Abstract
Analysis of the molecular factors determining hepatocyte survival or death in response to inflammatory stimuli is essential for understanding the pathogenesis of inflammatory liver disease and for identifying novel therapeutic approaches. c-Jun N-terminal kinase (JNK) is a major mediator of cytokine-induced cell death during hepatitis, but the signaling pathways downstream of JNK remain less well defined. Here we show that the transcription factor c-Jun/AP-1, a prototypic target of JNK, is strongly expressed in the liver of patients with acute liver injury. The molecular function of c-Jun in inflammatory liver disease was analyzed in mice by using the Con A model of T cell-mediated hepatitis. Mice lacking c-Jun in hepatocytes display increased liver cell death and mortality upon Con A injection. This phenotype is caused by impaired expression of inducible nitric oxide synthase (nos2), a direct transcriptional target of c-Jun, and reduced production of hepatoprotective nitric oxide (NO). Moreover, increased hepatotoxicity in mutant mice is likely caused by hypoxia and oxidative stress and can be rescued pharmacologically by liver-specific NO delivery. These findings demonstrate that c-Jun/AP-1 is hepatoprotective during acute hepatitis by regulating nos2/NO expression and thus functionally antagonizes the cell death-promoting functions of JNK.
Keywords: activator protein 1, concanavalin A, hypoxia
Exploration of the molecular pathways that determine liver cell survival or death during hepatitis is essential for understanding the molecular pathogenesis of inflammatory liver disease and for identifying novel therapeutic strategies. Mouse models of hepatitis induced by TNFα, which is involved in the pathogenesis of various inflammatory liver disorders (1, 2), have been particularly useful to identify pathways mediating inflammation-associated liver cell death and survival (3). In rodents, treatment with TNFα or bacterial lipopolysaccharide (LPS), a strong inducer of TNFα release in macrophages, does not induce apoptosis unless transcription of hepatoprotective genes is blocked. Con A is therefore widely used to study acute hepatitis in mice. The pathogenesis of Con A-mediated hepatitis involves T cells and various cytokines, including TNFα, and recapitulates several aspects of viral and autoimmune hepatitis in humans (4). During Con A-mediated hepatitis, binding of TNFα to its receptors TNFR1 and TNFR2, respectively, unambiguously results in activation of c-Jun N-terminal kinase (JNK), nuclear factor κB (NF-κB), and activator protein 1 (AP-1) (5, 6). JNK activity closely correlates with cell death because Con A-mediated hepatitis is prevented in mice lacking either JNK1 or JNK2, the isoforms expressed in liver (5). JNK activity is negatively regulated by NF-κB, and inhibition of NF-κB consistently results in sustained JNK activity and increased cell death after Con A injection (5). The signaling pathways downstream of JNK that ultimately cause hepatocyte death are less well characterized, and it is not clear whether JNK may promote hepatocyte apoptosis by AP-1-dependent gene transcription, in particular by activating its prototypic target c-Jun.
AP-1 is a dimeric transcription factor composed of Jun (c-Jun, JunB, and JunD) and Fos proteins (c-Fos, FosB, Fra-1, and Fra-2) and can either promote or antagonize cell death in a tissue- and stress stimulus-dependent manner (7, 8). c-Jun mediates JNK-dependent apoptosis in TNFα-stimulated thymocytes and kainate-treated neurons (9, 10). However, several reports demonstrated protective functions of c-Jun also, e.g., in the liver. Mice lacking c-jun die at midgestation and display increased apoptosis of fetal liver cells (11, 12). Moreover, mice with conditional inactivation of c-jun in hepatocytes (c-junΔli) have no overt phenotype under resting conditions but show impaired liver regeneration upon partial hepatectomy (13, 14). During chemical carcinogenesis, c-Jun promotes hepatocyte survival by inhibition of p53 and its proapoptotic target gene noxa (15). The contribution of c-Jun to TNFα-dependent apoptosis has mainly been addressed in vitro and remains controversial because c-Jun was shown to promote (16), or antagonize (15), or not to influence hepatic cell death (17). In this work, we investigated whether c-Jun is involved in JNK-mediated cell death or hepatocyte survival during acute hepatitis in vivo, and we found that c-Jun mediates hepatocyte survival by regulating transcription of the inducible nitric oxide synthase nos2 (also known as iNOS).
Results
c-Jun/AP-1 Is Highly Expressed During Acute Liver Injury in Humans.
