Abstract
The roles of serine proteases and protease activated receptors have been extensively studied in coagulation, wound healing, inflammation, and neurodegeneration. More recently, serine proteases have been suggested to influence synaptic plasticity. In this context, we examined the role of protease activated receptor 1 (PAR1), which is activated following proteolytic cleavage by thrombin and plasmin, in emotionally-motivated learning. We were particularly interested in PAR1 because its activation enhances the function of NMDA receptors, which are required for some forms of synaptic plasticity. We examined several baseline behavioral measures, including locomotor activity, expression of anxiety-like behavior, motor task acquisition, nociceptive responses, and startle responses in C57Bl/6 mice in which the PAR1 receptor has been genetically deleted. In addition, we evaluated learning and memory in these mice using two memory tasks, passive avoidance and cued fear-conditioning. Whereas locomotion, pain response, startle, and measures of baseline anxiety were largely unaffected by PAR1 removal, PAR1−/− animals showed significant deficits in a passive avoidance task and in cued fear conditioning. These data suggest that PAR1 may play an important role in emotionally-motivated learning.
1. Introduction
Several reports suggest that proteins primarily known for their role in coagulation and neurodegeneration are also important for central nervous system (CNS) function, including stress-induced alterations in learning and memory (Pawlak et al., 2002, 2005; Pang et al., 2004). For example, Pawlak et al. (2005) have demonstrated that a stress-induced decrease in NMDA receptor expression is enhanced by plasmin expression. Mice deficient in the precursor to plasmin, plasminogen or the enzyme that converts plasminogen to plasmin, tissue plasminogen activator (tPA), were protected from this decrement in NMDA receptor expression, as well as the cognitive decline associated with stress (Pawlak et al., 2005). tPA−/− mice also showed deficits in hippocampal learning that were reversed by tPA administration (Pawlak et al., 2002). Both tPA −/− and plasminogen −/− mice showed deficits in conditioned reward (Nagai et al., 2004). Plasmin expression and function are also necessary for activation of brain-derived neurotrophic factor (BDNF) from its propeptide form. Without plasmin, BDNF-dependent late-phase long-term potentiation (LTP), which has been proposed be the molecular correlate of some forms of memory formation, is inhibited (Pang et al., 2004). We have previously demonstrated a critical role for BDNF within the amygdala in the acquisition of emotional learning in vivo (Rattiner et al., 2004a,b; Chhatwal et al 2006), raising the possibility that protease-mediated activation of BDNF may partially underlie these effects. Studies of LTP suggested that removal of tPA, which converts plasminogen to plasmin, can decrease late phase LTP in hippocampal slices (Baranes et al., 1998). These data are consistent with the idea that serine proteases may regulate synaptic plasticity in the CNS.
PAR1 is a member of a family of four G-protein coupled receptors that are activated by proteolytic cleavage of their amino terminus by serine proteases such as thrombin, trypsin, and plasmin. This cleavage reveals a new amino terminus that acts as a tethered ligand to activate the receptor (Macfarlane et al., 2001; Coughlin et al., 2000). Upon activation, PAR1 couples to multiple heterotrimeric G-proteins, including Gαq/11, Gαi/0, and Gα12/13, and their respective intracellular signaling pathways. PAR1 is highly-expressed in the CNS in rodents and humans, with the highest levels of expression seen in astrocytes, as well as dopaminergic neurons in the ventral tegmental area (Weinstein et al., 1995; Niclou et al., 1998; Junge et al., 2004; Hamill et al., 2005; Nicole et al., 2005). Notably, in adult animals, PAR1 is expressed at moderate to high levels in brain regions involved with emotional learning, including hippocampus and amygdala (Striggow et al., 2001). PAR1 has been extensively studied for its role in coagulation and hemostasis (Coughlin, 2000; Macfarlane et al., 2001), as well as in the survival of neurons following ischemic, traumatic, or neurotoxic insults (Gingrich & Traynelis, 2000a; Shibata et al., 2001; Riewald et al., 2002; Suo et al., 2002; Cheng et al., 2003; Junge et al., 2003; Xi et al., 2003; Guo et al., 2004; Olson et al., 2004; Hamill et al., 2005; Nicole et al., 2005). Surprisingly little, however, is known about PAR1’s roles in normal brain function.
