimulations were applied to the following regions: parietal association cortex (AP −3.8, ML 2.5, DV −1.5), ventroposteromedial nucleus of the thalamus (AP −3.8, ML 2.5, DV −6.0), zona incerta (AP −3.8, ML 2.5, DV −7.0), and then STN (AP −3.8, ML 2.5, DV −8.0). (iii) To construct peristimulus-time histograms, low-frequency stimulation (1 Hz for 3–5 min) was applied while recording from a DRN 5-HT neuron.
Computation of Electrophysiological Data.
Stimulation artifacts (<0.5 ms in duration) were removed from spike trains by off-line analysis. Specifically, single-unit activity was isolated with standard “spike sorting” procedures, including template-matching, principal component analysis, and supervised clustering (Spike2). Each trace was then visually inspected to ensure the quality of spike identification. The firing rate of each neuron was then quantified in the final 60 s of each baseline, stimulation, and poststimulation period. Regularity of firing was calculated for the same periods by standard coefficient of variation analysis. Spike waveform width was calculated from the average of waveforms recorded during the final 60 s of baseline recording for each neuron, with width determined as the time between a 5% positive deviation from baseline to the return to baseline after a negative phase (20). Peristimulus time histograms were constructed (Spike 2) and assessed according to published criteria (58).
Intra-STN Infusion of Muscimol.
Muscimol, or saline vehicle, was infused into the STN during recordings. Before recordings, bilateral cannulae were stereotactically implanted into the STN and left in place for the remainder of the experiment. Cannulae were connected via tubing to a 50-μl syringe driven by a microinfusion pump. After a baseline recording of 2–3 min, either muscimol (0.1 μg in 0.5 μl of saline) or saline (0.5 μl) were infused (0.25 μl/min), and recordings were continued for up to 20 min.
Experimental Models of PD.
For chronic dopamine neuron lesions, rats (n = 8) were pretreated with desipramine (25 mg/kg i.p.) and anesthetized with halothane before infusion (over 2 min) of 6-hydroxydopamine (250 μg in 10 μl, n = 5) or vehicle (1% ascorbic acid/saline, n = 3) into the lateral ventricle (AP −0.9, ML 1.4, DV −4.0) (21). Electrophysiological experiments were carried out 14–16 days after surgery. Compared with vehicle controls, animals treated with 6-hydroxydopamine showed a significant (P < 0.05) loss of striatal dopamine (−82%) and dihydroxyphenylacetic acid (−89%), as measured by HPLC with electrochemical detection.
The effect of acute dopamine depletion on STN-evoked inhibition of 5-HT cell firing was also investigated. Animals were treated with reserpine (5 mg/kg, i.p., 20 h before recording) and then α-methyl-p-tyrosine (250 mg/kg i.p., 4 h before recording) according to Garcia et al. (22). Rats were strikingly akinetic before anesthesia.
FST.
Bilateral stimulation electrodes were stereotaxically implanted into the STN of anesthetized rats (90 mg/kg ketamine/10 mg/kg xylazine, i.p.). After recovery (1 week) animals were stimulated via externalized leads while performing behavioral tasks (31, 32).
In the case of the modified FST (59), testing was carried out by using a transparent Perspex cylinder (50 × 20 cm) surrounded by black side walls and placed on a black base 60 cm from the floor. The cylinder was filled with tap water (25 ± 1°C) to a depth of 30 cm.
In a pretest session, each rat was placed in the water for 15 min. The following day and 2 weeks later, rats were placed in the water for 5 min. STN stimulation commenced 2 min before testing and continued for the duration of the test. Recordings of behavior were taken by a digital camera placed above the cylinders. The duration of the following behaviors was timed by observers blind to treatments: “immobility” (no movements or small and infrequent movements performed solely to maintain the nose above the water), “swimming” (active swimming with the forepaws), and “climbing” (scratching of cylinder walls using both forepaws and hindpaws). Whereas previous studies have associated climbing behavior with noradrenergic mechanisms (59), our measures of immobility and climbing (and swimming) were not completely independent variables, and any reversal of climbing is likely, at least in part, linked to the reversal of immobility.
FST Protocol.