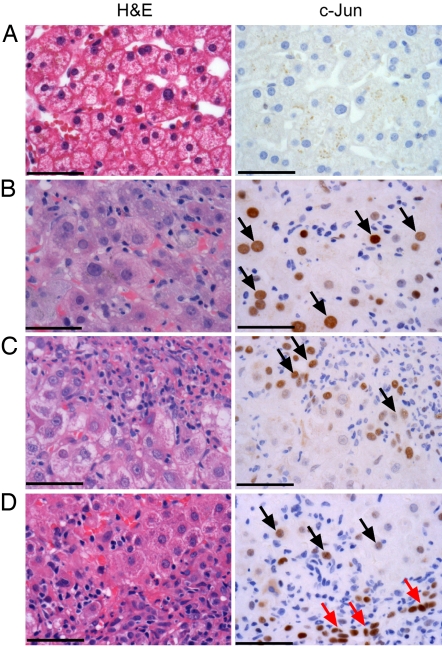
The expression of c-Jun was analyzed by immunohistochemistry in liver biopsies from patients with severe drug- or hepatitis C virus-induced liver injury displaying confluent hepatocyte necroses. Livers without signs of inflammation or hepatocyte damage were all negative for c-Jun (Fig. 1A). In contrast, c-Jun was strongly expressed in all livers with severe injury and predominantly localized in hepatocytes rather than inflammatory cells. Distinct patterns of c-Jun expression were observed either in scattered hepatocytes throughout the lobule (Fig. 1B) or in close proximity to inflammatory cells in areas of interface hepatitis (Fig. 1 C and D). These findings suggest that c-Jun is involved in the regulation of cell death or survival. In some cases, strong positive reaction for c-Jun was seen in proliferating ductular cells (Fig. 1D, red arrows), suggesting that expression of c-Jun might also be linked to regenerative responses.
Fig. 1.
c-Jun is highly expressed during severe liver injury in humans. Liver biopsies from patients without (A; n = 5) or with severe liver injury (B–D; n = 9) were analyzed by H&E staining (Left) or immunohistochemistry for c-Jun (Right). Note the distinct expression patterns of c-Jun in scattered hepatocytes throughout the lobule (arrows in B), in hepatocytes in vicinity of inflammatory cells (arrows in C and D), or in proliferating ductular cells (red arrows in D). (Scale bars: 50 μm.)
c-Jun Mediates Hepatocyte Survival During Con A-Mediated Hepatitis.
The biological functions of c-Jun/AP-1 were further investigated in mice by using the Con A model of acute T cell-mediated and TNFα-dependent hepatitis. Mice harboring floxed alleles of c-jun (c-junf/f) were crossed with Alfp-Cre transgenic mice to obtain animals lacking c-jun specifically in hepatocytes (c-junΔli) (13). Con A injection into control mice resulted in a strong induction of c-jun, junB, junD, c-fos, and fra-2 RNAs (Fig. 2A). Hepatic expression of c-jun RNA was hardly detectable in c-junΔli mice, whereas expression of c-fos RNA was significantly elevated compared with Con A-treated controls (Fig. 2A), as observed earlier upon partial hepatectomy (13). Immunohistochemistry revealed that c-Jun was expressed in hepatocytes and inflammatory cells during Con A-mediated hepatitis and that its residual expression in c-junΔli mice occurred exclusively in nonparenchymal cells (Fig. 2B).
Fig. 2.
c-Jun mediates hepatocyte survival during Con A-mediated hepatitis. (A) Expression of AP-1 genes 8 h after injection of 0.9% NaCl or Con A (10 mg/kg) in control mice (c-junf/f) and mice lacking c-Jun in hepatocytes (c-junΔli) as assessed by RNase protection assay. Expression of gapdh was used as loading control. (B) Immunohistochemistry for c-Jun 8 h after injection of NaCl or Con A. Note the respective c-Jun expression in hepatocytes (black arrows) and inflammatory cells (red arrows). (Scale bars: 50 μm.) (C) Kaplan–Meier plot showing increased mortality of c-junΔli mice during Con A-mediated hepatitis; n = 24 mice per genotype, P ≥ 0.05. (D) Areas of confluent cell death as assessed by TUNEL assays. (Upper) Eight hours after Con A injection, nuclei were counterstained with DAPI). (Lower) H&E staining 24 h after Con A injection. (Scale bars: 100 μm.) The area of confluent necrosis was quantified on serial sections stained by H&E (24 h after Con A injection) and is given in percentage of total liver area. Data represent mean ± SEM, n = 10 livers per genotype. *, P ≤ 0.05. (E) Concentrations of the serum ALT in untreated or Con A-treated mice of the indicated genotype 8 h after Con A injection. Data represent mean ± SEM from n ≥ 10 mice per genotype. §, P ≤ 0.01.
Importantly, injection of Con A caused increased mortality in c-junΔli mice (Fig. 2C). Histological analysis revealed areas of confluent cell death in c-junΔli mice predominantly localized in the subcapsular regions (Fig. 2D). The area of necrotic liver tissue was increased 4-fold in c-junΔli mice compared with Con A-treated controls (Fig. 2D) and correlated with a 6-fold increased concentration of serum alanine transaminase (ALT) (Fig. 2E). A similar phenotype was observed upon injection of Con A into mice lacking c-jun in both hepatocytes and hematopoietic cells (Mx-Cre c-junf/f or c-junΔli*), in which the amount of cell death and ALT concentrations were also strikingly increased [supporting information (SI) Fig. 6]. This finding indicates that increased liver damage was independent of c-Jun expression in nonparenchymal liver cells. Interestingly, the strong induction of junB observed upon Con A injection (Fig. 2A) was dispensable for hepatocyte survival because injection of Con A into mice lacking junB in hepatocytes did not result in increased liver damage (data not shown). These findings indicate that expression of c-Jun in hepatocytes is specifically required to promote cell survival during Con A-mediated hepatitis.