Several studies have examined the effects of PAR1 activation on NMDA receptor function (Gingrich et al. 2000b; Lee et al., 2007; Mannaioni et al., 2007). In hippocampal slices, application of both thrombin and plasmin potentiate current responses of CA1 pyramidal cells to localized NMDA application (Gingrich et al., 2000b; Mannaioni et al., 2007). PAR1 activation also can potentiate synaptic NMDA receptor function through a relief of Mg2+ block that appears secondary to spine depolarization (Lee et al., 2007). This potentiation can also be produced by peptides that directly activate PAR1 by mimicking the newly formed N-terminal following cleavage (Natarajan et al., 1995; Hollenberg et al., 1996; Hollenberg et al., 1997). Thrombin potentiation of NMDA receptor function is greatly reduced in PAR1−/− mouse hippocampal neurons (Gingrich et al., 2000b), and plasmin potentiation is blocked by BMS200261, a potent PAR1 antagonist (Mannaioni et al., 2007). In addition, endogenous brain-derived protease plasmin can activate PAR1 (Junge et al., 2003) and potentiate synaptic NMDA receptor function in brain tissue (Mannaioni et al., 2007). Taken together, these data raise the possibility that PAR1 expressed in brain parenchyma may influence learning and memory. To evaluate this possibility, we tested PAR1−/− mice and age-matched control mice in a series of NMDA receptor-dependent learning and memory tasks (Venable and Kelly, 1990; Danysz, 1991; Sharma and Kulkarni, 1991; Gewirtz and Davis, 1997; Lee and Kim, 1998; Schauz and Koch, 2000; Fendt, 2001; Savenko et al., 2003). These experiments were supported by a number of baseline behavioral assays in PAR1−/− and wild-type mice that measured locomotion, anxiety-like behavior, pain processing, and startle responses. The results of these tests demonstrate that PAR1−/− mice show deficits in passive avoidance and conditioned fear learning, raising the idea that PAR1 may be important for emotional learning.
2. Materials and Methods
2.1. Animals
PAR1−/− and wild-type mice were obtained by crossing PAR1+/− mice, a gift from Dr. Shaun Coughlin (University of California, San Francisco, CA; Connolly et al., 1996), with C57BL/6 wild-type mice from Jackson Laboratory (Bar Harbor, ME). A colony of homozygous PAR1−/− and wild-type mice that were >99% C57Bl/6 (>7 back-crossings) were derived from heterozygous breeding pairs. All mice were at least 90 days of age. No overt behavioral phenotype has previously been reported for adult PAR1−/− mice (Connolly et al., 1996). The subjects used for each behavioral test were separately housed male PAR1−/− and age-matched wild-type controls derived from the same PAR1+/− crossings. Mice were housed up to six per cage and maintained in the Emory University School of Medicine Division of Animal Resources. Food and water were available ad libitum. The colony room was maintained on a 12:12 light-dark cycle with lights on at 0700 h. This study was performed in full accordance with “Guide for the Care and Use of Laboratory Animals” (National Academy of Sciences, 1996), and the research protocols were approved by the Institutional Animal Care and Use Committee of Emory University.
2.2. Locomotor Activity Testing
Locomotor activity was measured using eight Accuscan Digiscan Activity Monitors (AccuScan Instruments, Inc., Columbus, OH) with the aid of the VersaMax® software (Version 1.30, Omnitech Instruments Inc; White et al., 2004). Each animal was tested in a 40 × 40 × 30 cm (high) clear acrylic chamber surrounded by a framework of infrared photobeams. Each chamber was individually housed in a ventilated, sound-attenuating cubicle that was illuminated by incandescent light (approximately 45 lux). The infrared photobeams were in a 16 × 16 array around the bottom of the box and 2.5 cm from the floor. Sixteen additional photobeams were mounted 10.5 cm above the bottom photobeams on the left and right sides of the box in order to measure vertical motor activity. Movements were determined by breaks in photobeams and were converted into locomotor activity counts with the aid of VersaDat® software (Version 1.3; AccuScan Instruments Inc.).
On testing days animals were taken from the colony room and moved to the testing room in their home cages. Animals were habituated to the testing room for at least 30 min before they were tested in activity chambers. Basal activity was measured for 1 hour. We analyzed three measures of motor activity provided by automated analysis. Two of these measures and associated behaviors were horizontal activity counts (ambulation) and vertical activity counts (rearing). In addition, the ratio of center distance to total distance traveled was analyzed as an index of emotional reactivity (Crawley, 1999; Harro, 1993; Kalinichev et al., 2000).
