Abstract
Nicotine, the main psychoactive ingredient of tobacco, induces negative emotional symptoms during abstinence that contribute to a profound craving for nicotine. However, the neurobiological mechanisms underlying how nicotine produces dependence remains poorly understood. We demonstrate one mechanism for both the anxiety-like symptoms of withdrawal and excessive nicotine intake observed after abstinence, through recruitment of the extrahypothalamic stress peptide corticotropin-releasing factor (CRF) system and activation of CRF1 receptors. Overactivation of the CRF–CRF1 system may contribute to nicotine dependence and may represent a prominent target for investigating the vulnerability to tobacco addiction.
Keywords: abstinence, addiction, amygdala, deprivation, stress
Tobacco addiction is the leading avoidable cause of disease and premature death in the U.S., responsible for >400,000 deaths annually (1, 2). The main psychoactive ingredient responsible for tobacco addiction has long been hypothesized to be nicotine. Nicotine acutely produces modest positive reinforcing effects (3, 4) by activating reward systems, including the mesolimbic dopamine system (5, 6). However, the transition from nicotine use to nicotine dependence has been hypothesized to result from neuroadaptative changes in the brain that produce a powerful need to continue tobacco use (7, 8). Such neuroadaptation may involve the mechanisms responsible for the negative emotional states observed during abstinence from nicotine in dependent individuals (9, 10). The negative emotional state produced by nicotine withdrawal is hypothesized to represent a powerful source of negative reinforcement leading to excessive drug intake.
Spontaneous and precipitated (using nicotinic receptor antagonists such as mecamylamine) nicotine withdrawal dramatically decreases brain reward function and the efficacy of natural reinforcers in rodents (9, 11). These effects occur despite the initial, weak reinforcing effect of nicotine, suggesting there must be other mechanisms driving the development of nicotine dependence. The general hypothesis tested here is that chronic nicotine use recruits a major brain stress system, the extrahypothalamic corticotropin-releasing factor (CRF) system (7, 12–15), which contributes critically to the motivation to continue tobacco use. To this end, we tested whether (i) nicotine withdrawal activates the CRF system in the central nucleus of the amygdala, (ii) CRF overactivity, via CRF type 1 receptors (CRF1), induces an anxiety-like state, a component of the negative emotional state hypothesized to drive nicotine dependence, and (iii) nicotine abstinence increases the motivation to take nicotine, by means of a CRF1-dependent mechanism.
Results
Precipitated Withdrawal Increases CRF Levels in the Central Nucleus of the Amygdala.
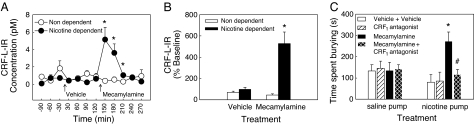
To test the hypothesis that nicotine withdrawal activates the extrahypothalamic CRF system, CRF levels in the central nucleus of the amygdala were measured by using in vivo microdialysis and RIA. CRF levels were assessed before and after precipitated withdrawal by administering mecamylamine to block nicotine receptors in rats with chronic administration of nicotine (nicotine-dependent rats) or saline (nondependent rats), delivered by osmotic minipumps (9). In dependent rats, mecamylamine robustly increased CRF-like immunoreactivity (CRF-L-IR) in the central amygdala (by >500% compared with baseline, Fig. 1B), with levels returning to baseline after 2 h (Fig. 1A). This increase was not observed in saline-treated rats, injected with mecamylamine, and baseline levels did not differ between the two groups.
Fig. 1.
Effects of mecamylamine-precipitated nicotine withdrawal on extracellular levels of CRF-L-IR in the central nucleus of the amygdala and CRF antagonist blockade of precipitated withdrawal-induced anxiety-like behavior in rats by using the defensive burying test. (A) Effect of mecamylamine (1.5 mg/kg, i.p.) -precipitated withdrawal on extracellular levels of CRF-L-IR in the central nucleus of the amygdala as measured by in vivo microdialysis in chronic nicotine pump-treated (nicotine-dependent, n = 7) and chronic saline pump-treated (nondependent, n = 6) rats (*, P < 0.05 vs. nondependent). (B) CRF-L-IR levels expressed as percentage of baseline (first three samples) during the first four samples after vehicle or mecamylamine injections (*, P < 0.05 vs. vehicle). (C) CRF1 antagonist blockade of precipitated withdrawal-induced anxiety-like behavior in rats by using the defensive burying test. Mecamylamine (1.5 mg/kg, i.p.) injection in nicotine-dependent rats increased the time spent burying (*, P < 0.05 vs. vehicle), an effect blocked by pretreatment with the CRF1 antagonist (MPZP, 4 mg/kg s.c., −1 h) (n = 7–9 per group, #, P < 0.05 vs. mecamylamine). Data represent mean ± SEM.
Precipitated Withdrawal Increases Anxiety-Like Behavior Through Activation of CRF1 Receptors.
