Abstract
Observations in hemodialysis patients suggest a survival advantage associated with activated vitamin D therapy. Left ventricular (LV) structural and functional abnormalities are strongly linked with hemodialysis mortality. Here, we investigated whether paricalcitol (PC, 19-nor-1,25(OH)2D2), an activated vitamin D compound, attenuates the development of LV abnormalities in the Dahl salt-sensitive (DSS) rat and whether humans demonstrate comparable findings. Compared with DSS rats fed a high-salt (HS) diet (6% NaCl for 6 weeks), HS+PC was associated with lower heart and lung weights, reduced LV mass, posterior wall thickness and end diastolic pressures, and increased fractional shortening. Blood pressures did not significantly differ between the HS groups. Plasma brain natriuretic peptide levels, and cardiac mRNA expression of brain natriuretic peptide, atrial natriuretic factor, and renin were significantly reduced in the HS+PC animals. Microarray analyses revealed 45 specific HS genes modified by PC. In a retrospective pilot study of hemodialysis patients, PC-treated subjects demonstrated improved diastolic function and a reduction in LV septal and posterior wall thickness by echocardiography compared with untreated patients. In summary, PC attenuates the development of LV alterations in DSS rats, and these effects should be examined in human clinical trials.
Keywords: cardiac hypertrophy, heart failure, paricalcitol, renal failure
The rate of cardiovascular-related mortality in hemodialysis patients is 10–20 times higher than that observed in the general population (1). Left ventricular hypertrophy (LVH) and diastolic dysfunction are present in >50% of patients at dialysis initiation, and these abnormalities are strongly linked with dialysis-related mortality (2). Currently, there are no well accepted means to modify cardiac structural and functional alterations in renal-failure patients.
We recently demonstrated in observational studies that therapy with activated vitamin D to chronic hemodialysis patients is associated with reduction in cardiovascular-related mortality (3, 4). Conversion of nutritional vitamin D (25(OH)D3) to the hormonally active form of vitamin D (1,25(OH)2D3) occurs primarily in the kidney; thus, patients with kidney failure commonly present with altered vitamin D status (5). There is growing evidence that vitamin D either directly or indirectly affects cardiac structure and function. The vitamin D receptor knockout mouse model demonstrates increased cardiac renin expression and marked cardiomyocyte hypertrophy (6), and 1,25(OH)2D3 attenuates cardiomyocyte proliferation (7) and hypertrophy (8) in vitro. Here, we demonstrate that treatment with an activated vitamin D compound attenuates the development of cardiac hypertrophy and dysfunction in a recognized animal model of such abnormalities and that comparable findings are evident in humans.
Results
Effect of Paricalcitol (PC) on Cardiac Hypertrophy and Dysfunction.
To evaluate the effect of activated vitamin D on cardiac structure and function, we chose the previously characterized high-salt (HS)-induced model of hypertension and LVH in which Dahl salt-sensitive (DSS) rats develop LVH and signs of diastolic dysfunction as early as 6–8 weeks after HS exposure (9, 10). Organ weights were adjusted to the tibial length (Table 1). Rats receiving HS+vehicle (V) for 6 weeks developed a significant increase in heart and lung weights compared with their normal-diet littermates (Table 1). In HS animals, PC treatment resulted in a 13% and 30% reduction in normalized heart and lung weights, respectively, compared with HS+V-treated animals. Specific examination of individual cardiomyocyte size showed a 71% increase after HS+V that was decreased by 21% in the HS+PC model [supporting information (SI) Fig. 5A and SI Text]. Overall myocardial fibrosis was mild and did not differ between groups (SI Fig. 5 B and C).
Table 1.
Morphometry analysis of DSS rats treated with PC
| Normal diet |
HS diet |
|||
|---|---|---|---|---|
| C | PC | C | PC | |
| Heart weight, g | 1.3 ± 0.02 | 1.2 ± 0.01 | 1.7 ± 0.02* | 1.4 ± 0.05† |
| Lung weight, g | 1.7 ± 0.11 | 1.4 ± 0.03 | 2.2 ± 0.13 | 1.5 ± 0.06† |
| Liver weight, g | 14.0 ± 0.6 | 13.1 ± 0.2 | 16.1 ± 0.2 | 14.8 ± 0.7 |
| Tibial length, mm | 38 ± 0.2 | 37 ± 0.2 | 37 ± 0.3 | 37 ± 0.2 |
| HW/TL, mg/mm | 34.5 ± 0.5 | 32.3 ± 0.1 | 44.8 ± 0.4* | 39.1 ± 1.2*† |
| LuW/TL, mg/mm | 44.3 ± 3.1 | 36.76 ± 0.8 | 58.8 ± 3.4* | 41.1 ± 1.6† |
| LiW/TL, mg/mm | 364 ± 13 | 352 ± 4 | 436 ± 5* | 406 ± 19* |
Results are presented as mean ± SEM.,
*, P < 0.05 vs. normal diet treated with V.
