Abstract
Patterns in food-web structure have frequently been examined in static food webs, but few studies have attempted to delineate patterns that materialize in food webs under nonequilibrium conditions. Here, using one of nature's classical nonequilibrium systems as the food-web database, we test the major assumptions of recent advances in food-web theory. We show that a complex web of interactions between insect herbivores and their natural enemies displays significant architectural flexibility over a large fluctuation in the natural abundance of the major herbivore, the spruce budworm (Choristoneura fumiferana). Importantly, this flexibility operates precisely in the manner predicted by recent foraging-based food-web theories: higher-order mobile generalists respond rapidly in time and space by converging on areas of increasing prey abundance. This “birdfeeder effect” operates such that increasing budworm densities correspond to a cascade of increasing diversity and food-web complexity. Thus, by integrating foraging theory with food-web ecology and analyzing a long-term, natural data set coupled with manipulative field experiments, we are able to show that food-web structure varies in a predictable manner. Furthermore, both recent food-web theory and longstanding foraging theory suggest that this very same food-web flexibility ought to be a potent stabilizing mechanism. Interestingly, we find that this food-web flexibility tends to be greater in heterogeneous than in homogeneous forest plots. Because our results provide a plausible mechanism for boreal forest effects on populations of forest insect pests, they have implications for forest and pest management practices.
Keywords: budworm, food-web theory, foraging theory, herbivore–natural enemy interactions, insect outbreaks
Food webs are among nature's most complex creations. For example, Fig. 1 depicts the complex tapestry of species interactions that stems from a single tree species (the balsam fir). Although food webs provide humans with essential ecosystem services (1), they are relatively unstudied and poorly understood empirically. An essential task of science is to delineate the underlying natural structures that impart the stability and resilience needed to sustain ecosystems and their accompanying services (2–6). Although of critical importance, this is neither a simple theoretical nor an empirical problem.
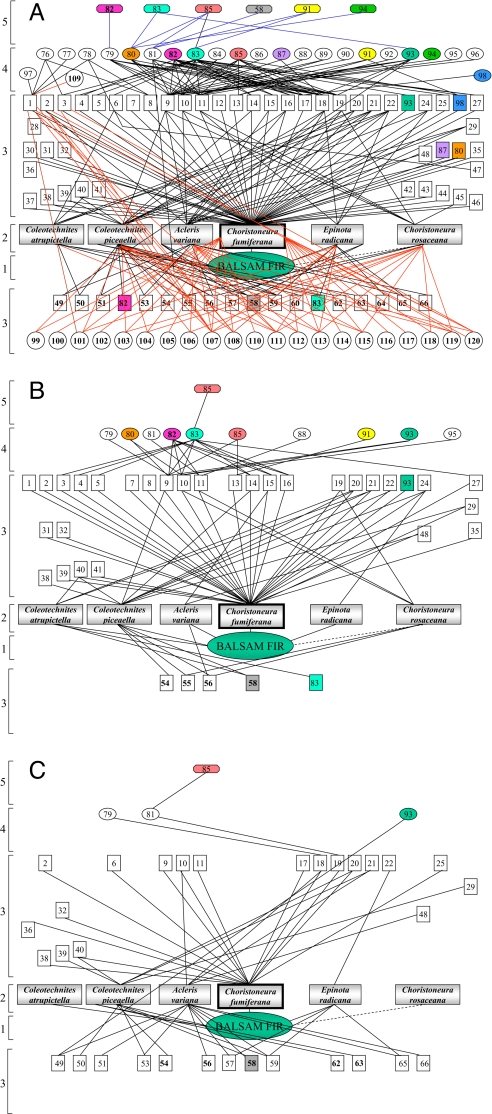
Fig. 1.
Structure of the balsam fir source food web. (A) Total food web from all samples collected throughout entire study in all plot-years (1983–1993) and in all field experiments. (B) Food web occurring at the peak budworm density using weekly samples in all plots, showing the presence of several hyperparasitoid species (data from Plot 1, 1985; Plot 2, 1986; Plot 3, 1991; see SI Fig. 6). (C) Food web occurring at a lower declining density using weekly samples in all plots, showing a much smaller assemblage of hyperparasitoids than in B, but a larger assemblage of primary parasitoids attacking non-budworm herbivores (data from Plot 1, 1989; Plot 2, 1989; Plot 3, 1993; see SI Fig. 6). Primary parasitoids are represented by squares, secondary parasitoids are represented by ovals, tertiary parasitoids are represented by octagons, and entomopathogens are represented by circles connected by red lines to hosts. The brackets and numbers on the far left identify trophic level. For clarity, those primary parasitoids (third trophic level) attacking budworm and other herbivore species are placed above the herbivore species, whereas those attacking only the non-budworm herbivore species are placed below the herbivore species. Omnivore species occur in more than one trophic level and are represented by a solid color (not white). [Identification codes are shown in SI Table 3. Primary parasitoids attacking unidentifiable herbivores (numbers 67–75) are not shown.]