Increased Hepatocellular Damage in c-junΔli Mice Is Not Caused by Increased JNK Activity and Is Independent of p53.
Liver cell death during Con A-mediated hepatitis is strongly influenced by the severity of inflammation and the subsequent activation of MAP kinase signaling. No difference in hepatocellular expression of phosphorylated JNK was observed in both genotypes upon Con A treatment (SI Fig. 7A). Only slight differences in the kinetics of JNK kinase activity were observed during early time points. Importantly, JNK activity was induced to the same extent 8 h after Con A injection when cell death became apparent and was equally reduced thereafter (Fig. 3A and data not shown). Con A injection resulted in phosphorylation of ERK1/2, whereas phosphorylation of p38α was not increased in livers from both genotypes (Fig. 3A). Importantly, serum concentrations of TNFα, IFNγ, IL-1β, IL-4, IL-5, IL-6, and IL-10 as well as the amount of liver-infiltrating CD3-positive T lymphocytes were not significantly increased in c-junΔli mice (SI Fig. 7B and data not shown). These findings suggest that increased cell death in c-junΔli livers was most likely not the result of altered MAP kinase signaling or increased inflammation.
Fig. 3.
Increased hepatic cell death in c-junΔli mice is not caused by sustained JNK activity or impaired NF-κB activity and is independent of p53. (A) MAPK activity upon Con A injection in livers of the indicated genotypes. JNK activity was determined by kinase assay (KA). Average activities of three experiments are indicated below. p38α and ERK phosphorylation (arrows) were assessed by Western blotting (WB). The asterisk indicates an unspecific band. (B) qPCR for hepatic expression of NF-κB and p53 target genes (8 h after Con A injection). Data are normalized to untreated controls of the same genotype and are given as mean ± SD from n ≥ 4 livers per genotype. *, P ≤ 0.05; §, P ≤ 0.01. (C) Increased liver cell death in c-junΔli mice is not rescued by additional knockout of p53 in hepatocytes. Representative TUNEL stainings (average area of ≥3 livers is indicated as a percentage), and serum ALT concentrations are shown (mean ± SEM from n ≥ 11 mice per genotype. §, P ≤ 0.01).
To analyze whether increased hepatic cell death was caused by impaired activation of NF-κB, immunohistochemistry for the NF-κB subunit p65 was performed. Nuclear translocation of p65 upon treatment with Con A was observed in both genotypes (SI Fig. 7C). Hepatic expression of the NF-κB target genes gadd45β, bcl-XL, a20, and ho-1 was induced to the same extent in both genotypes at 4 h and even elevated in c-junΔli mice at 8 h after Con A injection (Fig. 3B and data not shown). These findings suggest that hepatic NF-κB activity was increased in c-junΔli mice, most likely as a consequence of increased cell death in the absence of c-Jun. RNase protection analysis confirmed the induction of bcl-XL and revealed that hepatic expression of several other apoptosis-related genes was not altered in c-junΔli mice (SI Fig. 7D). Because primary hepatocytes lacking c-jun exhibit increased p53-dependent apoptosis upon TNFα stimulation in vitro (15), a detailed analysis of the p53 pathway was performed in c-junΔli mice after Con A treatment. Expression of p53 RNA was rather decreased, whereas expression of the p53 target genes p21, mdm2, and noxa was induced to the same extent in c-junΔli livers as in controls (Fig. 3B). Moreover, p53 protein was hardly detectable by Western blotting, and increased cell death in c-junΔli livers was not rescued in double knockout mice lacking both c-jun and p53 in hepatocytes (Fig. 3C, compare with Fig. 2 D and E). These results indicate that the survival functions of c-Jun during acute hepatitis are independent of p53.
c-Jun Regulates the Expression of nos2 During Acute Hepatitis.
Nos2 is an essential modulator of the immune response and has been implicated as a potential NF-κB target gene in the pathogenesis of various liver diseases. Interestingly, induction of nos2 RNA after Con A injection was strikingly reduced in the absence of c-Jun (Fig. 4A). Nos2 protein expression largely followed the kinetics of c-Jun expression in controls and was drastically reduced and delayed in c-junΔli livers (Fig. 4B). Immunohistochemistry confirmed that Nos2 was expressed in hepatocytes and inflammatory cells during Con A-mediated hepatitis and that the hepatocellular expression was strikingly reduced in c-junΔli mice (Fig. 4C). Hepatic expression of nos2 RNA was also impaired in c-junΔli mice upon treatment with LPS, another model of TNFα-mediated hepatitis (Fig. 4D).