†, P < 0.05 vs. HS diet treated with V. C, V control; HW, heart weight; LuW, lung weight; LiW, liver weight; TL, tibial length.
We next examined cardiac parameters using M-mode echocardiogram measures. In HS+V animals, anterior and posterior wall thickness was increased in the setting of reduced fractional shortening (FS) (Table 2). In contrast, alternations in both wall thickness and FS were markedly attenuated in the HS+PC group (Table 2 and Fig. 1A). In addition, LV mass was elevated in the HS+V group, and significantly reduced by PC treatment (Table 2). Thus, PC resulted in significant attenuation of cardiac and cardiomyocyte hypertrophy and cardiac dysfunction.
Table 2.
Echocardiographic analysis of DSS rats treated with PC
| Normal diet |
HS diet |
|||
|---|---|---|---|---|
| C | PC | C | PC | |
| HR, beats per min | 394 ± 13 | 405 ± 8 | 352 ± 22* | 360 ± 8 |
| AWd, mm | 1.72 ± 0.07 | 1.65 ± 0.06 | 2.14 ± 0.04* | 1.91 ± 0.05*† |
| PWd, mm | 1.82 ± 0.06 | 1.81 ± 0.07 | 2.37 ± 0.10* | 1.88 ± 0.04† |
| LVDd, mm | 7.82 ± 0.18 | 7.73 ± 0.22 | 7.80 ± 0.27 | 8.35 ± 0.05 |
| LVDs, mm | 3.70 ± 0.14 | 3.73 ± 0.11 | 4.25 ± 0.22 | 3.93 ± 0.08 |
| LV mass, g | 0.83 ± 0.03 | 0.79 ± 0.02 | 1.17 ± 0.05* | 1.01 ± 0.02*† |
| FS, % | 52.7 ± 1.1 | 51.6 ± 0.8 | 45.7 ± 1.6* | 52.9 ± 1.0† |
Results are presented as mean ± SEM.
*, P < 0.05 vs. normal diet treated with V.
†, P < 0.05 vs. HS diet treated with V. C, V control; HR, heart rate; AWd, anterior wall thickness in diastole; PWd, posterior wall thickness in diastole; LVDd, LV dimension in diastole; LVDs, LV dimension in systole, LV mass, calculated LV mass; FS, fractional shortening.
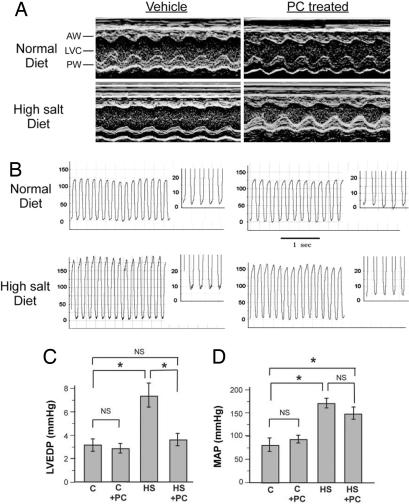
Fig. 1.
Echocardiographic and hemodynamic measures. (A) Representative M-mode images of the LV of DSS rats fed normal and HS diets. The increases in LV wall thickness and lower LV wall motion in HS animals are improved in the HS+PC group. AW, anterior wall; PW, posterior wall; LVC, left ventricular cavity. (B) Representative LV pressure tracings. (C) LVEDP measurement. LVEDP is significantly higher in HS group, whereas in HS+PC animals, it is similar to controls. (D) Noninvasive MAP measurements. MAP is significantly elevated in HS diet groups, but not modified by PC treatment. n = 3–10 in each group. *;, P < 0.05.
Effect of PC on LV Hemodynamics and Blood Pressure.
LV end-diastolic pressure (LVEDP), a measure of LV filling pressure reflecting LV dysfunction, was significantly elevated in the HS+V-treated group (Fig. 1 B and C). In contrast, HS+PC animals had nearly normal LVEDP measures, suggesting that PC prevented LV hemodynamic alterations in the setting of HS. We then ascertained whether PC exerted its effect through changes in blood pressure. In both HS+V and HS+PC animals, tail-cuff-measured mean arterial pressures (MAPs) increased compared with controls. PC did not significantly reduce MAPs in either the HS or normal-diet animals (Fig. 1D). To more precisely exclude the effect of blood pressure explaining our results, we used continuous wireless telemetry for seven consecutive days in nonanesthetized animals fed HS diet for 5 weeks and injected with PC or V (SI Fig. 6 and SI Text). Blood pressures were similar, providing further evidence against the possibility that PC effects were mediated by changes in blood pressure.