Of those empirical ecologists who have begun tackling this vexing problem, most have attempted to delineate patterns in food-web structure by documenting static food webs (refs. 7 and 8; also see Winemiller, ref. 9). Recently, ecologists have begun to recognize that important stabilizing structures likely materialize under nonequilibrium conditions (9–16). Arguably, one of the main mechanisms for stability under such dynamic conditions is the tendency for complex systems to adapt to changing conditions (11, 17). In this article, we attempt to harness nature's nonequilibrium properties, the periodic and relatively predictable spruce budworm [Choristoneura fumiferana (Clem.), hereafter, “budworm”] outbreaks in the temperate, Acadian forest ecosystem of New Brunswick, Canada, to study the flexibility (i.e., changes in food-web topology in response to changing conditions) of the balsam fir [Abies balsamea (L.) Mill.] food web. Our data show significant and consistent food-web changes across a natural gradient in density of a major food-web player, the budworm. The changing food-web structure we document agrees with recent nonequilibrium food-web theory that suggests higher-order mobile generalists will rapidly respond to changing resource conditions (11, 12, 17). Importantly, such behavioral responses by higher-order predators can play a major role in maintaining stable or persistent ecosystems (11, 12, 18).
There is a long-held view that homogeneous environments promote more oscillatory or less stable dynamics in ecological systems (2, 13). This idea has crossed into the insect outbreak literature, where a number of studies suggest that homogeneous stands are more vulnerable to outbreaks and insect damage than heterogeneous stands (19–21); however, the underlying mechanisms remain largely unresolved [although, see Montoya et al. (22) for arguments that increased diversity weakens parasitism rates]. Recent theory (11–13, 15, 17) suggests that the ability of mobile predators, or parasitoids, to respond to changing prey densities plays a crucial role in stabilizing food webs [see details in supporting information (SI) Fig. 5]. More specifically, because mobility and foraging behavior tend to scale with trophic position, this theory predicts that higher trophic levels ought to have the greatest response to changing prey conditions on the landscape (12). This theory further postulates that heterogeneity in resources allows mobile generalist predators to stabilize food webs relative to spatially homogeneous habitats. The argument is that varying prey densities across the landscape attract predators (i.e., the “birdfeeder effect,” whereby high local-prey densities attract regional predators) and thus minimize the severity of outbreaks. Effectively, if one habitat has increasing prey density and another has decreasing prey density, then, consistent with optimal foraging theory (14), mobile organisms ought to move to the increasing-prey habitat relative to the decreasing-prey habitat (hereafter, “switching behavior”). Thus, the predators increase prey consumption during the increase phase and decrease prey consumption during the decrease phase, precisely the behavior required to mute the magnitude of prey outbreak densities. Clearly, homogeneous sites have no such resource heterogeneity, and, so, the switching behavior of mobile generalist parasitoids is limited, leading to weaker species' responses across prey densities. Additionally, homogeneous habitats are not as likely as heterogeneous habitats to contain as many alternate and alternative prey (herbivore) species on which the parasitoids can feed (20, 21), thus further weakening the overall potential for parasitoid diversity and abundance to respond to changing prey conditions. We assessed this theory by monitoring the changes in food-web attributes at three sites varying in habitat heterogeneity over a budworm outbreak and decline (see SI Materials and Methods and SI Table 1 for details on sites, insects, sampling, rearing, and identification methodologies; see SI Fig. 6 for annual budworm densities). We found that increasing budworm density corresponds to a cascading increase in insect diversity and food-web complexity (i.e., increased species diversity, trophic position, and numbers of generalists) and that this food-web flexibility is more pronounced in heterogeneous than in homogeneous forest plots.
Results and Discussion
Food-Web Diversity and Budworm Density.