To test the hypothesis that withdrawal-induced increases in CRF activity, through activation of the CRF1 receptor, might be a mechanism responsible for the appearance of a negative emotional state, we measured anxiety-like behavior during precipitated withdrawal in nicotine-dependent rats and nondependent rats, using the defensive burying test (16, 17). In dependent rats, mecamylamine injection increased the time spent burying (+243%), and decreased the latency to bury (−70%), two markers of active anxiety-like behavior (17), compared with vehicle injection (Fig. 1C) without affecting general activity (rearing), nonanxiety behaviors (resting, grooming), or a passive form of anxiety-like behavior (freezing) [see also supporting information (SI) Table 2]. Mecamylamine injection did not alter anxiety-like behavior in nondependent rats, consistent with the differential effects of mecamylamine on extracellular amygdala levels of CRF-L-IR (Fig. 1 A and B). Pretreatment with a selective small-molecule nonpeptide CRF1 receptor antagonist (N,N-bis(2-methoxyethyl)-3-(4-methoxy-2-methylphenyl)-2,5-dimethyl-pyrazolo [1,5α] pyrimidin-7-amine (MPZP), 4 mg/kg, s.c.)§,¶ blocked the anxiogenic-like effect of mecamylamine to increase burying in nicotine-dependent rats (Fig. 1C).
To confirm that increases of CRF in the central nucleus of the amygdala elicit anxiety-like behavior, we measured anxiety-like behavior using the defensive burying test, after bilateral infusion of CRF (30 pmol total dose) in the central nucleus of the amygdala of naïve rats. CRF administered directly into the amygdala increased the time spent burying and decreased the latency to bury during the first 5 min after infusion (Table 1), without affecting other behaviors (rearing, grooming, resting, and freezing). The low burying baseline observed in these animals can be explained by the extensive handling, and the higher body weight of the rats (≈600g) in this experiment, two factors known to decrease baseline level of burying (18, 19). Such a low baseline allows for anxiogenic-like effects to be detected more easily and has been reported previously in young rats under different conditions (20). Thus, increased CRF release, and CRF1 receptor activation during abstinence appears to mediate anxiety-like behavior during precipitated withdrawal in nicotine-dependent rats. This hypothesis predicts that, in dependent rats, a period of abstinence may lead to an increase in nicotine intake during the subsequent access to nicotine and that blocking the action of CRF using a CRF1 receptor antagonist could prevent this increase in nicotine intake.
Table 1.
Effect of CRF infusion in the central nucleus of the amygdala on defensive burying
| Treatment | Burying, s | Latency to bury, s | Grooming, s | Rearing, s |
|---|---|---|---|---|
| Vehicle | 0.1 ± 0.2 | 341 ± 78 | 21 ± 17 | 103 ± 15 |
| CRF (30 pmol) | 7.1 ± 4.7* | 165 ± 91* | 30 ± 27 | 91 ± 15 |
CRF (30 pmol) or vehicle (PBS) were infused in the central nucleus of the amygdala 1 min before the beginning of the defensive burying test. Behaviors were recorded during the first 5 min of the test. Resting and freezing did not differ between groups.
*, P < 0.05 vs. vehicle.
Abstinence Increases Nicotine Intake in Rats Given Extended Access to Self-Administration.
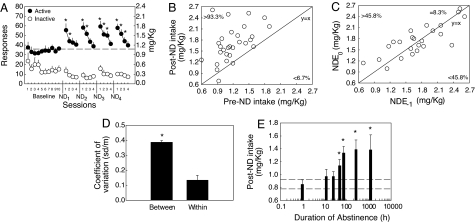
To evaluate the effect of abstinence on nicotine intake, we used an animal model of intermittent exposure to 23-h extended access to nicotine self-administration. The intermittent access consisted of four consecutive days of self-administration at a constant unit dose (0.03 mg/kg per injection), followed by 3 days of abstinence, because 3 days of abstinence from chronic nicotine administration increases anxiety-like behavior in rats (21, 22). Nicotine intake significantly increased during the first session after each cycle of abstinence (Fig. 2A) and returned to baseline by the 4th day of nicotine self-administration. The “nicotine-deprivation effect” reflected mainly increased drug intake during the active (dark) period, which represents ≈80% of daily nicotine intake, but a significant increase, albeit smaller, was also observed during the light period (data not shown). Rats exhibited “drug-loading” behavior during the beginning of the active period such that the nondeprived baseline amount of intake normally requiring 12 h was attained in only 6.4 ± 1.2 h. Scatter plot of pre- vs. postabstinence nicotine intake shows that the majority (>93%) of postabstinence nicotine intakes were higher than preabstinence nicotine intakes, demonstrating the robustness of the phenomenon (Fig. 2B). The fact that postabstinence nicotine intakes measured during the four successive cycles were (i) highly correlated with each other (mean r = 0.81, range: 0.72–0.92, all P < 0.05; Fig. 2C) and (ii) evenly distributed around the y = x line, and (iii) that the coefficient of variation between subjects was three times higher than the coefficient of variation within subjects (Fig. 2D) demonstrate the existence of reliable interindividual differences in the effect of abstinence on nicotine intake.
Fig. 2.
Characterization of the nicotine-deprivation effect. (A) Total (23-h) active and inactive responses after repeated cycles of 72 h of nicotine deprivation (ND), followed by 4 days of self-administration (*, P < 0.05 vs. baseline). (B) Robustness of the nicotine-deprivation effect. Scatter plot of nicotine intakes observed during the first session before (pre-ND) and after (post-ND) each of the four cycles of nicotine deprivation. The numbers represent the percentage of measures above and below the y = x line. (C) Reliability of the nicotine-deprivation effect. Correlation of post-ND nicotine intakes between each of the four cycles (ND(−1) vs. ND(0) = ND1 vs. ND2, ND2 vs. ND3, and ND3 vs. ND4). (D) Coefficient of variation of post-ND intakes between subjects vs. within subjects (*, P < 0.05). (E) Effect of duration of abstinence (h) on active responses during the subsequent 12-h period of nicotine access. (*, P < 0.05 vs. 1 h). Note logarithmic time scale. Dotted lines represent mean ± SEM of the 1-h time point (*, P < 0.05 vs. 1 h). Data represent mean ± SEM.