Biochemical and Molecular Measures of Cardiac Dysfunction Attenuated by PC.
Brain natriuretic peptide (BNP) and atrial natriuretic factor (ANF) are reliable early measures of ventricular myocyte stress and antedate cardiac hypertrophy and heart failure. HS+V animals demonstrated a marked late increase in plasma BNP levels compared with HS+PC animals (Fig. 2A). Similarly, cardiac ventricle mRNA expression of ANF and BNP was increased in the HS+V group and markedly attenuated in the HS+PC animals (Fig. 2 B and C). Because the vitamin D receptor (VDR)KO model demonstrates cardiac myocyte hypertrophy and an increase in cardiac renin gene expression (6), and local renin-angiotensin activation is directly linked with cardiac hypertrophy and heart failure (11), we also characterized renin mRNA expression in ventricular tissue. Overall, renin expression in controls was low as suggested by previous studies (12). In contrast, renin expression by RT-PCR was significantly increased in the HS+V group and markedly attenuated in the HS+PC model (Fig. 2 D and E). Real-time qPCR for renin mRNA expression confirmed these findings: HS+V, 11 ± 3-fold increase compared with controls; HS+PC significantly repressed this alteration to 5 ± 1-fold increase compared with controls; HS+V vs. HS+PC, P < 0.05. Thus, PC significantly attenuated the biochemical and molecular measures of cardiac ventricular stress and hypertrophy.
Fig. 2.
Plasma BNP and LV gene expression. (A–C) Plasma BNP level (ELISA) (A), and relative ANF and BNP (B and C) mRNA level in LV tissue, respectively, (real time RT-PCR). C, control vehicle group. n = 4–11 in each group. *, P < 0.05. (D) Semiquantitative RT-PCR of LV renin mRNA expression. Actin expression is shown below for loading control. (E) Quantification of renin mRNA expression normalized to actin mRNA expression. *, P < 0.05.
Serum creatinine (HS+V, 1.7 ± 0.2 vs. HS+PC, 1.8 ± 0.2 mg/dl; P > 0.05) and calcium (13.6 ± 2.1 vs. 15.3 ± 2.0 mg/dl, respectively; P > 0.05) levels did not significantly differ between the two HS groups. Finally, after HS diet, the DSS model is known to develop 25(OH)D3 deficiency (13), a finding we verified after 6 weeks of HS (SI Fig. 7).
Effect of PC on Gene Expression Profiles.
In an effort to examine the potential mediators of PC's effect, we first compared the cardiac ventricle gene expression profiles of normal diet+V and HS+V animals. Among the 15,866 sequences evaluated, 1,338 genes meeting a significance of P < 0.001 were altered by HS: 741 (4.7%) down- and 597 (3.8%) up-regulated (Fig. 3A). By comparison, in the normal diet+V compared with the HS+PC group, only 290 (1.8%) sequences were significantly down- and 231 (1.5%) up-regulated. Clustering the two data sets (normal vs. HS+V and normal vs. HS+PC) suggested indirectly that PC had a “normalizing” effect on several HS-induced genes (Fig. 3B and SI Table 3). We examined cardiac microarrays from animals after 3 weeks of HS to better elucidate the potential mechanisms affected by PC. Less than 5% of all expression changes observed at 6 weeks in the HS+V animals were altered at 3 weeks, and none met a significance of P < 0.001. Nevertheless, gene expression of muscle contractile proteins did demonstrate early alterations (SI Table 4).
Fig. 3.
Summary of gene expression changes. (A) Normal diet vs. HS+V “M/A” plot [average probe intensity (x axis) vs. log ratio (y axis)] using a P < 0.001 as cut-off for significance. Specifically, 1,338 sequences are regulated (597 up- and 741 down-regulated) by HS compared with normal diet at 6 weeks. Scale is log ratio −2.0 to +2.0 (i.e., −100- to +100-fold). (B) Agglomerative hierarchical clustering using the 1,338 HS sequences and two samples (normal diet vs. HS+V; normal diet vs. HS+PC). Scale is log ratio −0.7 to +0.7 (i.e., −5- to +5-fold). Red, up-regulation and blue, down-regulation. A sequence that failed to meet the P value <0.001 cut-off was colored black. (C) “M/A” plot of the 6-week gene expression pattern for HS+V vs. HS+PC using the 6-week 1,338 HS sequences (P < 0.001). Of the 1,338 sequences regulated at 6 weeks, only 45 are regulated specifically by PC (22 up- and 23 down-regulated).