Fig. 1A reveals a remarkably complex and diverse assemblage of species interacting at five trophic levels (1 host plant, 6 herbivores, 66 primary parasitoids and 21 primary entomopathogens, 23 secondary parasitoids and 1 secondary entomopathogen, and 6 tertiary parasitoids); in fact, it rivals that of many tropical food webs in terms of the total number of parasitoid species (23, 24). Although this food web provides valuable insight into the numerous players and interactions likely to be found in a static balsam fir food web, the actual structural diversity seen at any given time depends greatly on when in the budworm outbreak cycle the food web is monitored. To demonstrate this visually, we constructed food webs at peak and low, declining budworm densities. The food web is clearly more diverse and complex at peak (high) budworm densities (Fig. 1B) than at low, declining budworm densities (Fig. 1C)
To investigate this more rigorously, we examined food-web structure across a range of budworm densities. Rarefaction curves were constructed to facilitate comparisons across space and time (see Methods and SI Fig. 7). Regression analysis was performed on both transformed (to account for autocorrelations) and untransformed data (trends appeared cointegrated; see Methods for details). Our results are consistent across both methodologies, and we present both for completeness. As budworm density increases, there is a strong, significant increase in the number of primary parasitoid species attacking only budworm (Fig. 2 A and A′). This increase is accompanied by an equally strong, significant decrease in the number of primary parasitoid species attacking non-budworm herbivores (Fig. 2 B and B′). Because there is no relationship between the total number of primary parasitoid species attacking all herbivores and budworm density (Fig. 2 C and C′), and no sampling bias of non-budworm herbivores occurred with changes in budworm density (regression; R2, 0.1471; P, 0.095; df, 19), these results suggest that, with a natural decline in the most abundant host (budworm), more primary parasitoids shift their diet to the other non-budworm herbivores (alternatives). In fact, our web diagrams clearly show that more primary parasitoids were attacking non-budworm herbivores at declining (Fig. 1C) than at peak budworm densities (Fig. 1B).
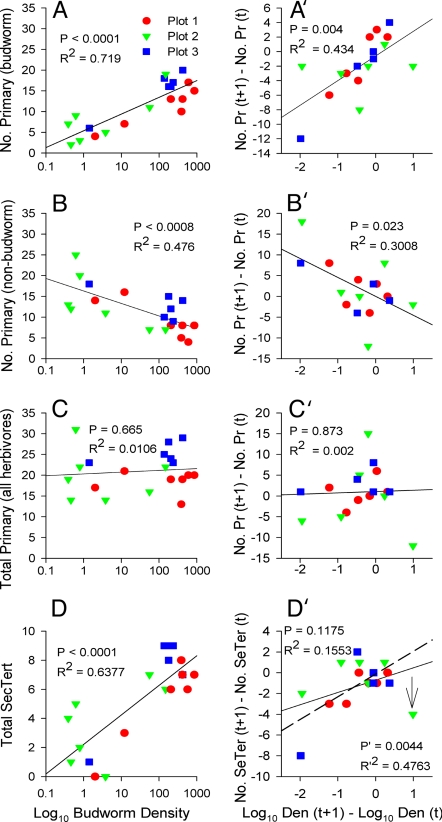
Fig. 2.
Food-web diversity and budworm density. A–D are based on untransformed data, and A′–D′ are based on transformed data. Similar results were obtained by both methods. (A) Number of primary parasitoids attacking only budworm increases significantly with increasing budworm density. (B) Number of primary parasitoids attacking one or more non-budworm herbivores decreases significantly with increasing budworm density. (C) Total number of primary parasitoids attacking all herbivores does not change in response to increasing budworm density. (D) Total number of secondary and tertiary parasitoids increases significantly with increasing budworm density. Results in D′ are not significant. This is mainly because of an “outlier” (indicated by arrow) caused by a sharp increase in budworm (second-instar budworm) density in Plot 2 in 1990 (see SI Fig. 6). This increase was caused by an invasion of budworm adults (moths) into the plot in 1989 from an other location(s). These invading moths laid eggs, thereby augmenting the local egg populations, resulting in a much greater number of budworm (second-instar budworm) in the following spring (1990) than normally would have occurred that year. This “artificial” (positive) deviation from the local host population trend did not result in a corresponding positive response by the secondary and tertiary parasitoid complex (i.e., parasitoid numbers continued to decline as expected from the trend). Results were significant when “outlier” was removed (dashed line; note P and R2 values in D′). (In A–D, n = 20; in A′–D′, n = 17). SecTert and SeTer, secondary and tertiary; Pr, primary; Den, density.