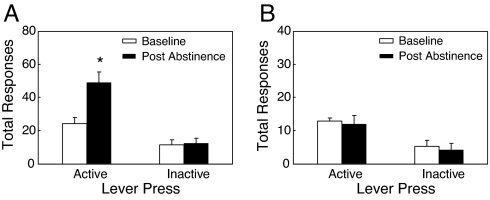
We then evaluated the time course of appearance of the nicotine-deprivation effect by exposing rats to different durations of abstinence, from 1 h to ≈2 months (1,201 h). Abstinence-induced increase in nicotine intake was significant after 48 h, reached a maximum after 3 days of abstinence, and remained elevated even after 2 months of abstinence (Fig. 2E). To test the relevance of the nicotine-deprivation effect to nicotine dependence, we tested the effect of 3 days of abstinence in rats given limited access to nicotine self-administration (1 h per session), a condition known not to induce any spontaneous signs of withdrawal (23). We found that abstinence had no effects in rats with limited access (Fig. 3B), whereas, as observed in the previous experiments, abstinence markedly increased nicotine responding in rats with extended access (23 h per session) (Fig. 3A). Inactive lever responses were not affected by abstinence.
Fig. 3.
Specificity of the nicotine-deprivation effect. Total responses during the entire session in rats given extended (23 h, n = 7) (A), or limited (1 h, n = 6) (B) access to nicotine before and after 72 h of abstinence (*, P < 0.05 vs. baseline). Data represent mean ± SEM.
Antagonism of CRF1 Receptor Prevents Abstinence-Induced Increases in Nicotine Intake.
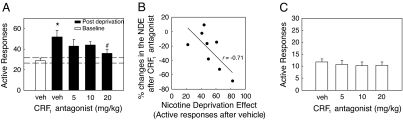
To evaluate the role of the CRF–CRF1 system in the nicotine-deprivation effect, we tested the effect of the CRF1 receptor antagonist MPZP on nicotine responding in rats with intermittent access to extended nicotine self-administration (23 h, 4 d/week). After abstinence, pretreatment with the CRF1 antagonist dose-dependently decreased nicotine intake (Fig. 4A) compared with vehicle-treated rats, and blocked the nicotine-deprivation effect compared with baseline levels. As expected, the CRF1 receptor antagonist decreased nicotine self-administration during the active (dark) period when abstinence-induced escalation of nicotine intake occurred but not during the inactive (light) period (data not shown). Efficacy of the CRF1 receptor antagonist correlated with the magnitude of the nicotine-deprivation effect observed in any given subject (Fig. 4B). CRF1 receptor antagonist efficacy did not correlate with the magnitude of baseline responding (r = 0.05, not significant) and had no effect in rats given limited access to nicotine (1 h) (Fig. 4C), supporting a specific relation to abstinence responding and nicotine dependence.
Fig. 4.
Abstinence-induced escalation of nicotine intake is blocked by a CRF1 receptor antagonist. (A) Effect of a CRF1 antagonist (MPZP, s.c., −1 h) on nicotine self-administration during the active period in rats given extended access to nicotine (*, P < 0.05 vs. baseline; #, P < 0.05 vs. after-abstinence vehicle treatment, n = 8). (B) Correlation between magnitude of the nicotine-deprivation effect and percentage changes in the nicotine-deprivation effect after CRF1 antagonist. The higher the nicotine-deprivation effect, the more effectively the antagonist blocked self-administration of nicotine (r = −0.71, P < 0.05). The x axis represents active responses after vehicle injection, and the y axis represents the reduction in active responses after the highest dose of MPZP (20 mg/kg), in percentage changes compared with active responses after vehicle injection. (C) Lack of effect of the CRF1 receptor antagonist (MPZP, s.c., −1 h) on baseline nicotine self-administration responding in rats given limited access to nicotine (n = 10). Data represent mean ± SEM.
Discussion
This report demonstrates that precipitated withdrawal, in nicotine dependent rats, increases CRF release in the central nucleus of the amygdala and increases anxiety-like behavior by means of a CRF1-dependent mechanism. Nicotine abstinence produces a robust increase in nicotine intake in rats allowed extended access to nicotine self-administration. Finally, the increased nicotine intake can be blocked by pretreatment with a specific CRF1 receptor antagonist.