To more directly examine PC's effect, we then focused on the 1,338 HS sequences that retained a P < 0.001 when comparing gene expression differences between HS+V and HS+PC groups (Fig. 3C). This analysis yielded 45 specific HS sequences that were significantly modulated by PC (SI Table 5). Among these, possible candidates for PC's effect included genes related to cell signaling (Nkiras2), cell adhesion (Itgb1, Nrp1), stress responses (Hmox1), and muscle contractile function (Myh7, Tpm1). Importantly, myosin heavy-chain (MHC) isoform switch (increased β-MHC with decreased α-MHC expression), an established fetal-type gene reexpression characteristic of load-dependent hypertrophy (14), was reversed after PC treatment.
PC Is Associated with Improved LV Parameters in Hemodialysis Patients.
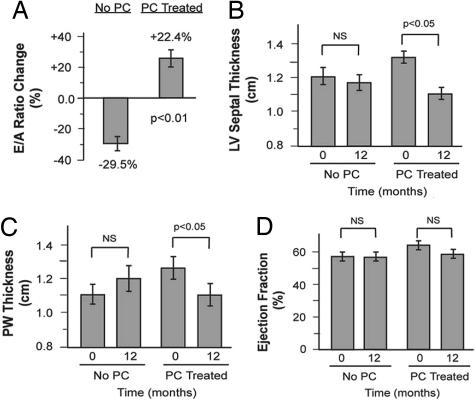
In the U.S., PC is routinely used among chronic hemodialysis patients (3), a population with a high prevalence of cardiac hypertrophy, diastolic dysfunction, and profound vitamin D deficiency similar to our animal model (15). Therefore, we retrospectively examined baseline and 12-month cardiac echocardiograms in adult hemodialysis patients treated with or without PC (SI Table 6 for baseline characteristics). Among patients untreated for the entire duration, E/A ratios decreased, suggesting a worsening of diastolic dysfunction, whereas, in treated patients (duration of PC, 4.3 ± 1.2 months; average dose, 13 ± 7 μg/week), E/A ratios increased (Fig. 4A). Furthermore, treatment was associated with a 15% and 11% reduction in septal and posterior wall thickness, respectively (Fig. 4 B and C). Ejection fractions were within the normal range at baseline and follow-up (Fig. 4D). Although observational and retrospective, these findings suggest that our animal results may translate to humans.
Fig. 4.
Quantitative analysis of echocardiogram parameters in hemodialysis patients. Changes in E/A ratio (A), LV septal thickness (B), posterior wall (PW) thickness (C), and ejection fraction (D) in patients with (n = 15) and without (n = 6) PC treatment.
Discussion
We have shown that in the DSS model, PC markedly attenuates the development of the anatomic, functional, biochemical, molecular, and genetic alterations characteristic of cardiac hypertrophy and dysfunction. The DSS model has been reproducibly used to investigate cardiac hypertrophy, diastolic dysfunction, and transition to heart failure (9, 16). Therapies shown to attenuate cardiac dysfunction in this model (17, 18) ameliorate comparable cardiac abnormalities in humans (19). Importantly, although our retrospective clinical data of activated vitamin D-treated hemodialysis subjects involved small numbers, the observed significant improvement in cardiac alterations is encouraging and suggestive of a potential for clinical intervention. These results, the accumulating data on the cardiovascular effects of activated vitamin D, and studies from our group and others suggesting a survival benefit associated with activated vitamin D therapy in dialysis patients (3, 4, 20) strongly support the basis for an interventional trial to directly assess the cardiac-specific effects of activated vitamin D administration in renal-failure subjects.
Skin-derived 7-dehydrocholesterol undergoes liver hydroxylation to form 25(OH)D3, the storage form of vitamin D (21). The kidney hydroxylates 25(OH)D3 to 1,25(OH)2D3, the active form of vitamin D that exerts its effect through binding the nuclear VDR present in cells, including smooth muscle and endothelial cells, and possibly cardiomyocytes (7, 22, 23). A 25(OH)D3 deficiency leads to derangements in cardiac myocyte contraction (24), proliferation (25), and cardiac collagen accumulation (26). Furthermore, 1,25(OH)2D3 blocks endothelin-induced ventricular myocyte hypertrophy (8), and VDRKO mice develop cardiomyocyte hypertrophy (6). Comparable human data has been limited. Nutritional vitamin D-deficiency rickets is associated with a dilated cardiomyopathy (27). Patients with heart failure have reduced circulating levels of 25(OH)D3 (28), and small uncontrolled studies suggest that 1,25(OH)2D3 therapy reduces LV hypertrophy in hemodialysis patients (29). Nevertheless, before this study, the link between animal and human studies had been tentative.