2.3. Elevated Plus-Maze
The apparatus (Hamilton-Kinder, Poway, CA) was constructed of black Plexiglas and was approximately 87 cm off the floor. It had four arms (50×10 cm) radiating outward from a central open square (10×10 cm). Two were open-sided runway-style arms, and two arms were enclosed with 40 cm walls. Activity was measured and analyzed using MotorMonitor System and software (Hamilton-Kinder, Poway, CA), which was interfaced with a microcomputer. Movements were determined by breaks in an array of 48 photobeams (24 × 24; 6 cm apart). During the experiment the maze was illuminated by fluorescent lights to 330 lux on the open arms and 315 lux in the central open square as measured by a dual range digital light meter (VWR Scientific, Model 62344–944, Atlanta, GA).
The general testing procedure has been described elsewhere in rats (Kalinichev et al., 2000) and mice (Holmes et al, 2000). Briefly, each mouse was placed in the plus-maze, facing a closed arm, and was allowed to explore freely the plus-maze for 10 min. The system provided automated calculations of several measures of activity. We focused on three measures of activity: the number entries into open arms, the total time spent on open arms, and distance (cm) traveled in the closed arms. Animals were tested once.
2.4. Rotarod
A rotarod apparatus (Columbus Instruments, Columbus, OH) was used to measure motor coordination and balance. Following previously described methods (Crawley, 1999; Olson et al., 2004), mice were trained for 2 days prior to testing. A training session consisted of a 60 s trial on a rod rotating at constant speed of 10 rotations per minute (rpm), followed by a 60 s rest. Animals were then subjected to an open ended time trial on an accelerating rod until they fell (10 rpm plus 0.1 rpm/s). Animals were rested for 5 minutes, and then subjected to the same training session (60 s trial at 10 rpm, 60 s rest, and accelerating open-ended time trial until fall). Training sessions were conducted on two sequential days. On the third test day, a test session was performed that consisted of a 60 s trial at 10 rpm, a 60 s rest period, followed by an open-ended time trial on an rod accelerating from 10 rpm at 0.1 rpm/s until the animal fell from the rod. After a 5 minute rest, this procedure was repeated (60 s 10 rpm, 60 s rest, open ended time trial on accelerating rod until fall). The latency to fall was recorded for both acceleration segments in the experimental session on the third day, and the two scores were averaged.
2.5. Hot Plate and Shock Threshold Tests
To determine potential genotype-related differences in nociception, stimulus intensity-response latency curves were constructed using the hot plate test as described elsewhere (White et al., 2004). The surface of the hot plate (Model 39D, Hot Plate Analgesia Meter; IITC, Inc., Woodland Hills, CA) measured 26.5×29×3 cm and was surrounded by 28.5-cm-high Plexiglas walls and removable cover. The surface temperature of the plate using ranged from 46.0–52.0 ±0.2°C. The stimuli were presented in ascending order of intensity at 30-min intervals. Two response latencies were recorded per stimulus intensity for each subject and averaged. The test was stopped when an animal licked its hind paws, jumped off the surface, or if a response was not made within 30 s (i.e. 30 s cutoff).
As an additional test for nociception, the shock threshold test was used to determine sensitivity to electric shock applied to the foot as measured by the extent of flinching, jumping, and vocalization in response to increasing foot shock intensities (Weeber et al., 2000). Mice were placed in the electrified compartment of the passive avoidance chamber described below and one second long foot shocks were administered from 0-1.5 mA in 0.05 mA increments.
2.6. Prepulse Inhibition
Startle reflexes were measured in eight identical startle response systems (SR-LAB, SDI, San Diego, CA). Each system consisted of a nonrestrictive Plexiglas cylinder, 5.5 cm in diameter and 13 cm long, mounted on a Plexiglas platform located in a ventilated, sound-attenuated chamber. Cylinder movements were detected by a piezoelectric accelerometer mounted under each platform and were digitized and stored by an interfacing computer assembly. Movements were sampled each millisecond (ms) and startle amplitude was defined as the peak accelerometer voltage that occurred during the first 100 ms after the onset of the startle stimulus. Response sensitivities were calibrated (SR-LAB Startle Calibration System) to be nearly identical in each of the eight startle cylinders. Startle, prepulse and background stimuli were presented through a high-frequency speaker located 15 cm above the startle chambers. Stimuli intensities were verified with the microphone of a sound level meter (Radio Shack, #33-2055) placed inside of the cylinder. Stimuli presentation and data acquisition were controlled by a computer using SR-Lab software.