Nicotine withdrawal, precipitated by mecamylamine, increased CRF release in the central nucleus of the amygdala in rats chronically exposed to nicotine. Interstitial amygdalar CRF concentration reached a maximum 30 min after mecamylamine injection, with levels returning to baseline after 2 h. This pattern may be explained by the short pharmacokinetic half-life of mecamylamine (≈1 h) (24), and the constant exposure to nicotine. Withdrawal-induced CRF release in nicotine-dependent rats and intraamygdala infusion of CRF in naïve rats, were both associated with an increase in time spent burying, and a decreased latency to bury in the defensive burying test, whereas the CRF1 receptor antagonist MPZP reversed the increase in defensive burying observed during mecamylamine-precipitated nicotine withdrawal. The defensive burying test possesses face and predictive validity as an animal model of normal and pathological anxiety; in particular, time spent burying reflects an active coping strategy to an anxiogenic environment, and is respectively decreased and increased by anxiolytic- and anxiogenic-like compounds (16, 17, 25). Administration of mecamylamine, MPZP, or CRF did not change general activity or nonanxiety behaviors such as rearing, resting, and grooming. This argues against nonspecific effects, and confirming an earlier report showing that CRF infusion in the central nucleus of the amygdala does not alter feeding or grooming behavior (26). Also, the increase of defensive burying observed after mecamylamine or CRF administration was not associated with an increase in the time spent freezing. Freezing under these conditions may represent a different measure of anxiety related to passive and not active avoidance and can be dissociated pharmacologically from the active form of anxiety measured by the time spent burying or the latency to bury (17). Perhaps the CRF–CRF1 system is not involved in all aspects of negative emotions, and some forms of anxiety-like behavior may be unchanged during nicotine withdrawal. The CRF system has been implicated in anxiety-like behavior, and studies of CRF1 receptor antagonists have promising potential for anxiolytic drug development (13). Our findings with nicotine add to reports showing increased amygdalar CRF release, and anxiety-like behaviors following withdrawal from other drugs of abuse, including ethanol, cocaine, opiates, and cannabinoids (27–32), and suggest that overactivation of the extrahypothalamic CRF–CRF1 system may constitute a common denominator of motivational aspects of drug withdrawal. Overactivation of the CRF–CRF1 system during withdrawal is also associated with a hypoactivation of the dopaminergic system in the central nucleus of the amygdala (33), suggesting that both systems may interact to mediate anxiety-like behavior during withdrawal. However, whether the increase in CRF and the decrease in dopamine are causally linked is unknown and needs further investigation.
We recently showed that an escalating-dose regimen of nicotine associated with intermittent abstinence periods produces high levels of nicotine intake, suggesting that abstinence may increase subsequent nicotine intake (34). New data presented herein extend this finding by showing that at a constant unit dose, three days of forced abstinence induces a marked increase of nicotine intake. The nicotine-deprivation effect was mainly observed during the early active period (dark). This situation is very similar to the human condition, where abstinence is followed by an increase in smoking, during the early active period (light), followed by a titration period of nicotine intake (35, 36). The time course of recovery to the original basal levels of intake if deprivation is not initiated has not been fully investigated, but preliminary results suggest that recovery time will depend on the duration of withdrawal, the magnitude of the deprivation effect, the number of self-administration sessions, and the period of nicotine intake (light vs. dark). The nicotine-deprivation effect was found to be a long-lasting phenomenon that progressively develops during the first week of abstinence and remains robust for at least 2 months. The time course of the nicotine-deprivation effect is similar to the phenomenon of incubation of reward craving (37), where an increase in responding for cues related to drug delivery after withdrawal has been observed across several drugs (cocaine, heroin, methamphetamine) and natural rewards (sucrose) as well (37–41). However, the increase in responding with the incubation effect is observed under extinction and reinstatement sessions but not after reexposure to the drug itself (37), and there is little evidence to date suggesting that the incubation effect leads to increased drug self-administration. The present results demonstrate that the potential for nicotine self-administration progressively develops during withdrawal and leads to increased nicotine intake during renewed drug access. In this regard, the nicotine-deprivation effect may be more comparable to the alcohol-deprivation effect (42).
Moreover, reliable interindividual differences were observed in the magnitude of the nicotine-deprivation effect, suggesting that this measure may represent a relevant marker of individual vulnerability to nicotine dependence. This hypothesis is supported by the fact that the nicotine-deprivation effect was not observed in rats with limited (1 h) access to nicotine, a condition known not to induce spontaneous signs of withdrawal, a central aspect of nicotine dependence (23), but a condition sufficient to produce the reward incubation effect, see above. The nicotine-deprivation effect is unlikely to result from a sensitized reward state for nicotine or a loss of tolerance to the effect of nicotine during withdrawal (both of which would have led to a decrease in nicotine intake compared with baseline) but may be better explained by a negative reinforcement construct. Here, dependent rats may be hypothesized to escalate their nicotine intake after abstinence to obtain relief from a resulting CRF–CRF1-mediated anxiety-like state.
The role of the CRF–CRF1 system in the nicotine-deprivation effect was confirmed by the experiment showing that the increased nicotine intake observed after abstinence was dose-dependently blocked by pretreatment with MPZP. This result is reinforced by the fact that the CRF1 receptor antagonist was more effective at reducing nicotine intake in individual animals exhibiting a high nicotine-deprivation effect, again suggesting that the magnitude of the nicotine-deprivation effect may be a marker of individual vulnerability to nicotine dependence. The potential role of CRF2 receptors in these effects is currently unknown and would require further investigation. However, inactivation of CRF2 receptor is more likely to produce a stress-like response than an antianxiety-like effect, based on pharmacological and knockout studies (43, 44). Antagonism of CRF1 receptors prevents deficits in brain reward function (15) and increases in anxiety-like behavior (present report) associated with precipitated nicotine withdrawal. Thus, MPZP administration may be hypothesized to block abstinence-induced increases in nicotine intake through a reduction of negative reward and anxiety-like states contributing to the negative emotional state associated with nicotine abstinence.