Fig. 4.
c-Jun transcriptionally controls the expression of nos2 during Con A-mediated hepatitis. (A) Impaired hepatic expression of nos2 RNA in c-junΔli mice 4 h after Con A injection as assessed by RNase protection assay. l32 was used as a loading control. (B) Western blotting of total liver lysates demonstrating impaired and delayed hepatic expression of Nos2 in c-junΔli mice. Actin was used as loading control. (C) Immunohistochemistry demonstrating cytoplasmic Nos2 expression in hepatocytes (black arrows) and inflammatory cells (red arrows) 4 h after Con A injection. (Scale bars: 50 μm.) (D) Relative induction of nos2 RNA in livers of the indicated genotypes 4 h after injection of LPS (100 mg/kg) as assessed by qPCR. Relative expression is given as mean ± SD from n = 5 livers per genotype; *, P ≤ 0.05. (E) Schematic organization of the murine nos2 promoter with previously described AP-1 sites and the localization of the primers. Chromatin immunoprecipitation reveals binding of c-Jun to the nos2 promoter in Con A-treated control livers but not in untreated livers or livers lacking c-jun as assessed by PCR. Enrichment of nos2 promoter sequences was analyzed by qPCR and normalized to untreated controls. Specificity of antibody binding is shown by immunoprecipitation with control IgG. n.d., not detectable. (F) N-terminal phosphorylation of c-Jun is not required for regulating nos2 expression. Relative induction of nos2 RNA in livers of the indicated genotypes 4 h after injection of Con A (10 mg/kg), as assessed by qPCR, is shown. Expression is normalized to untreated livers of the respective genotype and is given as mean ± SD from n = 5 livers per genotype.
Nos2 expression is primarily controlled at the transcriptional level, but little is known about the contribution of c-Jun to nos2 transcription in vivo. Therefore, chromatin immunoprecipitation using a c-Jun antibody or control IgG was performed in liver lysates. nos2 promoter sequences could be immunoprecipitated in liver lysates from control mice treated with Con A but not from untreated controls or c-junΔli mice treated with Con A (Fig. 4E). Similar results were obtained by using PCR primers flanking the proximal and distal AP-1 sites, respectively (data not shown). No PCR product was obtained by using primers for an unrelated gene (Apc), and control IgG failed to precipitate nos2 promoter sequences (Fig. 4E and data not shown). These findings indicate that c-Jun binds directly to the nos2 promoter during acute hepatitis and induces its expression, thereby mediating hepatoprotection.
The transactivation potential of c-Jun is enhanced by N-terminal phosphorylation through JNK. Interestingly, expression of c-jun and subsequent nos2 transcription were not impaired in Con A-treated mice lacking both N-terminal phosphorylation sites [c-junAA/AA (Fig. 4F and SI Fig. 8A)]. Moreover, Con A-mediated liver damage as assessed by ALT concentrations was also not increased in c-junAA/AA mice (SI Fig. 8B). These findings indicate that c-Jun-mediated regulation of nos2 transcription is independent of JNK.
Nitric Oxide Mediates Hepatocyte Survival During Con A-Mediated Hepatitis by Protecting Against Hypoxia and Oxidative Stress.
Impaired hepatic nos2 expression in c-junΔli mice resulted in decreased production of NO as determined by the serum concentrations of its metabolites nitrate and nitrite (Fig. 5A). To find out whether the hepatoprotective functions of c-Jun are mediated by NO, mice were injected with Con A and the liver-specific NO donor V-Pyrro/NO, which restored the impaired NO production in c-junΔli mice (Fig. 5A). Increased liver cell death in Con A-treated c-junΔli mice was rescued upon coadministration of V-Pyrro/NO, as assessed by ALT concentrations, TUNEL assays, and expression of the stress-related genes gadd45β, a20, and ho-1 (Fig. 5B and data not shown). NO is thought to promote hepatocyte survival by protecting against oxidative stress or by improving hepatic microcirculation (18). Coadministration of Con A and the antioxidant N-acetylcysteine (NAC; 400 mg/kg) rescued increased cell death observed by TUNEL assays and resulted in a reduction of ALT concentrations in c-junΔli mice to levels of Con A-treated controls (Fig. 5B and data not shown). This finding suggests that oxidative stress contributes to increased hepatic cell death in c-junΔli mice.
Fig. 5.