Four groups of six rats were tested in the FST as follows: (i) no STN stimulation with saline, (ii) no STN stimulation with citalopram, (iii) STN stimulation (130 Hz, 150 μA) with saline, and (iv) STN stimulation (130 Hz, 150 μA) with citalopram. Citalopram treatment comprised 10 mg/kg s.c. once daily for 14 days. Saline treatment comprised once daily injections for 14 days. A challenge dose of 10 mg/kg s.c. of citalopram increases extracellular 5-HT in prefrontal cortex of freely moving rats by 2- to 3-fold (60).
The effect of STN stimulation on immobility time was also tested in rats with a dopamine neuron lesion. This experiment comprised two groups of six STN electrode-implanted, dopamine-denervated rats; one group was stimulated (130 Hz, 150 μA) during the FST, and the other received no stimulation.
Open-Field Test.
Rats with bilateral STN electrodes were placed in a square open-field arena (inner dimensions 50 × 50 × 50 cm) and the distance moved over 10 min was recorded by digital camera (Ethovision software; Noldus Information Technology, Wageningen, The Netherlands) placed above the arena. Open-field activity was measured during one session when electrodes were not stimulated and another session 2 weeks later when the electrodes were stimulated (130 Hz, 150 μA).
Histology.
At the end of electrophysiological and behavioral experiments, rats were perfused transcardially with PBS and then fixative containing 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.6). Brains were removed and postfixed for 2 h, followed by overnight immersion in 20% sucrose at 4°C. Brain tissue was stored (−80°C) subsequent to sectioning (30 μm) by using a cryostat. Standard hematoxylin–eosin and standard cresyl violet counterstains were used to locate the stimulating and recording electrode tips.
Statistical Analysis.
Electrophysiological data were analyzed statistically by one-way ANOVA with repeated measures. STN stimulation or drug treatment (muscimol) were used as the repeated-measures factor, followed by a Duncan's post hoc test. Behavioral data were analyzed by two-way ANOVA with “stimulation” and “treatment” being used as repeated measures, followed by a Duncan's post hoc test. A P value <0.05 was considered significant.
Acknowledgments
We thank Eva van Donkelaar, Rob Hameleers, Katie Jennings, and Josie Raley for helpful discussions and technical assistance. This work was supported by European Community Grant LSHM-CT-2004-503474 (Integrated Network, NEWMOOD) (to T.S. and H.W.M.S.), the Dutch Medical Research Council (ZonMw) (Y.T.), the Dutch Brain Foundation (Hersenstichting Nederland) (Y.T. and H.W.M.S.), the Medical Research Council U.K. (P.J.M.), and the Royal Dutch Academy of Sciences (KNAW) (Y.T.).
Abbreviations
- 5-HT
5-hydroxytryptamine
- STN
subthalamic nucleus
- FST
forced swim test
- DRN
dorsal raphe nucleus
- HFS
high-frequency stimulation
- PD
Parkinson's disease
- AP
anteroposterior
- DV
dorsoventral
- ML
mediolateral.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Wichmann T, Delong MR. Neuron. 2006;52:197–204. doi: 10.1016/j.neuron.2006.09.022. [DOI] [PubMed] [Google Scholar]