Taken together, these results suggest that a key mechanism in nicotine dependence is withdrawal-induced overactivation of the CRF–CRF1 receptor system, which contributes to the increased negative emotional state that drives subsequent nicotine intake. The recruitment of such a negative emotional system may explain one site of vulnerability for the transition from nicotine use to nicotine dependence and suggests a new target for nonnicotine pharmacotherapy for tobacco addiction.
Materials and Methods
All animal-use procedures were approved by The Scripps Research Institute's Animal Care and Use Committee and were in accordance with the National Institutes of Health guidelines. Effort was made to reduce the number of animals by using both between- and within-subject design studies. Adult male Wistar rats (Charles River Laboratories, Wilmington, MA) were housed in a temperature-controlled vivarium with a 12-h/12-h light/dark cycle. Tests were performed at the beginning of the dark cycle (10:00 a.m.).
Drugs.
Nicotine hydrogen tartrate salt (Sigma, Natick, MA) was dissolved in saline at pH 7.4 and experimenter-administered via minipump or self-administered via indwelling jugular catheter. Doses are expressed as free base. Mecamylamine (Sigma) was dissolved in saline and administered i.p. (1 ml/kg). The CRF1 antagonist (N,N-bis(2-methoxyethyl)-3-(4-methoxy-2-methylphenyl)-2,5-dimethyl-pyrazolo [1,5α] pyrimidin-7-amine, or MPZP) was synthesized at The Scripps Research Institute by P. Wirshing, dissolved in 20% hydroxypropyl β-cyclodextrin (Cavitron; Cargill, Wayzata, MN) in isotonic saline at pH 4.5 and administered s.c. (2 ml/kg, 45–60 min before testing). Rat/human CRF was supplied by Jean Rivier (The Salk Institute, La Jolla, CA). CRF was dissolved in 1× PBS (pH 7.4) and prepared fresh a few minutes before intracerebral injection. The doses and time of injections were selected based on previous studies§,¶.
Microdialysis.
The changes in interstitial levels of CRF-L-IR in the central nucleus of the amygdala were examined during mecamylamine-precipitated withdrawal (1.5 mg/kg, i.p.). Rats were s.c. implanted with osmotic minipumps (model 2ml2, 14 days, 5 μl/h; Durect, Palo Alto, CA) delivering either saline (nondependent, n = 5) or nicotine (nicotine-dependent, n = 7) (3.16 mg/kg/day, free base, s.c.) and a microdialysis guide cannula (SciPro, Sanborn NY) stereotaxically positioned 1 mm above the central nucleus of the amygdala by using the following coordinates [anteroposterior (AP) −3.3 mm; mediolateral (ML) ± 4.2 mm; ventral (V) −6.5 mm, from dura with flat skull]. After 14 days of pump exposure, a microdialysis probe (1-mm polyestersulfone membrane, 15-kDa molecular mass cutoff; SciPro) was lowered into the guide cannula and allowed to equilibrate for 12 h (1 μl/min flow rate, artificial cerebrospinal fluid). Subsequently dialysate samples (30-min fractions) were collected for a period of baseline sampling and after saline and mecamylamine challenge injections by using a within-subjects design. Sample tubes were kept on wet ice during collection and were then frozen on dry ice until later analysis by RIA.
CRF Immunoassay.
Dialysate CRF-like immunoreactivity was quantified with a sensitive and specific solid-phase RIA adapted from Zorrilla et al. (45) to increase sensitivity. Immulon-4 96-well plates (Dynatech, Chantilly, VA) were coated with protein A/G (1 μg/100 μl, 1 M NaHC03 per well, pH 9.0; Calbiochem, La Jolla, CA) overnight. Plates were rinsed with wash buffer (0.15 M K2HPO4 supplemented with 0.2 mM ascorbic acid and 0.1% Tween-20, pH 7.5) to dislodge loose Protein A/G. Wells were incubated 48 h at 4°C with 50 μl of anti-CRF serum (rC68, generously provided by W. Vale, The Salk Institute) at a titer of 1:300,000 in gelatin assay buffer. After three rinses to dislodge loose antibody, 50 μl of dilute sample (in duplicate) or standard (3–1,000 pg/ml, in quadruplicate) were incubated overnight at 4°C. After incubation, 50 μl of [125I-Tyr0]r/hCRF (≈4,000 cpm/50 μl; New England Nuclear, Boston, MA) were added to each well and incubated for an additional 24 h at 4°C. Wells were rinsed, blotted dry, and separated, and residual radioactivity was counted by a γ-counter for 5 min per well. Sensitivity of the assay is ≈0.1 fmol per well, and inter- and intraassay coefficients of variation at the ED50 dose range from 7–11%.
Defensive Burying Behavior.
Rats were s.c. implanted with osmotic minipumps delivering either saline (n = 33) or nicotine (n = 31) (3.16 mg/kg/day, free base, s.c.) as described above. After 14 days of pump exposure, testing was performed 5–8 h into the dark cycle in a standard cage with 2 in of bedding (wood shavings) along the bottom and a small hole centered in one side 1 inch above the bedding to accommodate the shock probe. Rats were habituated (45 min) to the test cage for 2 days before testing. On the test day, mecamylamine or its vehicle were administered 30 min before behavioral testing, and the CRF1 antagonist or its vehicle were administered 45 min before behavioral testing (n = 7–9 per group). On contact with the probe and shock delivery (using a Coulbourn precision shocker, 1.5 mA, AC, <1 s), verified by a startle response, the probe was deactivated. The latency and duration of probe-directed burying, rearing, resting, grooming, and freezing were measured from videotape over a 10-min period by an experimenter blind to the subject treatment condition and using a computer program.