Contrary to the primary parasitoids, there is a strong positive increase in the total number of secondary and tertiary parasitoid species (hereafter, “hyperparasitoids”) in the community as a whole as budworm density increases (Fig. 2 D and D′). This increase is most likely because the abundance of many of the primary parasitoid hosts of the secondary parasitoids, and thus the tertiaries as well, is strongly tied to budworm abundance. Interestingly, the most heterogeneous plot (Plot 3) has a more diverse set of higher-order parasitoids (i.e., hyperparasitoids) than the most homogeneous plot (Plot 1; P, 0.025). Even greater parasitoid diversity is seen in Plot 3 than in Plot 1 when the number of parasitoid species unique to “unidentifiable” herbivores is included in the food webs (SI Table 1).
Importantly, all of these relationships are consistent among plots, indicating that food-web diversity changes in a consistent manner in both time and space with changing budworm density. In effect, fluctuations in budworm density cause diversity cascades in food-web trophic levels positioned above the herbivores, where high budworm densities have a birdfeeder-like effect, attracting primary parasitoids, followed rapidly by an assemblage of hyperparasitoids. This behavior is precisely that predicted by current food-web theory.
Generalism, Omnivory, and Budworm Density.
The degree of flexibility in resource use by a parasitoid species is governed by biological characteristics and various outside factors, including host abundance. As such, theory (11–14, 17) predicts that high-budworm-density food webs should contain more higher-order generalist parasitoids and have a higher mean trophic position than low-budworm-density food webs.
Considering first the total number of generalist parasitoids in the food web (i.e., all trophic levels combined), we found that the total number of generalists increased in a nearly significant manner with increasing budworm density (Fig. 3 A and A′). Primary generalists did not contribute to this positive response (Fig. 3 B and B′); however, as theory predicts, this positive response by all generalists in the food webs appears to be largely driven by hyperparasitoids (Fig. 3 C and C′). Consistent with this, we find that increases in budworm density in the plots are also accompanied by a strong increase in average trophic position (i.e., the food webs become more top heavy; Fig. 3 D and D′). These results indicate that food webs are more diverse and top heavy at high budworm densities, becoming less diverse and more bottom heavy as budworm densities decline and higher-order parasitoids leave. Moreover, in accordance with theory, the food webs in the heterogeneous landscapes (Plots 2 and 3) tended to show greater amounts of generalism over the range in budworm densities than the food web in the homogeneous landscape (Plot 1; Fig. 3 C and C′; P < 0.01). These results suggest that the diversity of generalist hyperparasitoids is greater at high than at low budworm densities, and that generalists respond more rapidly to increasing budworm density in heterogeneous than homogeneous landscapes, precisely as predicted by food-web theory.
Fig. 3.
Generalism and budworm density. A–D are based on untransformed data, and A′–D′ are based on transformed data. Similar results were obtained by both methods. (A) Total number of generalist parasitoid species (i.e., species that attack more than one host species) increases with increasing budworm density. (B) Number of primary generalist species does not change with increasing budworm density. (C) Number of secondary and tertiary generalists increases significantly with increasing budworm density. (D) Mean trophic position increases significantly with increasing budworm density. Results in D′ are not significant for the reasons stated in Fig. 2D′ above. However, when “outlier” (indicated by arrow) was removed, results were significant (dashed line; note P′ and R′2 values). (In A–D, n = 20; in A′–D′, n = 17.) SecTert, secondary and tertiary; Den, density; TotGen, total generalists; GenPrim, primary generalists; GenSeTe, secondary and tertiary generalists; TP, trophic position.
Omnivores, by virtue of being able to feed on hosts at more than one trophic level, can influence food-web structure through a variety of trophic pathways. Because the number of generalists in our food web responds positively to budworm density, we also examined trophic generalism (omnivory). As predicted by recent food-web theory (12), hyperparasitoids significantly change their foraging patterns under low budworm densities by increasingly attacking primary parasitoids [two-tailed t test, assuming unequal variances; P, 0.0012; df, 8; comparing average percentage of hyperparasitoids feeding as secondaries (as opposed to percentage feeding as tertiaries) at log budworm densities ≤2.0 and >2.0]. This pattern is consistent with the generalism pattern; webs get longer when budworm densities are high (higher-order species focus more attention on secondary parasitoids) and then truncate during periods of low budworm density. Interestingly, less omnivory occurred at high budworm densities in the homogenous plot (Plot 1) than in the most heterogeneous plot (Plot 3) (two-tailed t test assuming unequal variances; P, 0.0262; df, 6).