- 2.Deuschl G, Schade-Brittinger C, Krack P, Volkmann J, Schafer H, Botzel K, Daniels C, Deutschlander A, Dillmann U, Eisner W, et al. N Engl J Med. 2006;355:896–908. doi: 10.1056/NEJMoa060281. [DOI] [PubMed] [Google Scholar]
- 3.Benabid AL, Chabardes S, Seigneuret E. Curr Opin Neurol. 2005;18:623–630. doi: 10.1097/01.wco.0000186839.53807.93. [DOI] [PubMed] [Google Scholar]
- 4.Bevan MD, Magill PJ, Terman D, Bolam JP, Wilson CJ. Trends Neurosci. 2002;25:525–531. doi: 10.1016/s0166-2236(02)02235-x. [DOI] [PubMed] [Google Scholar]
- 5.Lozano AM, Dostrovsky J, Chen R, Ashby P. Lancet Neurol. 2002;1:225–231. doi: 10.1016/s1474-4422(02)00101-1. [DOI] [PubMed] [Google Scholar]
- 6.Bergman H, Wichmann T, DeLong MR. Science. 1990;249:1436–1438. doi: 10.1126/science.2402638. [DOI] [PubMed] [Google Scholar]
- 7.Plenz D, Kital ST. Nature. 1999;400:677–682. doi: 10.1038/23281. [DOI] [PubMed] [Google Scholar]
- 8.Kass JI, Mintz IM. Proc Natl Acad Sci USA. 2006;103:183–188. doi: 10.1073/pnas.0506781102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Temel Y, Kessels A, Tan S, Topdag A, Boon P, Visser-Vandewalle V. Parkinsonism Relat Disord. 2006;12:265–272. doi: 10.1016/j.parkreldis.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 10.Bejjani BP, Damier P, Arnulf I, Thivard L, Bonnet AM, Dormont D, Cornu P, Pidoux B, Samson Y, Agid Y. N Engl J Med. 1999;340:1476–1480. doi: 10.1056/NEJM199905133401905. [DOI] [PubMed] [Google Scholar]
- 11.Piasecki SD, Jefferson JW. J Clin Psychiatry. 2004;65:845–849. doi: 10.4088/jcp.v65n0617. [DOI] [PubMed] [Google Scholar]
- 12.Temel Y, Blokland A, Steinbusch HW, Visser-Vandewalle V. Prog Neurobiol. 2005;76:393–413. doi: 10.1016/j.pneurobio.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 13.Chaudhuri KR, Healy DG, Schapira AH. Lancet Neurol. 2006;5:235–245. doi: 10.1016/S1474-4422(06)70373-8. [DOI] [PubMed] [Google Scholar]
- 14.Turner MS, Lavin A, Grace AA, Napier TC. J Neurosci. 2001;21:2820–2832. doi: 10.1523/JNEUROSCI.21-08-02820.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhagwagar Z, Whale R, Cowen PJ. Br J Psychiatry. 2002;180:24–28. doi: 10.1192/bjp.180.1.24. [DOI] [PubMed] [Google Scholar]
- 16.Mann JJ. Nat Rev Neurosci. 2003;4:819–828. doi: 10.1038/nrn1220. [DOI] [PubMed] [Google Scholar]
- 17.Smith KA, Fairburn CG, Cowen PJ. Lancet. 1997;349:915–919. doi: 10.1016/s0140-6736(96)07044-4. [DOI] [PubMed] [Google Scholar]
- 18.Wrase J, Reimold M, Puls I, Kienast T, Heinz A. Cogn Affect Behav Neurosci. 2006;6:53–61. doi: 10.3758/cabn.6.1.53. [DOI] [PubMed] [Google Scholar]
- 19.Booij L, Swenne CA, Brosschot JF, Haffmans PM, Thayer JF, Van der Does AJ. Biol Psychiatry. 2006;60:507–514. doi: 10.1016/j.biopsych.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 20.Allers KA, Sharp T. Neuroscience. 2003;122:193–204. doi: 10.1016/s0306-4522(03)00518-9. [DOI] [PubMed] [Google Scholar]
- 21.Rodriguez Diaz M, Abdala P, Barroso-Chinea P, Obeso J, Gonzalez-Hernandez T. Behav Brain Res. 2001;122:79–92. doi: 10.1016/s0166-4328(01)00168-1. [DOI] [PubMed] [Google Scholar]