Intracerebral Cannulations and CRF Infusions.
Rats were anesthetized with an isoflurane–oxygen mixture, and 26-gauge stainless steel guide cannulas (Plastics One, Roanoke, VA) aimed 2 mm above the central nucleus of the amygdala stereotaxically were implanted bilaterally: AP −2.6 mm; ML ±4.2 mm; V −5.2 mm, from dura, with flat skull (46). The guide cannulas were secured to the skull with dental cement and anchor screws, and guide cannulas were maintained with stylets. Intracerebral injections were administered with the use of injectors (33-gauge; Plastics One) that projected 2 mm past the guide cannula to the central nucleus of the amygdala. The injectors were attached to 70 cm of calibrated polyethylene-20 tubing preloaded with drug solution. This cohort of rats had been extensively handled previously in the context of a food intake study, in which they received administration of a CRF1 receptor antagonist s.c. and into the central nucleus of the amygdala in a Latin-square design. A washout period of 7 days was imposed before the present study with all subjects maintained on chow, during which time, animals were handled daily. Rats were randomly assigned to CRF vs. vehicle conditions balanced for previous diet history, which was statistically unrelated to performance in the defensive burying test. The CRF group (n = 5) was infused bilaterally (30 pmol total dose) with a volume of 0.25 μl per side over 30 s by using Hamilton microsyringes and two infusion pumps (Harvard Apparatus, Holliston, MA). The control group (n = 5) received the same volume of PBS. Injectors were removed from guide cannulae 1 min after the end of the infusions, and rats were returned to the home cage for 1 min before being tested in the defensive burying test.
Nicotine Self-Administration.
The apparatus and detailed procedures for both i.v. catheterization and self-administration of nicotine have been described (34). Adult male Wistar rats (280–330 g) were first allowed to nose-poke for food and water in 23-h sessions before and after recovery from surgical implantation of jugular catheters. After acquisition of these operant responses, rats were allowed to self-administer nicotine (0.03 mg/kg/100 μl/1 s, free base, fixed ratio = 1 lever-response, time out = 20 s) under different paradigms.
Experiment A: Effect of MPZP in ShA rats.
Rats were allowed to acquire nicotine self-administration during daily 1 h, “short-access” sessions (ShA, n = 10) for at least 10 days. The CRF1 antagonist MPZP (0, 5, 10, 20 mg/kg) was then administered by using a Latin square design, with 1–2 intervening treatment-free days.
Experiment B: Effect of nicotine deprivation in ShA and LgA rats.
ShA (n = 6) and LgA rats (n = 7) were allowed to self-administer nicotine during daily sessions during at least 10 days. Then, they were submitted to 3 days of abstinence in their home cage, followed by one session of nicotine self-administration to assess the magnitude of the nicotine-deprivation effect. ShA and LgA rats were then left undisturbed in the vivarium for a 1-month period and used for experiment C. Catheter patency was tested by using an ultrashort-acting barbiturate, Brevital (methohexital sodium, 10 mg/ml, 2 mg per rat), and only rats with a fully patent catheter were used.
Experiment C: Effect of MPZP on the nicotine-deprivation effect in LgA rats.
After completion of experiment B, rats were allowed to self-administer nicotine during daily 23-h, “long-access” sessions (LgA, n = 8) for at least 10 days. Then, they were allowed to respond on a lever for nicotine self-administration in four 4-day cycles, each separated by three intervening days of abstinence in their home cage. MPZP was administered before the first session after each cycle of abstinence by using a Latin square design.
Experiment D: Further characterization of the nicotine-deprivation effect.
After completion of experiment C, rats were submitted to nine successive cycles of nicotine self-administration periods and abstinence periods. The different durations of abstinence were tested in the following order 72 h, 48 h, 265 h, 12 h, 1,201 h. After each abstinence period, rats were allowed to self-administer nicotine until they reached their predeprivation baseline (range 3–5 days). The 72-h cycle period was repeated four times to analyze the reproducibility of the results (Fig. 2A).
Statistical Analysis.
Results were analyzed with SPSS software using ANOVA (SPSS, Chicago, IL. In all cases, a normality test and an equal variance test were performed before the ANOVA to ensure its validity. The following variables (dependent/nondependent: two levels; sham/abstinence: two levels; duration of nicotine access: two levels; pharmacological treatments: two or four levels; active/inactive response: two levels) were used as between-subjects factor. Depending on the analysis, the condition (baseline/postabstinence: two levels) and the time (number of self-administration sessions or number of microdialysis samples) were used as within-subjects factors. Post hoc Newman–Keuls tests and Pearson correlations were used when necessary. When assumptions of ANOVA were violated, the nonparametric Kruskal–Wallis test was used, followed by Welch's t test. Data are shown as mean ± SEM.
Supplementary Material
Acknowledgments
We thank Katy Rahmani, Robert Lintz, Yanabel Grant, Thomas Greenwell, and Molly Brennan for technical assistance; Frederic Ambroggi and Luigi Pulvirenti for helpful discussions; and Michael Arends for editorial assistance. This is publication number 18662 of the Committee on the Neurobiology of Addictive Disorders from The Scripps Research Institute. We also thank the Tobacco Etiology Research Network of the Robert Wood Johnson Foundation for discussion and support. This work was supported by Tobacco Related Disease Research Program (TRDRP) of the State of California Grant 12RT-0099, the National Institute of Diabetes and Digestive and Kidney Diseases Grant DK26741, and the Pearson Center for Alcoholism and Addiction Research.