The food webs in the heterogeneous plots were also characterized by a lower peak budworm density (and thus lower budworm damage) than in the homogeneous plot (SI Table 1). In a recent large-scale survey of mixed balsam fir–hardwood stands in New Brunswick, Canada (19), it was hypothesized that the lower levels of damage from budworm feeding observed in heterogeneous (i.e., greater hardwood and less balsam fir content) than in homogeneous stands were due to greater numbers and/or diversity of natural enemies in heterogeneous stands. Our results confirm that host-plant diversity likely influences primary parasitoid populations (20, 25, 26), and they further suggest that higher-order generalist species respond to spatial variability by moving to where higher budworm densities occur. This switching behavior, augmented by the fact that heterogeneous plots also contain more plant diversity to support more and diverse alternate and alternative host species than homogeneous plots, may be extremely important in muting budworm damage in heterogeneous plots.
Manipulative Field Experiments at Low (Endemic) Budworm Densities.
Our foregoing empirical field data on natural budworm populations suggest that the complexity of the food web swings from being diverse, complex, and reticulate (highly connected) when budworm are at peak densities to simpler and less reticulate when densities are in decline. To further examine this phenomenon at very low (endemic) budworm densities, we conducted two manipulative field experiments in Plot 2: (i) several thousand second-instar budworms were released on several trees each spring (1990–1995), creating a birdfeeder effect (mass implanting) and (ii) individual larvae and pupae were released at the rate of one specimen per tree (individual implanting) throughout each season (1992–1995), simulating natural low densities (see SI Materials and Methods and SI Fig. 6). Although the food webs in these experiments are restricted to just one herbivore (budworm), it is clearly evident that many hyperparasitoids were attracted to primary parasitoids in the mass implanting experiment (Fig. 4A), whereas no hyperparasitoids were found in the individual implanting experiment (Fig. 4B) (two-tailed t test assuming unequal variances; P, 0.0013; df, 5). Thus, consistent with our empirical field data and food-web theory, an assemblage of generalist hyperparasitoids is capable of responding rapidly to high budworm densities even when surrounding field populations are naturally low and attracting few, if any, hyperparasitoids. These experimental results provide further evidence, together with the transformed results in Figs. 2 and 3, that the changes in food-web complexity that we observed are not simply a numerical response of consumers to changes in budworm density.
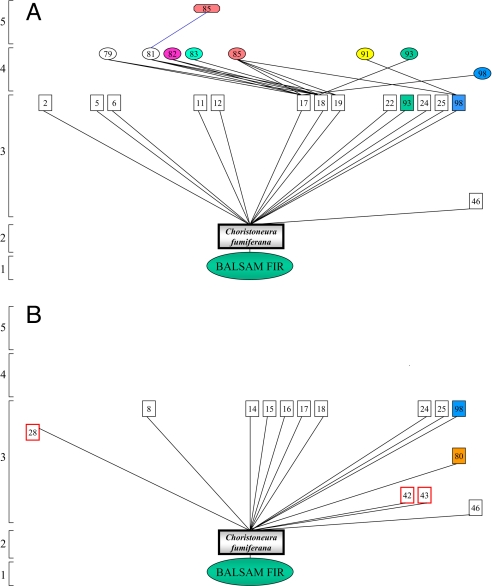
Fig. 4.
Food webs from manipulative field experiments. (A) Food web generated by implanting several thousand overwintering budworm (second-instar budworm) (mass implanting) on 10–20 balsam fir trees each year from 1990 to 1995, showing the presence of several hyperparasitoid species. (B) Food web generated by implanting individual budworm larvae/pupae (individual implanting) on 150–200 balsam fir trees each year from 1992 to 1995. In contrast to the former experiment, no hyperparasitoids were found. Results of both experiments differed significantly regardless of temporal sequences examined [i.e., 1990–1995 (mass implanting) vs. 1992–1995 (individual implanting) or 1992–1995 for both experiments]. Primary parasitoids enclosed in red squares in individual implanting experiment were found only at low budworm densities. (All codes and symbols are the same as those used in Fig. 1.)
Consistent with the birdfeeder effect, parasitism rates were also considerably higher at high than at low budworm densities (>70% at high natural budworm densities but <10% at low densities in the individual implanting experiment). Moreover, parasitism at high densities was due mainly to a complex of generalist parasitoids (tachinid flies and braconid and ichneumonid wasps), whereas at low densities, parasitism was due mainly to the generalist chalcidoid wasp, Elachertus cacoeciae. Similar results were reported in earlier budworm studies (27–30).