NO mediates hepatocyte survival during Con A-mediated hepatitis by protecting against hypoxia and oxidative stress. (A) Systemic NO concentrations in serum were assessed indirectly by measuring NO metabolites nitrate and nitrite in untreated mice and 8 h after treatment with Con A or Con A and V-Pyrro/NO (V-P/NO). Data are given as mean ± SEM from n ≥ 4 mice per genotype. *, P ≤ 0.05. (B) Con A-mediated liver toxicity is rescued pharmacologically by hepatocyte-specific NO delivery or antioxidant treatment as assessed by analysis of serum ALT concentrations. Mice were injected either with V-P/NO (n ≥ 7 mice per genotype) or NAC (n = 4 mice per genotype). Data are given as mean ± SEM. §, P ≤ 0.01. (C) Immunohistochemistry demonstrating increased hypoxia in livers from c-junΔli mice treated with Con A. (Scale bars: 200 μm.) (D) Expression of hypoxia-inducible genes was assessed by qPCR and is given as mean ± SD from n = 4 livers per genotype. (E) Schematic model of c-Jun/AP-1-dependent functions during acute hepatitis. c-Jun mediates hepatocyte survival by regulating the expression of nos2, subsequent release of NO, and protection of the liver against ischemia/hypoxia and oxidative stress (ROS). Thus, c-Jun antagonizes the cell death-promoting functions of JNK and acts synergistically with NF-κB, which, among other functions, negatively regulates JNK activity and promotes hepatocyte survival.
The finding that cell death during Con A-mediated hepatitis occurs primarily in the subcapsular, i.e., the least well perfused regions, suggested that necrosis could arise as a consequence of ischemia with subsequent hypoxia. Con A indeed caused increased hepatic hypoxia and expression of hypoxia-induced genes in c-junΔli mice that was reversible upon injection of V-Pyrro/NO (Fig. 5 C and D). Hypoxia in c-junΔli mice was most prominent in the subcapsular areas and intermediate zones of the lobules, and this distribution correlated with necrosis at later time points (Fig. 2D and SI Fig. 9). These findings indicate that c-Jun-dependent release of NO mediates hepatocyte survival by protecting the liver from hypoxia and oxidative stress (Fig. 5E).
Discussion
The transcription factor AP-1 is a major regulator of cell death and survival (7), although its functions during acute liver injury are largely unknown. Here we show that hepatocytes strongly express c-Jun in response to acute liver injury in humans, suggesting a link between c-Jun expression and hepatic stress responses. Employing a mouse model of T cell and TNFα-dependent hepatitis revealed that c-Jun-dependent expression of Nos2 is an essential mediator of hepatocyte survival. We reported previously that c-Jun promoted hepatocyte survival during chemical carcinogenesis or TNFα treatment in vitro by antagonizing the proapoptotic functions of p53 (15). However, increased cell death observed in c-junΔli mice upon Con A treatment was not rescued by additional inactivation of p53, suggesting that the interaction of c-Jun and p53 is not functional in vivo during acute hepatitis.
Nos2 is the major source of NO during inflammation and is strongly expressed in hepatocytes (19, 20). Genetic and pharmacological approaches have revealed both protective and toxic effects of NO and have suggested that its functions greatly depend on NO concentrations and the underlying cell injury (18). Increased toxicity of Con A in c-junΔli mice correlated with impaired nos2 expression, reduced NO production, and was rescued by liver-specific NO delivery using V-Pyrro/NO. These findings are consistent with genetic evidence that Nos2 protects primary hepatocytes against TNFα in vitro and antagonizes cytokine-mediated liver cell death after partial hepatectomy (21, 22). Moreover, NO-releasing agents such as V-Pyrro/NO have been shown to protect against liver injury induced by LPS/galactosamine (23) and display antiapoptotic functions even at very high concentrations (18). It is intriguing that nos2−/− mice are protected against Con A-mediated hepatitis because the inverse phenotype would have been predicted (24). However, TNFα production is strongly impaired in Con A-treated nos2−/− mice, suggesting that reduced toxicity is likely caused by an attenuated inflammatory response rather than by direct effects of NO on hepatocyte survival. Expression of Nos2 is mainly controlled at the transcriptional level, and the human and murine nos2 promoters contain several binding sites for AP-1 and NF-κB (25, 26). Both AP-1 and NF-κB have been shown to be required for efficient nos2 transcription in vitro (27, 28). Here we demonstrate that c-Jun binds to the nos2 promoter in vivo and is required for efficient expression of nos2 during Con A- or LPS-mediated hepatitis, thus identifying c-Jun as an essential modulator of nos2 transcription in vivo. Previous studies indicated that NF-κB significantly contributes to nos2 expression in the liver (21, 29), although it may be dispensable in specific situations (30). Our data suggest that hepatic NF-κB activity was increased in c-junΔli mice most likely as a result of increased necrosis, implying that reduced nos2 expression was not caused by impaired activation of NF-κB.
Several mechanisms can mediate the effects of NO in the liver (18). NO substantially improves liver microcirculation (31) and may antagonize the microcirculatory disturbance and subsequent ischemia/hypoxia observed in Con A-mediated hepatitis. Hypoxia and expression of hypoxia-inducible genes were consistently increased in the liver of Con A-treated c-junΔli mice and rescued by V-Pyrro/NO, thus underlining the importance of maintaining the hepatic microcirculation during acute liver failure. NO may also protect hepatocytes against oxidative stress, an essential mediator of hepatocyte damage during hepatitis (18, 32). Because increased cell death in c-junΔli mice was rescued by coadministration of the antioxidant NAC, we propose that the hepatoprotective functions of c-Jun during acute hepatitis are partly mediated by protection against oxidative stress.