- 22.Garcia L, Audin J, D'Alessandro G, Bioulac B, Hammond C. J Neurosci. 2003;23:8743–8751. doi: 10.1523/JNEUROSCI.23-25-08743.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mehta A, Chesselet MF. Exp Neurol. 2005;193:110–117. doi: 10.1016/j.expneurol.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 24.Mehta A, Menalled L, Chesselet MF. Neuroscience. 2005;131:769–778. doi: 10.1016/j.neuroscience.2004.11.036. [DOI] [PubMed] [Google Scholar]
- 25.Feger J, Robledo P. Eur J Neurosci. 1991;3:947–952. doi: 10.1111/j.1460-9568.1991.tb00030.x. [DOI] [PubMed] [Google Scholar]
- 26.Meissner W, Leblois A, Hansel D, Bioulac B, Gross CE, Benazzouz A, Boraud T. Brain. 2005;128:2372–2382. doi: 10.1093/brain/awh616. [DOI] [PubMed] [Google Scholar]
- 27.Benazzouz A, Tai CH, Meissner W, Bioulac B, Bezard E, Gross C. FASEB J. 2004;18:528–530. doi: 10.1096/fj.03-0576fje. [DOI] [PubMed] [Google Scholar]
- 28.Tai CH, Boraud T, Bezard E, Bioulac B, Gross C, Benazzouz A. FASEB J. 2003;17:1820–1830. doi: 10.1096/fj.03-0163com. [DOI] [PubMed] [Google Scholar]
- 29.Cryan JF, Markou A, Lucki I. Trends Pharmacol Sci. 2002;23:238–245. doi: 10.1016/s0165-6147(02)02017-5. [DOI] [PubMed] [Google Scholar]
- 30.Rygula R, Abumaria N, Flugge G, Hiemke C, Fuchs E, Ruther E, Havemann-Reinecke U. Behav Pharmacol. 2006;17:19–29. doi: 10.1097/01.fbp.0000186631.53851.71. [DOI] [PubMed] [Google Scholar]
- 31.Temel Y, Visser-Vandewalle V, Aendekerk B, Rutten B, Tan S, Scholtissen B, Schmitz C, Blokland A, Steinbusch HW. Exp Neurol. 2005;193:43–52. doi: 10.1016/j.expneurol.2004.12.025. [DOI] [PubMed] [Google Scholar]
- 32.Desbonnet L, Temel Y, Visser-Vandewalle V, Blokland A, Hornikx V, Steinbusch HW. Brain Res. 2004;1008:198–204. doi: 10.1016/j.brainres.2004.02.032. [DOI] [PubMed] [Google Scholar]
- 33.Timmermann L, Wojtecki L, Gross J, Lehrke R, Voges J, Maarouf M, Treuer H, Sturm V, Schnitzler A. Mov Disord. 2004;19:1328–1333. doi: 10.1002/mds.20198. [DOI] [PubMed] [Google Scholar]
- 34.Benabid AL. Curr Opin Neurobiol. 2003;13:696–706. doi: 10.1016/j.conb.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 35.Berney A, Vingerhoets F, Perrin A, Guex P, Villemure JG, Burkhard PR, Benkelfat C, Ghika J. Neurology. 2002;59:1427–1429. doi: 10.1212/01.wnl.0000032756.14298.18. [DOI] [PubMed] [Google Scholar]
- 36.Peyron C, Petit JM, Rampon C, Jouvet M, Luppi PH. Neuroscience. 1998;82:443–468. doi: 10.1016/s0306-4522(97)00268-6. [DOI] [PubMed] [Google Scholar]
- 37.Monkul ES, Hatch JP, Nicoletti MA, Spence S, Brambilla P, Lacerda AL, Sassi RB, Mallinger AG, Keshavan MS, Soares JC. Mol Psychiatry. 2007;12:360–366. doi: 10.1038/sj.mp.4001919. [DOI] [PubMed] [Google Scholar]
- 38.Porsolt RD, Le Pichon M, Jalfre M. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- 39.Cryan JF, O'Leary OF, Jin SH, Friedland JC, Ouyang M, Hirsch BR, Page ME, Dalvi A, Thomas SA, Lucki I. Proc Natl Acad Sci USA. 2004;101:8186–8191. doi: 10.1073/pnas.0401080101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Slattery DA, Desrayaud S, Cryan JF. J Pharmacol Exp Ther. 2005;312:290–296. doi: 10.1124/jpet.104.073536. [DOI] [PubMed] [Google Scholar]