Species composition at low budworm densities is somewhat different from that at high and declining budworm densities, as is also seen in the European fir budworm (Choristoneura murinana) food web (31). For instance, our experiments revealed that certain primary parasitoid species (e.g., Meteorus trachynotus), common at high and declining budworm densities, were not found at low densities, whereas a few other primary parasitoids were present only at low densities (e.g., Euplectrus maculiventris, Ischnus inquisitorius, and Phytodietus vulgaris). This finding further emphasizes the point that species composition, as well as overall structure of the food web, likely depends profoundly on the phase of budworm outbreak cycle that is examined. Thus, both diet shifts and changes in species composition are important in shaping the food-web restructuring pattern during a budworm outbreak cycle.
We can draw two general conclusions from our results. (i) The diversity and food-web complexity of a natural community change dramatically and consistently in both time and space in response to natural changes in the abundance of a dominant food-web player. Further, our results support current food-web theory in that diversity, degree of generalism, and trophic position are all higher at peak budworm densities relative to the declining densities in both homogeneous and heterogeneous landscapes. These results show an interesting combination of bottom-up influences (i.e., budworm density correlates with parasitoid diversity) and top-down influences (i.e., higher-order parasitoids behaviorally respond in space to dampen outbreaks). Such flexible changes in food-web structure across multiple trophic levels reveal the critical relationship between nonequilibrium dynamics and food-web structure. (ii) Because budworm outbreaks tend to occur periodically, on average every 35 years in the Acadian forest (32), changes in food-web complexity are also likely to occur in a similar periodic manner as budworm populations go through outbreak cycles. Thus, as theorized by Royama (30), knowing the status and composition of the food web will be critical for predicting what will happen in the budworm system at a given moment.
Our results also strongly suggest that host-plant diversity [i.e., mixed softwood–hardwood content (19)] influences parasitoid (and predator) diversity and movement. Thus, there may be strong dynamic links (coupling) between softwood and hardwood sub-food webs, each playing a significant role in determining the food-web structure of the other's insect community. We believe that it is now critically important to examine the trophic pathways linking these sub-food webs for a more complete understanding of the role of landscape diversity (space) in shaping forest insect food webs and individual species dynamics (15, 33).
Our results integrate foraging theory into food-web ecology and, in doing so, allow us to understand how food-web structure changes in time and space as it relates to budworm population cycles. Further, these results have important implications for forest management practices and conservation. These results suggest that any action that results in large-scale forest homogenization (for example, plantation forestry) could inhibit the inherent flexibility that underlies complex ecosystems. This effect, in turn, may lead to more-severe insect outbreaks because of greater restrictions on the switching ability of some parasitoids (33) and/or the lack of vegetational diversity supporting alternate/alternative hosts in homogeneous habitats. From a biodiversity viewpoint (34), our results further suggest that any action that adversely affects the dominant player of the food web (whether it is habitat fragmentation, degradation, or loss; climate change; or pollution) can profoundly affect the diversity and structure of food webs over multiple trophic levels, with repercussions that may even reach far beyond the species directly affected. In other words, a highly eruptive herbivore species, capable of inflicting widespread damage to economically valuable timber, and thus requiring control interventions, is nonetheless an integral and vital player in the forest ecosystem.
Methods
Estimating the Diversity of Interactions in Space and Time.
There exists a large and developing body of research delineating the appropriate methods for estimating diversity in comparative analysis (35, 36). Food-web ecologists have used similar techniques to compare food webs (e.g., ref. 37). Because we are seeking to delineate localized food-web interactions in space and time, we need to show that our estimates of the density of diversity and interactions are not biased in a manner that generates the relationships identified (e.g., results for low-density years are due to poor sampling effort). To accomplish this, we generated rarefaction curves of species density across time (budworm density) and space (plots represent different localized food web interactions) by using EstimateS v8.0.0 (38).
SI Fig. 7 depicts the rarefaction curves for three representative years from peak to low budworm density for each plot across sampling effort (i.e., number of branches sampled). In all cases, plots were sampled in intensity according to the relative annual density of budworm such that lower-density years received greater sampling effort than higher-density years (SI Table 2). Although it is impossible for any of the annual curves to actually saturate [even with frequent daily sampling at high budworm densities (see SI Fig. 7; 1985, all weekdays)] because of the appearance of different parasitoid species at the end of the univoltine budworm life cycle, there is a strong tendency for the lower-density years to show greater saturation effects. This finding indicates that our results are not due to sampling artifacts.