In another model of liver injury induced by LPS, c-Jun also regulates nos2 expression, although LPS did not induce significant cell death, suggesting that the protective functions of c-Jun and Nos2 are determined by the inflammatory stimulus. Moreover, the protective functions of c-Jun were independent of JNK. Nos2 expression and the severity of Con A-mediated liver damage were not altered in c-junAA/AA mice, indicating that JNK and c-Jun have rather distinct functions in the liver.
In conclusion, we propose a pathway for how c-Jun mediates hepatocyte survival during acute hepatitis (Fig. 5E). Inflammatory stimuli such as TNFα activate c-Jun and JNK. In contrast to JNK, which mediates hepatocyte death, c-Jun mediates hepatocyte survival by regulating the expression of nos2, subsequent release of NO, and protection of the liver against hypoxia and oxidative stress. If substantial cell death occurs, c-Jun is also likely required for inducing compensatory liver regeneration. These findings suggest that c-Jun, in concert with NF-κB, functionally antagonizes the cell death-promoting functions of JNK. Because c-Jun and Nos2 are strongly expressed during acute and chronic liver diseases (33, 34), these data provide a rationale for further exploring the use of hepatocyte-specific NO delivery to treat fulminant liver injury of inflammatory or toxic origin. It is very likely that our findings will be relevant for other diseases in which c-Jun and Nos2 are expressed, such as inflammatory bowel disease and cancer (8, 35).
Materials and Methods
Human Tissues.
Human liver biopsies were obtained from the biobank of the Institute of Pathology at the Medical University of Graz (Genome-Austria Tissue Bank). The use of the samples has been approved by the local ethical committee.
Animals.
c-junf/f mice were crossed with transgenic mice expressing either Alfp-Cre or Mx-Cre to obtain mice lacking c-Jun in the liver (13). Mx-Cre c-junf/f mice were injected twice with poly(I·C) at least 1 week before the experiment (13 mg/kg i.p.; Amersham Biosciences, Piscataway, NJ). c-junAA/AA and p53f/f mice have been described (10, 36). Mice were kept on mixed genetic backgrounds (C57BL/6 × 129/Sv × FVB/N for Alfp-Cre c-junf/f and Alfp-Cre c-junf/f p53f/f mice; C57BL/6 × 129/Sv for Mx-Cre c-junf/f and c-junAA/AA mice). Littermates were used as controls.
Liver Injury Models.
Seven- to 12-week-old mice were injected with Con A i.v. (10–15 mg/kg; Sigma, St. Louis, MO). V-Pyrro/NO (Cayman, Ann Arbor, MI) was administered s.c. at 10 mg/kg before Con A injection and then every 2 h until mice were killed. NAC (Sigma; 400 mg/kg) and LPS (Escherichia coli O55:B5, 100 mg/kg; Sigma) were injected i.p. Animal experiments were performed in accordance with local and institutional regulations.
Histology and Immunohistochemistry and TUNEL Assay.
Livers were fixed in 4% neutral buffered formaldehyde at 4°C and embedded in paraffin. The area of necrotic liver tissue was measured histologically on H&E-stained sections by using ImageJ software (National Institutes of Health, Bethesda, MD). Immunohistochemistry was performed with antibodies for c-Jun (CS9165; Cell Signaling, Danvers, MA), Nos2 (AB5382; Chemicon, Temecula, CA), phospho-JNK (V793B; Promega, Madison, WI), or p65 (SC109; Santa Cruz Biotechnology, Santa Cruz, CA). TUNEL staining was performed by using the in situ cell death detection kit (Roche, Indianapolis, IN). Hypoxia was detected by using the Hypoxyprobe kit (Chemicon).
RNase Protection Assay (RPA) and Quantitative PCR (qPCR).
Total RNA was isolated by using TRIzol (Invitrogen, Carlsbad, CA). RPA was performed by using the Riboquant multiprobe RPA systems mFos/Jun, mAPO2, and mAPO3 (BD Biosciences Pharmingen, San Diego, CA) or a probe detecting nos2 (Cayman). qPCR was performed with SYBR Green on an Opticon 2 monitor fluorescence thermocycler (MJ Research, Waltham, MA). Loading was normalized to hprt expression, and results are normalized to untreated mice. Primer sequences are available upon request.
Serum Analysis.
Serum ELISA was performed by using Bioplex mouse cytokine arrays (Bio-Rad, Hercules, CA) or DuoSet ELISA kits (R&D, Minneapolis, MN) according to the manufacturers' instructions. Serum ALT concentrations were measured 8 h after Con A injection (Reflotron; Roche). Serum nitrite and nitrate concentrations were analyzed with a colorimetric assay kit (Cayman).
Western Blotting.
Liver lysates were analyzed by JNK kinase assays (Cell Signaling) or Western blotting with antibodies for JNK1/2 (554285; BD Biosciences Pharmingen), p38α (CS9212; Cell Signaling), phospho-p38α (CS9211; Cell Signaling), phospho-ERK (sc7383; Santa Cruz Biotechnology), ERK2 (sc154; Santa Cruz Biotechnology), c-Jun (610327; BD Biosciences, Sparks, MD), Nos2 (610332; BD Biosciences), and actin (Sigma).