- 41.Thobois S, Mertens P, Guenot M, Hermier M, Mollion H, Bouvard M, Chazot G, Broussolle E, Sindou M. J Neurol. 2002;249:529–534. doi: 10.1007/s004150200059. [DOI] [PubMed] [Google Scholar]
- 42.Ostergaard K, Sunde N, Dupont E. Mov Disord. 2002;17:693–700. doi: 10.1002/mds.10188. [DOI] [PubMed] [Google Scholar]
- 43.Bymaster FP, Zhang W, Carter PA, Shaw J, Chernet E, Phebus L, Wong DT, Perry KW. Psychopharmacology (Berlin) 2002;160:353–361. doi: 10.1007/s00213-001-0986-x. [DOI] [PubMed] [Google Scholar]
- 44.Hjorth S, Bengtsson HJ, Milano S. Eur J Pharmacol. 1996;316:43–47. doi: 10.1016/s0014-2999(96)00779-0. [DOI] [PubMed] [Google Scholar]
- 45.Baunez C, Christakou A, Chudasama Y, Forni C, Robbins TW. Eur J Neurosci. 2007;25:1187–1194. doi: 10.1111/j.1460-9568.2007.05373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Evenden J. J Psychopharmacol. 1999;13:180–192. doi: 10.1177/026988119901300211. [DOI] [PubMed] [Google Scholar]
- 47.McCance-Katz EF, Marek KL, Price LH. Neurology. 1992;42:1813–1814. doi: 10.1212/wnl.42.9.1813. [DOI] [PubMed] [Google Scholar]
- 48.Garcia L, D'Alessandro G, Bioulac B, Hammond C. Trends Neurosci. 2005;28:209–216. doi: 10.1016/j.tins.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 49.Benazzouz A, Hallett M. Neurology. 2000;55:S13–6. [PubMed] [Google Scholar]
- 50.Filali M, Hutchison WD, Palter VN, Lozano AM, Dostrovsky JO. Exp Brain Res. 2004;156:274–281. doi: 10.1007/s00221-003-1784-y. [DOI] [PubMed] [Google Scholar]
- 51.Welter ML, Houeto JL, Bonnet AM, Bejjani PB, Mesnage V, Dormont D, Navarro S, Cornu P, Agid Y, Pidoux B. Arch Neurol. 2004;61:89–96. doi: 10.1001/archneur.61.1.89. [DOI] [PubMed] [Google Scholar]
- 52.Magarinos-Ascone C, Pazo JH, Macadar O, Buno W. Neuroscience. 2002;115:1109–1117. doi: 10.1016/s0306-4522(02)00538-9. [DOI] [PubMed] [Google Scholar]
- 53.Patel NK, Heywood P, O'Sullivan K, McCarter R, Love S, Gill SS. Brain. 2003;126:1136–1145. doi: 10.1093/brain/awg111. [DOI] [PubMed] [Google Scholar]
- 54.Alvarez L, Macias R, Lopez G, Alvarez E, Pavon N, Rodriguez-Oroz MC, Juncos JL, Maragoto C, Guridi J, Litvan I, et al. Brain. 2005;128:570–583. doi: 10.1093/brain/awh397. [DOI] [PubMed] [Google Scholar]
- 55.Smith Y, Bevan MD, Shink E, Bolam JP. Neuroscience. 1998;86:353–387. doi: 10.1016/s0306-4522(98)00004-9. [DOI] [PubMed] [Google Scholar]
- 56.Boothman LJ, Allers KA, Rasmussen K, Sharp T. Br J Pharmacol. 2003;139:998–1004. doi: 10.1038/sj.bjp.0705328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hajos M, Gartside SE, Villa AE, Sharp T. Neuroscience. 1995;69:189–197. doi: 10.1016/0306-4522(95)00227-a. [DOI] [PubMed] [Google Scholar]
- 58.Varga V, Kocsis B, Sharp T. Eur J Neurosci. 2003;17:280–286. doi: 10.1046/j.1460-9568.2003.02465.x. [DOI] [PubMed] [Google Scholar]
- 59.Cryan JF, Valentino RJ, Lucki I. Neurosci Biobehav Rev. 2005;29:547–569. doi: 10.1016/j.neubiorev.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 60.Huang M, Ichiwaka J, Li Z, Dai J, Meltzer HY. Psychopharmacology (Berlin) 2006;185:274–281. doi: 10.1007/s00213-005-0206-1. [DOI] [PubMed] [Google Scholar]