Abbreviations
- CRF
corticotropin-releasing factor
- MPZP
(N,N-bis(2-methoxyethyl)-3-(4-methoxy-2-methylphenyl)-2,5-dimethyl-pyrazolo[1,5α]pyrimidin-7-amine
- CRF-L-IR
CRF-like-immunoreactivity.
Footnotes
The authors declare no conflict of interest.
Richardson, H. N., Funk, C. K., Grant, Y., Zorrilla, E. P., Koob, G. F. (2006) Program No. 783 4 Neuroscience Meeting Planner, Atlanta, GA (Soc Neurosci, Washington, DC), www.sfn.org/am2006/.
Specio, S. E., Zorrilla, E. P., O'Dell, L. E., Boutrel, B., Smith, R. T., Grant, Y., Koob, G. F. (2004) Program No. 77711 Neuroscience Meeting Planner (Soc Neurosci, Washington, DC), http://sfn.scholarone.com/itin2004/.
This article contains supporting information online at www.pnas.org/cgi/content/full/0707585104/DC1.
References
- 1.Fellows JL, Trosclair A, Adams EK. Morb Mort Rep. 2002;51:300–303. [Google Scholar]
- 2.Henningfield JE, Fant RV, Gitchell J, Shiffman Sl. Ann NY Acad Sci. 2000;909:247–256. doi: 10.1111/j.1749-6632.2000.tb06686.x. [DOI] [PubMed] [Google Scholar]
- 3.Pomerleau CS, Pomerleau OF. Psychopharmacology (Berlin) 1992;108:460–465. doi: 10.1007/BF02247422. [DOI] [PubMed] [Google Scholar]
- 4.Grunberg NE. Addiction. 1994;89:1443–1446. doi: 10.1111/j.1360-0443.1994.tb03741.x. [DOI] [PubMed] [Google Scholar]
- 5.Mansvelder HD, Keath JR, McGehee DS. Neuron. 2002;33:905–919. doi: 10.1016/s0896-6273(02)00625-6. [DOI] [PubMed] [Google Scholar]
- 6.Nestler EJ. Nat Neurosci. 2005;8:1445–1449. doi: 10.1038/nn1578. [DOI] [PubMed] [Google Scholar]
- 7.Koob GF, Le Moal M. Nat Neurosci. 2005;8:1442–1444. doi: 10.1038/nn1105-1442. [DOI] [PubMed] [Google Scholar]
- 8.Tiffany ST, Conklin CA, Shiffman S, Clayton RR. Addiction. 2004;99:78–86. doi: 10.1111/j.1360-0443.2004.00734.x. [DOI] [PubMed] [Google Scholar]
- 9.Epping-Jordan MP, Watkins SS, Koob GF, Markou A. Nature. 1998;393:76–79. doi: 10.1038/30001. [DOI] [PubMed] [Google Scholar]
- 10.Hughes JR, Higgins ST, Bickel WK. Addiction. 1994;89:1461–1470. doi: 10.1111/j.1360-0443.1994.tb03744.x. [DOI] [PubMed] [Google Scholar]
- 11.LeSage MG, Burroughs D, Pentel PR. Pharmacol Biochem Behav. 2006;83:585–591. doi: 10.1016/j.pbb.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 12.Heinrichs SC, Koob GF. J Pharmacol Exp Ther. 2004;311:427–440. doi: 10.1124/jpet.103.052092. [DOI] [PubMed] [Google Scholar]
- 13.Zorrilla EP, Koob GF. Expert Opin Investig Drugs. 2004;13:799–828. doi: 10.1517/13543784.13.7.799. [DOI] [PubMed] [Google Scholar]
- 14.Sarnyai Z, Shaham Y, Heinrichs SC. Pharmacol Rev. 2001;53:209–244. [PubMed] [Google Scholar]
- 15.Bruijnzeel AW, Zislis G, Wilson C, Gold MS. Neuropsychopharmacology. 2006;32:955–963. doi: 10.1038/sj.npp.1301192. [DOI] [PubMed] [Google Scholar]
- 16.Basso AM, Spina M, Rivier J, Vale W, Koob GF. Psychopharmacology (Berlin) 1999;145:21–30. doi: 10.1007/s002130051028. [DOI] [PubMed] [Google Scholar]
- 17.De Boer SF, Koolhaas JM. Eur J Pharmacol. 2003;463:145–161. doi: 10.1016/s0014-2999(03)01278-0. [DOI] [PubMed] [Google Scholar]
- 18.Diamant M, Croiset G, de WD. Peptides. 1992;13:1149–1158. doi: 10.1016/0196-9781(92)90022-u. [DOI] [PubMed] [Google Scholar]
- 19.Pare WP. J Comp Physiol Psychol. 1969;69:214–218. [PubMed] [Google Scholar]
- 20.Richardson HN, Zorrilla EP, Mandyam CD, Rivier CL. Endocrinology. 2006;147:2506–2517. doi: 10.1210/en.2005-1054. [DOI] [PubMed] [Google Scholar]
- 21.Irvine EE, Cheeta S, File SE. Pharmacol Biochem Behav. 2001;68:319–325. doi: 10.1016/s0091-3057(00)00449-4. [DOI] [PubMed] [Google Scholar]
- 22.Irvine EE, Cheeta S, File SE. Behav Pharmacol. 1999;10:691–697. doi: 10.1097/00008877-199911000-00016. [DOI] [PubMed] [Google Scholar]