Chromatin Immunoprecipitation.
Total liver was isolated 4 h after Con A injection and homogenized in cross-linking buffer containing 1% formaldehyde for 15 min. After quenching with glycine, lysates were sonicated to yield chromatin fragments of 500–1,500 bp as visualized by agarose gel electrophoresis. Lysates were used for subsequent immunoprecipitation with an antibody for c-Jun (610327l; BD Biosciences) or control IgG. Immunoprecipitated chromatin was quantified by subsequent qPCR.
Statistical Analysis.
Data in bar graphs represent mean ± SEM or SD, as indicated. Statistical analysis was performed by using the nonparametric Mann–Whitney test or nondirectional two-tailed Student's t test as appropriate.
Supplementary Material
Acknowledgments
We thank Drs. R. Eferl, M. Sibilia, and members of the Wagner laboratory for critical reading of the manuscript and helpful discussions; A. Berns (The Netherlands Cancer Institute, Amersterdam, The Netherlands) for providing p53f/f mice; and H. Tkadletz for help with preparing the illustrations. The Institute of Molecular Pathology is funded by Boehringer Ingelheim (Ingelheim, Germany), and this work was also supported by the Austrian Industrial Research Promotion Fund. P.H. was supported by a postdoctoral fellowship from the German Research Foundation.
Abbreviations
- ALT
alanine transaminase
- AP-1
activator protein 1
- c-junAA/AA
c-jun lacking both N-terminal phosphorylation sites
- c-junf/f
floxed alleles of c-jun
- c-junΔli
hepatocyte-specific knockout of c-jun
- NAC
N-acetylcysteine
- qPCR
quantitative PCR.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0706272104/DC1.
References
- 1.Streetz K, Leifeld L, Grundmann D, Ramakers J, Eckert K, Spengler U, Brenner D, Manns M, Trautwein C. Gastroenterology. 2000;119:446–460. doi: 10.1053/gast.2000.9364. [DOI] [PubMed] [Google Scholar]
- 2.Tilg H, Diehl AM. N Engl J Med. 2000;343:1467–1476. doi: 10.1056/NEJM200011163432007. [DOI] [PubMed] [Google Scholar]
- 3.Schwabe RF, Brenner DA. Am J Physiol. 2006;290:G583–G589. doi: 10.1152/ajpgi.00422.2005. [DOI] [PubMed] [Google Scholar]
- 4.Tiegs G, Hentschel J, Wendel A. J Clin Invest. 1992;90:196–203. doi: 10.1172/JCI115836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maeda S, Chang L, Li ZW, Luo JL, Leffert H, Karin M. Immunity. 2003;19:725–737. doi: 10.1016/s1074-7613(03)00301-7. [DOI] [PubMed] [Google Scholar]
- 6.Streetz K, Fregien B, Plumpe J, Korber K, Kubicka S, Sass G, Bischoff SC, Manns MP, Tiegs G, Trautwein C. J Immunol. 2001;167:514–523. doi: 10.4049/jimmunol.167.1.514. [DOI] [PubMed] [Google Scholar]
- 7.Shaulian E, Karin M. Nat Cell Biol. 2002;4:E131–E136. doi: 10.1038/ncb0502-e131. [DOI] [PubMed] [Google Scholar]
- 8.Eferl R, Wagner EF. Nat Rev Cancer. 2003;3:859–868. doi: 10.1038/nrc1209. [DOI] [PubMed] [Google Scholar]
- 9.Behrens A, Sabapathy K, Graef I, Cleary M, Crabtree GR, Wagner EF. Proc Natl Acad Sci USA. 2001;98:1769–1774. doi: 10.1073/pnas.98.4.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Behrens A, Sibilia M, Wagner EF. Nat Genet. 1999;21:326–329. doi: 10.1038/6854. [DOI] [PubMed] [Google Scholar]
- 11.Hilberg F, Aguzzi A, Howells N, Wagner EF. Nature. 1993;365:179–181. doi: 10.1038/365179a0. [DOI] [PubMed] [Google Scholar]
- 12.Eferl R, Sibilia M, Hilberg F, Fuchsbichler A, Kufferath I, Guertl B, Zenz R, Wagner EF, Zatloukal K. J Cell Biol. 1999;145:1049–1061. doi: 10.1083/jcb.145.5.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Behrens A, Sibilia M, David JP, Mohle-Steinlein U, Tronche F, Schutz G, Wagner EF. EMBO J. 2002;21:1782–1790. doi: 10.1093/emboj/21.7.1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stepniak E, Ricci R, Eferl R, Sumara G, Sumara I, Rath M, Hui L, Wagner EF. Genes Dev. 2006;20:2306–2314. doi: 10.1101/gad.390506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eferl R, Ricci R, Kenner L, Zenz R, David JP, Rath M, Wagner EF. Cell. 2003;112:181–192. doi: 10.1016/s0092-8674(03)00042-4. [DOI] [PubMed] [Google Scholar]