We used a high threshold of statistical significance throughout our microarray gene analyses to minimize false-positive findings in the setting of multiple testing (30). In this context, a number of genes altered by HS diet were normalized by PC including the myosin heavy-chain isoform switch characteristic of load-dependent hypertrophy (10, 14). Changes in α-tropomyosin and in the NF-κB pathway, which have been linked with ventricular hypertrophy (31, 32), were also positively modified by PC. This is consistent with the finding that 1,25(OH)2D3 significantly blunts the activation of NF-κB signaling in vitro (33). Finally, cardiac renin gene expression was attenuated by PC, a finding consistent with Li et al. (6, 34) who demonstrated that 1,25(OH)2D3 is a negative regulator of renin gene synthesis. Whether the repression of cardiac renin by PC is cause or consequence of LV improvement remains unknown. Evidence for the presence of a functional VDR in cardiomyocytes is lacking, hence the effect of PC may be indirect. For example, mast cells release renin and activate the local cardiac RAS system (35), and mast cells contain the VDR (36). In the setting of cardiac hypertrophy, however, the precise role of locally derived renin compared with that released from the kidney continues to be debated (35, 37).
Importantly, DSS rats develop significant 25(OH)D3 deficiency (13), as we confirmed, and we suggest that activated vitamin D replacement significantly attenuates the LV alterations characteristic of this model. Furthermore, our animal findings may translate to humans with vitamin D deficiency and LV alterations such as those with kidney disease, although we acknowledge that the DSS model lacked the uremic milieu of renal failure, and our human data were observational and retrospective. Indeed, a more parallel comparison may be the examination of PC's effect in a uremic model of heart failure. Nonetheless, PC is already in routine clinical use among patients with renal disease, and thus an interventional clinical trial would be the next logical step. Although many unanswered questions remain in the rapidly expanding area of the “cardio-renal syndrome,” alterations in the vitamin D axis may be at least one integral mediator of this highly prevalent condition.
Materials and Methods
Animal Model.
Male DSS rats (Harlan Sprague–Dawley) were bred and fed a normal diet to 6 weeks of age. To generate pressure-overload cardiac hypertrophy, animals were fed a HS diet (6% NaCl) for the following 6 weeks. To study the effects of PC, the animals were divided into four groups: (i) normal diet+V, (ii) normal diet+PC (200 ng i.p. three times per wk), (iii) HS+V, and (iv) HS+ PC (200 ng i.p. three times per wk). PC (19-nor-1,25-(OH)2 D2), which is ≈10 times less potent in gastrointestinal calcium and phosphorus absorption than the endogenous form of active vitamin D calcitriol (1,25(OH)2 D3), was prepared with 95% propylene glycol/5% ethyl alcohol solution and initiated i.p. 3 days before the initiation of HS diet and continued at 200 ng three times per week (Monday, Wednesday, Friday) thereafter. Administration of 200 ng of PC three times per wk was based on comparable studies of nephrectomized uremic rats, where doses of up to 250 ng i.p. three times per week were safely administered without significant changes in blood pressure or serum calcium or phosphorus (38, 39). Nephrectomy was not part of this study. Controls were age-matched male DSS rats fed normal chow receiving equal volume of the V three times per week for 6 weeks starting at 6 weeks of age. After 6 weeks of normal diet or HS, morphometric measures, cardiac function using noninvasive and invasive physiologic methods, histopathology, and biochemical/gene changes related to cardiac stress were examined. The Institutional Animal Care and Use Committee (IACUC) of the Beth Israel Deaconess Medical Center approved this study.
Echocardiography.
Echocardiogram was performed under isoflurane anesthesia. An Agilent Sonos 5500 sector scanner equipped with a 7.5-MHz phased-array transducer was used to record 2D-guided M-mode tracings. The leading-edge method was used to determine anterior and posterior wall thickness and LV internal dimensions. LV mass was calculated by using the corrected American Society of Echocardiography (ASE) simplified cubed equation: LV mass (grams) = 0.8 [1.05 (LVDd+AWd+PWd)3 − (LVDd)3].
Blood Pressure and LV Pressure Measurements.
Noninvasive arterial blood pressure was measured with an automated noninvasive tail-cuff BP-2000 Blood Pressure Analysis System (Visitech Systems, Apex, NC) as described (40). An average of 10 readings per animal was assessed. Invasive LV pressure measurements were obtained by inserting a 1.4 French microtip pressure catheter (Millar Instruments, Houston, TX) through the right common carotid artery into the left ventricle. Data were recorded by using a PowerLab system and Chart 5 software (AD Instruments, Colorado Springs, CO). Heart rate, LV systolic pressure, and LVEDP were measured directly from LV pressure waveforms.
Plasma BNP.
Tail-vein blood was collected at baseline, 3, and 6 weeks (at sacrifice) after initiation of a HS diet. Plasma levels of BNP were measured by using BNP ELISA kit (Assaypro, St. Charles, MO) according to the manufacturer's instructions.
Real-Time PCR and RT-PCR Analyses.