- 23.Paterson NE, Markou A. Psychopharmacology (Berlin) 2004;173:64–72. doi: 10.1007/s00213-003-1692-7. [DOI] [PubMed] [Google Scholar]
- 24.Debruyne D, Sobrio F, Hinschberger A, Camsonne R, Coquerel A, Barre L. J Pharm Sci. 2003;92:1051–1057. doi: 10.1002/jps.10302. [DOI] [PubMed] [Google Scholar]
- 25.Gilligan PJ, Baldauf C, Cocuzza A, Chidester D, Zaczek R, Fitzgerald LW, McElroy J, Smith MA, Shen H-SL, Saye JA. Bioorg Med Chem. 2000;8:181–189. doi: 10.1016/s0968-0896(99)00271-0. [DOI] [PubMed] [Google Scholar]
- 26.Jochman KA, Newman SM, Kalin NH, Bakshi VP. Behav Neurosci. 2005;119:1448–1458. doi: 10.1037/0735-7044.119.6.1448. [DOI] [PubMed] [Google Scholar]
- 27.Weiss F, Ciccocioppo R, Parsons LH, Katner S, Liu X, Zorrilla EP, Valdez GR, Ben-Shahar O, Angeletti S, Richter RR. Ann NY Acad Sci. 2001;937:1–26. doi: 10.1111/j.1749-6632.2001.tb03556.x. [DOI] [PubMed] [Google Scholar]
- 28.Funk CK, O'Dell LE, Crawford EF, Koob GF. J Neurosci. 2006;26:11324–11332. doi: 10.1523/JNEUROSCI.3096-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Merlo PE, Lorang M, Yeganeh M, Rodriguez de Fonseca F, Raber J, Koob GF, Weiss F. J Neurosci. 1995;15:5439–5447. doi: 10.1523/JNEUROSCI.15-08-05439.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Contarino A, Papaleo F. Proc Natl Acad Sci USA. 2005;102:18649–18654. doi: 10.1073/pnas.0506999102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bruijnzeel AW, Gold MS. Brain Res Reviews. 2005;49:505–528. doi: 10.1016/j.brainresrev.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 32.Ambrosio E, Sharpe LG, Pilotte NS. Synapse. 1997;25:272–276. doi: 10.1002/(SICI)1098-2396(199703)25:3<272::AID-SYN6>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 33.Panagis G, Hildebrand BE, Svensson TH, Nomikos GG. Synapse. 2000;35:15–25. doi: 10.1002/(SICI)1098-2396(200001)35:1<15::AID-SYN3>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 34.O'Dell LE, Koob GF. Pharmacol Biochem Behav. 2007;86:346–353. doi: 10.1016/j.pbb.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Benowitz NL, Jacob P., III Clin Pharmacol Ther. 1984;35:499–504. doi: 10.1038/clpt.1984.67. [DOI] [PubMed] [Google Scholar]
- 36.Isaac PF, Rand MJ. Nature. 1972;236:308–310. doi: 10.1038/236308a0. [DOI] [PubMed] [Google Scholar]
- 37.Lu L, Grimm JW, Hope BT, Shaham Y. Neuropharmacology. 2004;47(Suppl 1):214–226. doi: 10.1016/j.neuropharm.2004.06.027. [DOI] [PubMed] [Google Scholar]
- 38.Grimm JW, Hope BT, Wise RA, Shaham Y. Nature. 2001;412:141–142. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shalev U, Morales M, Hope B, Yap J, Shaham Y. Psychopharmacology (Berlin) 2001;156:98–107. doi: 10.1007/s002130100748. [DOI] [PubMed] [Google Scholar]
- 40.Shepard JD, Bossert JM, Liu SY, Shaham Y. Biol Psychiatry. 2004;55:1082–1089. doi: 10.1016/j.biopsych.2004.02.032. [DOI] [PubMed] [Google Scholar]
- 41.Tran-Nguyen LT, Fuchs RA, Coffey GP, Baker DA, O'Dell LE, Neisewander JL. Neuropsychopharmacology. 1998;19:48–59. doi: 10.1016/S0893-133X(97)00205-4. [DOI] [PubMed] [Google Scholar]
- 42.Heyser CJ, Schulteis G, Koob GF. Alcohol Clin Exp Res. 1997;21:784–791. [PubMed] [Google Scholar]
- 43.Bale TL, Contarino A, Smith GW, Chan R, Gold LH, Sawchenko PE, Koob GF, Vale WW, Lee KF. Nat Genet. 2000;24:410–414. doi: 10.1038/74263. [DOI] [PubMed] [Google Scholar]
- 44.Valdez GR, Zorrilla EP, Rivier J, Vale WW, Koob GF. Brain Res. 2003;980:206–212. doi: 10.1016/s0006-8993(03)02971-8. [DOI] [PubMed] [Google Scholar]
- 45.Zorrilla EP, Valdez GR, Weiss F. Psychopharmacology (Berlin) 2001;158:374–381. doi: 10.1007/s002130100773. [DOI] [PubMed] [Google Scholar]
- 46.Paxinos G, Watson C. The Rat Brain in Stereotoxic Coordinates. San Diego: Academic; 1997. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.