- 16.Liu H, Lo CR, Czaja MJ. Hepatology. 2002;35:772–778. doi: 10.1053/jhep.2002.32534. [DOI] [PubMed] [Google Scholar]
- 17.Schwabe RF, Uchinami H, Qian T, Bennett BL, Lemasters JJ, Brenner DA. FASEB J. 2004;18:720–722. doi: 10.1096/fj.03-0771fje. [DOI] [PubMed] [Google Scholar]
- 18.Li J, Billiar TR. Am J Physiol. 1999;276:G1069–G1073. doi: 10.1152/ajpgi.1999.276.5.G1069. [DOI] [PubMed] [Google Scholar]
- 19.Lowenstein CJ, Padalko E. J Cell Sci. 2004;117:2865–2867. doi: 10.1242/jcs.01166. [DOI] [PubMed] [Google Scholar]
- 20.Bultinck J, Sips P, Vakaet L, Brouckaert P, Cauwels A. FASEB J. 2006;20:2363–2365. doi: 10.1096/fj.06-5798fje. [DOI] [PubMed] [Google Scholar]
- 21.Hatano E, Bennett BL, Manning AM, Qian T, Lemasters JJ, Brenner DA. Gastroenterology. 2001;120:1251–1262. doi: 10.1053/gast.2001.23239. [DOI] [PubMed] [Google Scholar]
- 22.Rai RM, Lee FYJ, Rosen A, Yang SQ, Lin SQ, Koteishi A, Liew FY, Zaragoza C, Lowenstein CJ, Diehl AM. Proc Natl Acad Sci USA. 1998;95:13829–13834. doi: 10.1073/pnas.95.23.13829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu J, Waalkes MP. Toxicology. 2005;208:289–297. doi: 10.1016/j.tox.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 24.Sass G, Koerber K, Bang R, Guehring H, Tiegs G. J Clin Invest. 2001;107:439–447. doi: 10.1172/JCI10613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chu SC, Marks-Konczalik J, Wu HP, Banks TC, Moss J. Biochem Biophys Res Commun. 1998;248:871–878. doi: 10.1006/bbrc.1998.9062. [DOI] [PubMed] [Google Scholar]
- 26.Xie QW, Whisnant R, Nathan C. J Exp Med. 1993;177:1779–1784. doi: 10.1084/jem.177.6.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marks-Konczalik J, Chu SC, Moss J. J Biol Chem. 1998;273:22201–22208. doi: 10.1074/jbc.273.35.22201. [DOI] [PubMed] [Google Scholar]
- 28.Gough DJ, Sabapathy K, Ko EY, Arthur HA, Schreiber RD, Trapani JA, Clarke CJ, Johnstone RW. J Biol Chem. 2007;282:938–946. doi: 10.1074/jbc.M607674200. [DOI] [PubMed] [Google Scholar]
- 29.Koerber K, Sass G, Kiemer AK, Vollmar AM, Tiegs G. Hepatology. 2002;36:1061–1069. doi: 10.1053/jhep.2002.36155. [DOI] [PubMed] [Google Scholar]
- 30.Beraza N, Ludde T, Assmus U, Roskams T, Vander Borght S, Trautwein C. Gastroenterology. 2007;132:2504–2517. doi: 10.1053/j.gastro.2007.03.045. [DOI] [PubMed] [Google Scholar]
- 31.DeLeve LD, Wang X, Kanel GC, Ito Y, Bethea NW, McCuskey MK, Tokes ZA, Tsai J, McCuskey RS. Hepatology. 2003;38:900–908. doi: 10.1053/jhep.2003.50383. [DOI] [PubMed] [Google Scholar]
- 32.Kamata H, Honda S, Maeda S, Chang L, Hirata H, Karin M. Cell. 2005;120:649–661. doi: 10.1016/j.cell.2004.12.041. [DOI] [PubMed] [Google Scholar]
- 33.Leifeld L, Fielenbach M, Dumoulin FL, Speidel N, Sauerbruch T, Spengler U. J Hepatol. 2002;37:613–619. doi: 10.1016/s0168-8278(02)00271-4. [DOI] [PubMed] [Google Scholar]
- 34.McNaughton L, Puttagunta L, Martinez-Cuesta MA, Kneteman N, Mayers I, Moqbel R, Hamid Q, Radomski MW. Proc Natl Acad Sci USA. 2002;99:17161–17166. doi: 10.1073/pnas.0134112100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hussain SP, Hofseth LJ, Harris CC. Nat Rev Cancer. 2003;3:276–285. doi: 10.1038/nrc1046. [DOI] [PubMed] [Google Scholar]
- 36.Jonkers J, Meuwissen R, van der Gulden H, Peterse H, van der Valk M, Berns A. Nat Genet. 2001;29:418–425. doi: 10.1038/ng747. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.