Total RNA was extracted from rat ventricle tissue by using TRIzol (GIBCO–BRL, Gaithersburg, MD). RT-PCR was performed by PCR amplification of cDNA reverse-transcribed from mRNA by using rat renin and actin primers (SI Table 7). Real-time PCR primers for rat BNP, ANF, renin, and actin were obtained from Applied Biosystems (Foster City, CA) (SI Table 7). Real-time PCR was performed with a 7500 Real-Time PCR System (Applied Biosystems). Each sample had a final volume of 20 μl, containing following: 1× one step SYBR green master mix, 3–5 pmol of primers for the gene of interest, and 2 μg of RNA. Samples were run in duplicate in optically clear 96-well plates. Data were calculated by 2−ΔCT method and presented as relative expression (RQ value) of transcripts normalized to β-actin.
Histological Analysis.
Cardiac histological analyses were performed as described (9, 10). H&E- and Masson's trichrome-stained sections were evaluated in a blinded fashion for pathological changes. Measurements were made at ×20 by using a calibrated digital camera and software (DP 70 and DPController, Olympus, Irving, TX).
RNA Isolation for Microarray Analysis.
Total RNA from left ventricles was isolated by using Qiagen RNeasy procedures (Qiagen, Valencia, CA). Hearts were removed rapidly, and the left ventricles were cut and snap-frozen in liquid nitrogen. Total RNA was isolated by homogenization in RLT buffer and purification performed on the RNeasy Mini column. Quality of recovered RNA was assessed with the Agilent 2100 Bioanalyzer; RIN scores were >9 for all samples.
Microarray Analysis.
Generation of biotinylated cRNA probe for the Affymetrix arrays was performed according to Affymetrix's protocols. The Affymetrix Rat Genome 230A GeneChip (Affymetrix, Santa Clara, CA) containing probe sets for ≈15,000 known sequences and expressed sequence tags (ESTs) was used for analysis. Hybridization and scanning were done per standard Affymetrix protocols. The resulting raw data files (.cel files) were first analyzed with the R/Bioconductor AffyQC package, and no outliers were observed with respect to percent present, 5′3/′ ratios or all pair-wise correlation analysis. Data were then loaded into the Rosetta Resolver system, where it was normalized, replicates combined, and ratios created by using the standard Affymetrix error model pipeline. Further analysis used the agglomerative clustering and data visualization tools in Resolver. The Database for Annotation, Visualization and Integrated Discovery (DAVID) (National Institute of Allergy and Infectious Diseases, Bethesda, MD) and GenMAPP programs (University of California, San Francisco, CA) were used for gene ontology analyses.
Human Studies.
Adult chronic hemodialysis patients at the Texas Diabetes Institute (San Antonio, TX) were enrolled in a 12-month study to assess the effects of hemodialysis on cardiac function (41). Baseline and 12-month transthoracic echocardiograms were performed by using standard techniques and reviewed by two independent cardiologists blinded to the examination date and therapy status. PC use was left up to the discretion of the treating physician. An initial cohort of 51 patients received thrice weekly conventional hemodialysis, and among these, 38 underwent a baseline and 12-month echocardiographic evaluation. We included all who received either no activated vitamin D therapy (n = 6) or who received only PC for at least one month (n = 15). The study was approved by the IRB of the University of Texas Health Science Center San Antonio, and all participants provided written informed consent.
Statistical Analysis.
Data were expressed as means ± SEM. Comparisons between and within groups were conducted with unpaired Student t tests and repeated-measures ANOVA, respectively. P values of <0.05 were considered statistically significant.
Supplementary Material
Acknowledgments
This work was supported in part by National Heart, Lung, and Blood Institute Grants R01 HL65742 (to P.M.K.) and DK71674 (to R.T.) and research support from Abbott (R.T.).
Abbreviations
- ANF
atrial natriuretic factor
- BNP
brain natriuretic peptide
- DSS
Dahl salt-sensitive
- HS
high salt
- LV
left ventricular
- LVEDP
LV end-diastolic pressure
- LVH
LV hypertrophy
- MAP
mean arterial pressure
- PC
paricalcitol
- V
vehicle
- VDR
vitamin D receptor.
Footnotes
Conflict of interest statement: The study was supported in part by Abbott Laboratories.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0611202104/DC1.