To rigorously compare years and plots, there are at least two options: (i) to compare years and plots at equal sampling effort (i.e., reflecting similar estimates of species density or interaction density) and (ii), which is more conservative, to compare endpoint diversity estimates, because these latter estimates likely reflect, more realistically, true species and interaction densities. Consistent with this latter approach, we also looked at the percentage of “rare” species caught in the different years and plots (SI Table 2). The percentage of rare species ought to be a reasonable metric of whether localized species density is well sampled such that increasingly catching rare species indicates that the density of species is well estimated. In all years and plots, we found a high percentage of rare species, but low-density periods showed a tendency to have a higher percentage of rare species (SI Table 2). This finding further suggests that the trends identified are real and not due to undersampling low-density years.
In addition, and importantly, the number of primary parasitoid species obtained per year at natural budworm densities of <1 per m2 [5.3 ± 1.7 (SE)] did not differ from the number obtained per year in our individual implanting experiment in which samples were “reared” in numbers comparable to those at high densities [6.5 ± 1.3 (SE)] (t test assuming unequal variances; P, 0.5763; df, 6). Extensive empirical and experimental studies conducted during the endemic phase of the previous budworm outbreak in New Brunswick also revealed a much-reduced parasitoid complex compared with the outbreak phase (28, 29). Thus, we can conclude that our results reflect events occurring throughout annual changes in budworm density in each plot.
Analyses.
All analyses were done by using the statistical package R. Time-series regression can be plagued by autocorrelation that can cause spurious correlations (39). To avoid this, we transformed the data (i.e., removed the trend) and examined the residuals. Nonetheless, detrending potentially loses important information and is not always necessary or correct (40, 41). Specifically, econometricians have pointed out that time series can be cointegrated (i.e., share common stochastic trends), and under these conditions, normal regression techniques or error-correction methods are appropriate (41). Preliminary examination of residuals on our regressions suggested potential cointegration (i.e., deviations from the long-run trend are stationary). Given this, we felt compelled to explore both the transformed and untransformed data. Importantly, relationships were consistent across both analyses, suggesting that our results are robust to analytical techniques. Finally, we tested differences between Plot 1 (homogeneous plot) and Plot 3 (heterogeneous plot) by detrending regressions to remove autocorrelations and then testing for differences by using two-tailed t tests assuming unequal variances.
Supplementary Material
Acknowledgments
We thank numerous summer student assistants for help with field and laboratory work; Barry Cooke and Gabriel Gellner for statistical discussions; Tom Royama, Steve Heard, Dan Quiring, Gaétan Moreau, and Wayne MacKinnon for providing valuable comments on the manuscript; and George Fanjoy for assistance with the figures. This study was funded by the Canadian Forest Service.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0704301104/DC1.
References
- 1.Daily GC, Alexander S, Ehrlich PR, Goulder L, Lubchenco J, Matson PA, Mooney HA, Postel S, Schneider SH, Tilman D, et al. Issues Ecol. 1977;1:1–18. [Google Scholar]
- 2.Elton CS. The Ecology of Invasions by Animals and Plants. New York: Kluwer; 1958. [Google Scholar]
- 3.Pimm SL. The Balance of Nature: Ecological Issues in the Conservation of Species and Communities. Chicago: Univ Chicago Press; 1991. [Google Scholar]
- 4.May RM. Stability and Complexity in Model Ecosystems. Princeton: Princeton Univ Press; 1974. [Google Scholar]