References
- 1.Foley RN, Parfrey PS, Sarnak MJ. Am J Kidney Dis. 1998;32:S112–S119. doi: 10.1053/ajkd.1998.v32.pm9820470. [DOI] [PubMed] [Google Scholar]
- 2.Parfrey PS, Foley RN, Harnett JD, Kent GM, Murray DC, Barre PE. Nephrol Dial Transplant. 1996;11:1277–1285. [PubMed] [Google Scholar]
- 3.Teng M, Wolf M, Lowrie E, Ofsthun N, Lazarus JM, Thadhani R. N Engl J Med. 2003;349:446–456. doi: 10.1056/NEJMoa022536. [DOI] [PubMed] [Google Scholar]
- 4.Teng M, Wolf M, Ofsthun MN, Lazarus JM, Hernan MA, Camargo CA, Jr, Thadhani R. J Am Soc Nephrol. 2005;16:1115–1125. doi: 10.1681/ASN.2004070573. [DOI] [PubMed] [Google Scholar]
- 5.Pitts TO, Piraino BH, Mitro R, Chen TC, Segre GV, Greenberg A, Puschett JB. J Clin Endocrinol Metab. 1988;67:876–881. doi: 10.1210/jcem-67-5-876. [DOI] [PubMed] [Google Scholar]
- 6.Xiang W, Kong J, Chen S, Cao LP, Qiao G, Zheng W, Liu W, Li X, Gardner DG, Li YC. Am J Physiol. 2005;288:E125–E132. doi: 10.1152/ajpendo.00224.2004. [DOI] [PubMed] [Google Scholar]
- 7.Nibbelink KA, Tishkoff DX, Hershey SD, Rahman A, Simpson RU. J Steroid Biochem Mol Biol. 2007;103:533–537. doi: 10.1016/j.jsbmb.2006.12.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu J, Garami M, Cheng T, Gardner DG. J Clin Invest. 1996;97:1577–1588. doi: 10.1172/JCI118582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kang PM, Yue P, Liu Z, Tarnavski O, Bodyak N, Izumo S. Am J Physiol. 2004;287:H72–H80. doi: 10.1152/ajpheart.00556.2003. [DOI] [PubMed] [Google Scholar]
- 10.Kong SW, Bodyak N, Yue P, Liu Z, Brown J, Izumo S, Kang PM. Physiol Genomics. 2005;21:34–42. doi: 10.1152/physiolgenomics.00226.2004. [DOI] [PubMed] [Google Scholar]
- 11.Dzau VJ. Arch Intern Med. 1993;153:937–942. [PubMed] [Google Scholar]
- 12.Bader M, Peters J, Baltatu O, Muller DN, Luft FC, Ganten D. J Mol Med. 2001;79:76–102. doi: 10.1007/s001090100210. [DOI] [PubMed] [Google Scholar]
- 13.Thierry-Palmer M, Cephas S, Sayavongsa P, Doherty A, Arnaud SB. Bone. 2005;36:645–653. doi: 10.1016/j.bone.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 14.Izumo S, Lompre AM, Matsuoka R, Koren G, Schwartz K, Nadal-Ginard B, Mahdavi V. J Clin Invest. 1987;79:970–977. doi: 10.1172/JCI112908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolf M, Shah A, Gutierrez O, Ankers E, Monroy M, Tamez H, Steele D, Chang Y, Camargo CA, Tonelli M, et al. Kidney Int. 2007 doi: 10.1038/sj.ki.5002451. in press. [DOI] [PubMed] [Google Scholar]
- 16.Doi R, Masuyama T, Yamamoto K, Doi Y, Mano T, Sakata Y, Ono K, Kuzuya T, Hirota S, Koyama T, et al. J Hypertens. 2000;18:111–120. doi: 10.1097/00004872-200018010-00016. [DOI] [PubMed] [Google Scholar]
- 17.Yamamoto K, Mano T, Yoshida J, Sakata Y, Nishikawa N, Nishio M, Ohtani T, Hori M, Miwa T, Masuyama T. J Hypertens. 2005;23:393–400. doi: 10.1097/00004872-200502000-00022. [DOI] [PubMed] [Google Scholar]
- 18.Noguchi T, Kihara Y, Begin KJ, Gorga JA, Palmiter KA, LeWinter MM, VanBuren P. Circulation. 2003;107:630–635. doi: 10.1161/01.cir.0000046267.16901.e9. [DOI] [PubMed] [Google Scholar]
- 19.Radford MJ, Arnold JM, Bennett SJ, Cinquegrani MP, Cleland JG, Havranek EP, Heidenreich PA, Rutherford JD, Spertus JA, Stevenson LW, et al. Circulation. 2005;112:1888–1916. doi: 10.1161/CIRCULATIONAHA.105.170073. [DOI] [PubMed] [Google Scholar]
- 20.Kalantar-Zadeh K, Kuwae N, Regidor DL, Kovesdy CP, Kilpatrick RD, Shinaberger CS, McAllister CJ, Budoff MJ, Salusky IB, Kopple JD. Kidney Int. 2006;70:771–780. doi: 10.1038/sj.ki.5001514. [DOI] [PubMed] [Google Scholar]
- 21.Holick MF. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 22.Walters MR, Wicker DC, Riggle PC. J Mol Cell Cardiol. 1986;18:67–72. doi: 10.1016/s0022-2828(86)80983-x. [DOI] [PubMed] [Google Scholar]
- 23.O'Connell TD, Simpson RU. Cell Biol Int. 1996;20:621–624. doi: 10.1006/cbir.1996.0081. [DOI] [PubMed] [Google Scholar]
- 24.Baksi SN, Hughes MJ. J Mol Cell Cardiol. 1986;18:653–656. doi: 10.1016/s0022-2828(86)80937-3. [DOI] [PubMed] [Google Scholar]
- 25.O'Connell TD, Berry JE, Jarvis AK, Somerman MJ, Simpson RU. Am J Physiol. 1997;272:H1751–H1758. doi: 10.1152/ajpheart.1997.272.4.H1751. [DOI] [PubMed] [Google Scholar]
- 26.Weishaar RE, Kim SN, Saunders DE, Simpson RU. Am J Physiol. 1990;258:E134–E142. doi: 10.1152/ajpendo.1990.258.1.E134. [DOI] [PubMed] [Google Scholar]
- 27.Abdullah M, Bigras JL, McCrindle BW. Can J Cardiol. 1999;15:699–701. [PubMed] [Google Scholar]
- 28.Zittermann A, Schleithoff SS, Tenderich G, Berthold HK, Korfer R, Stehle P. J Am Coll Cardiol. 2003;41:105–112. doi: 10.1016/s0735-1097(02)02624-4. [DOI] [PubMed] [Google Scholar]
- 29.Park CW, Oh YS, Shin YS, Kim CM, Kim YS, Kim SY, Choi EJ, Chang YS, Bang BK. Am J Kidney Dis. 1999;33:73–81. doi: 10.1016/s0272-6386(99)70260-x. [DOI] [PubMed] [Google Scholar]
- 30.Reiner A, Yekutieli D, Benjamini Y. Bioinformatics. 2003;19:368–375. doi: 10.1093/bioinformatics/btf877. [DOI] [PubMed] [Google Scholar]
- 31.Rajan S, Williams SS, Jagatheesan G, Ahmed RP, Fuller-Bicer G, Schwartz A, Aronow BJ, Wieczorek DF. Physiol Genomics. 2006;27:309–317. doi: 10.1152/physiolgenomics.00072.2006. [DOI] [PubMed] [Google Scholar]
- 32.Purcell NH, Tang G, Yu C, Mercurio F, DiDonato JA, Lin A. Proc Natl Acad Sci USA. 2001;98:6668–6673. doi: 10.1073/pnas.111155798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Z, Yuan W, Sun L, Szeto FL, Wong KE, Li X, Kong J, Li YC. Kidney Int. 2007;72:193–201. doi: 10.1038/sj.ki.5002296. [DOI] [PubMed] [Google Scholar]
- 34.Li YC, Kong J, Wei M, Chen ZF, Liu SQ, Cao LP. J Clin Invest. 2002;110:229–238. doi: 10.1172/JCI15219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mackins CJ, Kano S, Seyedi N, Schafer U, Reid AC, Machida T, Silver RB, Levi R. J Clin Invest. 2006;116:1063–1070. doi: 10.1172/JCI25713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baroni E, Biffi M, Benigni F, Monno A, Carlucci D, Carmeliet G, Bouillon R, D'Ambrosio D. J Leukoc Biol. 2007;81:250–262. doi: 10.1189/jlb.0506322. [DOI] [PubMed] [Google Scholar]
- 37.Parsons KK, Coffman TM. J Clin Invest. 2007;117:873–876. doi: 10.1172/JCI31856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Slatopolsky E, Cozzolino M, Lu Y, Finch J, Dusso A, Staniforth M, Wein Y, Webster J. Kidney Int. 2003;63:2020–2027. doi: 10.1046/j.1523-1755.2003.00029.x. [DOI] [PubMed] [Google Scholar]
- 39.Slatopolsky E, Cozzolino M, Finch JL. Kidney Int. 2002;62:1277–1284. doi: 10.1111/j.1523-1755.2002.kid573.x. [DOI] [PubMed] [Google Scholar]
- 40.Venkatesha S, Toporsian M, Lam C, Hanai J, Mammoto T, Kim YM, Bdolah Y, Lim KH, Yuan HT, Libermann TA, et al. Nat Med. 2006;12:642–649. doi: 10.1038/nm1429. [DOI] [PubMed] [Google Scholar]
- 41.Ayus JC, Mizani MR, Achinger SG, Thadhani R, Go AS, Lee S. J Am Soc Nephrol. 2005;16:2778–2788. doi: 10.1681/ASN.2005040392. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.