- 5.McCann KS. Nature. 2000;405:228–233. doi: 10.1038/35012234. [DOI] [PubMed] [Google Scholar]
- 6.Levin SA. PLoS Biol. 2006;4:1471–1472. [Google Scholar]
- 7.Goldwasser L, Roughgarden J. Ecology. 1993;74:1216–1233. [Google Scholar]
- 8.Raffaelli DG, Hall SJ. In: Food Webs: Integration of Patterns and Dynamics. Polis GA, Winemiller KO, editors. New York: Chapman and Hall; 1996. pp. 185–191. [Google Scholar]
- 9.Winemiller KO. Ecol Monogr. 1990;60:331–367. [Google Scholar]
- 10.Nicolis G, Prigogine I. Self-Organization in Nonequilibrium Systems. New York: Wiley and Sons; 1977. [Google Scholar]
- 11.Kondoh M. Science. 2003;299:1388–1391. doi: 10.1126/science.1079154. [DOI] [PubMed] [Google Scholar]
- 12.McCann KS, Rasmussen JB, Umbanhowar J. Ecol Lett. 2005;8:513–523. doi: 10.1111/j.1461-0248.2005.00742.x. [DOI] [PubMed] [Google Scholar]
- 13.Holt RD. Ecol Res. 2002;17:261–273. [Google Scholar]
- 14.Krivan V, Schmitz O. Evol Ecol Res. 2003;5:623–652. [Google Scholar]
- 15.Rooney N, McCann KS, Gellner G, Moore JC. Nature. 2006;442:265–269. doi: 10.1038/nature04887. [DOI] [PubMed] [Google Scholar]
- 16.Martinez ND, Dunne JA. In: Ecological Scale: Theory and Application. Peterson DL, Parker VT, editors. New York: Columbia Univ Press; 1998. pp. 207–226. [Google Scholar]
- 17.Beckerman AP, Petchey OL, Warren PH. Proc Natl Acad Sci USA. 2006;103:13745–13749. doi: 10.1073/pnas.0603039103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Polis G, Strong D. Am Nat. 1996;147:813–846. [Google Scholar]
- 19.Su G, MacLean DA, Needham TD. Can J For Res. 1996;26:1620–1628. [Google Scholar]
- 20.Cappuccino N, Lavertu D, Bergeron Y, Régnière J. Oecologia. 1998;114:236–242. doi: 10.1007/s004420050441. [DOI] [PubMed] [Google Scholar]
- 21.Jactel H, Goulard M, Menassieu P, Goujon G. J Appl Ecol. 2002;39:618–628. [Google Scholar]
- 22.Montoya JM, Rodriquez MA, Hawkins BA. Ecol Lett. 2003;6:587–593. [Google Scholar]
- 23.Lewis OT, Memmott J, Lasalle J, Lyal CHC, Whitefoord C, Godfray HCJ. J Anim Ecol. 2002;71:855–873. [Google Scholar]
- 24.Godfray HCJ, Lewis OT, Memmott J. Philos Trans R Soc London Ser B. 1999;354:1811–1824. doi: 10.1098/rstb.1999.0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simmons GA, Leonard DE, Chen CW. Environ Entomol. 1975;4:832–836. [Google Scholar]
- 26.Lill JT, Marquis RJ, Ricklefs RE. Nature. 2002;417:170–173. doi: 10.1038/417170a. [DOI] [PubMed] [Google Scholar]
- 27.Miller CA. Mem Entomol Soc Can. 1963;31:228–244. [Google Scholar]
- 28.Miller CA, Renault T. Internal Report M-11. Fredericton, NB: Can For Serv; 1966. [Google Scholar]
- 29.Miller CA, Renault T. Information Report M-X-115. Fredericton, NB: Can For Serv; 1981. [Google Scholar]
- 30.Royama T. Analytical Population Dynamics. London: Chapman and Hall; 1992. [Google Scholar]
- 31.Mills NJ, Kenis M. Bull Entomol Res. 1991;81:429–436. [Google Scholar]
- 32.Royama T, MacKinnon WE, Kettela EG, Carter NE, Hartling LK. Ecology. 2005;86:1212–1224. [Google Scholar]
- 33.Roland J, Taylor PD. Nature. 1997;386:710–713. [Google Scholar]
- 34.Memmott J, Alonso D, Berlow EL, Dobson A, Dunne JA, Solé R, Weitz J. In: Ecological Networks: Linking Structure to Dynamics in Food Webs. Pascual M, Dunne JA, editors. Oxford: Oxford Univ Press; 2005. pp. 325–347. [Google Scholar]
- 35.Gotelli NJ, Colwell RK. Ecol Lett. 2001;4:379–391. [Google Scholar]
- 36.Colwell RK, Mao CX, Chang J. Ecology. 2004;85:2717–2727. [Google Scholar]
- 37.Woodward G, Hildrew AG. J Anim Ecol. 2001;70:273–288. [Google Scholar]
- 38.Colwell RK. EstimateS. Storrs, CT: Univ of Connecticut; 2006. Version 8.0.0. [Google Scholar]
- 39.Chatfield C. The Analysis of Time Series. New York: Chapman and Hall; 2004. [Google Scholar]
- 40.Engle RF, Granger CWJ. Econometrica. 1987;55:277–304. [Google Scholar]
- 41.Endler W. Applied Econometric Time Series. New York: Wiley and Sons; 1995. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.