Pre-pulse inhibition (PPI) testing is described in detail in Heldt et al. (2004). Briefly, 12 behaviorally-naive mice (6 per genotype) were placed in startle testing/training chambers (see above), and presented with either startle stimuli alone (110 dB, 50 ms) or startle stimuli preceded by white noise pre-pulses (20 ms) of 2, 4, 8, 10, or 12 dB above a 63 dB white noise background (i.e., 65, 67, 71, 73, or 75dB) with a fixed interval (100 ms) between onsets of the pre-pulse and startle stimulus. Each session began with a 5 min acclimation period followed by the five different trial types presented in random order 15 times, for a total of 75 trials (an additional 15 startle alone trials were embedded within the test). Inter-trial intervals ranged from 20 to 40 s. Startle-amplitudes were calculated using the peak output detected by piezoelectric accelerometers built-in to the startle chambers (San Diego Instruments, San Diego, CA) during the 100 ms following each startle stimulus (sampling rate of 100 Hz).
2.7. Passive Avoidance
The passive avoidance procedure has been previously described (D'Hooge et al., 2005). Briefly, the test apparatus consisted of a standard mouse operant conditioning chamber (18 × 18 × 20 cm) divided into two equal compartments by a guillotine doorway (7.6 × 8.9 cm opening). One compartment was brightly lit (ambient light) with clear Plexiglas walls and an open top, while the other compartment was dark with black walls and a black lid. The floor consisted of metal bars spaced 0.5 cm apart. The dark side floor was electrified. During training, mice were placed in the bright side of the apparatus facing away from the doorway. The duration of time before entry into the dark side of the box (entry latency) was then recorded. When all four feet crossed the threshold, the guillotine door was closed, confining the mouse in the dark side of the apparatus. Approximately seven seconds after the door was closed an electrical stimulus was delivered (0.7 mA, 1 s) using an A-M Systems Isolated Pulse Stimulator (Model 2100; Sequim, WA). The subject was then removed from the apparatus. Subjects were tested 1 day later using the same procedure with a 300 s cut-off.
2.8. Conditioned Freezing
Similar to standard protocols (Anagnostaras et al. 2000; Jones et al., 2005; Heldt et al., 2007), male wild-type and PAR1−/− mice were trained in eight identical startle response systems (SR-LAB, SDI, San Diego, CA). Each system consisted of a nonrestrictive Plexiglas cylinder, 5.5 cm in diameter and 13 cm long, mounted on a Plexiglas platform located in a ventilated, sound-attenuated chamber. The tone conditioned stimulus (CS) was a 70-dB SPL, 6-kHz tone generated by a Tektronix function generator audio oscillator (Model CFG253, Beaverton, OR) and was delivered through a high frequency speaker (Motorola, Model 948) located 13 cm above each cylinder. The tone CS was 30 s in duration. The unconditioned stimulus (US) was a scrambled shock generated by programmable constant current shock generators (SDI, San Diego, CA) located outside the isolation chambers. Footshock intensity was 0.7mA. Shock levels were verified by using a 1 kOhm resistor across the bars of the shock grids and measuring the voltage drop between the bars to calculate the constant current. The chamber ventilation fans produced a 55 dB white-noise background. Stimuli presentation and data acquisition during training were controlled by an IBM PC-compatible computer using SR-Lab software. Mice were trained using five 30 s, 6 kHz tones co-terminating with 500 ms, 0.7 mA foot shocks separated by a five min inter-trial interval. During fear acquisition, cylinder movements were sampled each millisecond (ms) by a piezoelectric accelerometer mounted under each platform.
Mice were tested in a different, rectangular plexiglass chamber in a separate room to minimize the contribution of learned fear to the training chamber (i.e., contextual fear). They were tested for conditioned freezing behavior in standard rodent modular test chambers (ENV-008-VP; Med Associates Inc. Georgia, VT) with an inside area of 30.5 cm (L) × 24.1cm (W) × 21.0 cm (H). The tone conditioned stimulus (CS) was a 30 s, 6-kHz tone, delivered through a high frequency speaker (Motorola, Model 948) attached to side of each chamber. Stimuli presentations were controlled by an IBM PC-compatible computer using MED-PC® IV, software interfaced to chambers. Conditioned freezing responses were recorded manually with video cameras mounted in front of each conditioning apparatus. Testing consisted of 3–5 30 s 6 kHz tones with 2 min inter-trial intervals. Mice were tested 1 day following training. Conditioned freezing to the tone was assessed manually by measuring the percent of time spent motionless during the 30 s tones presented during the videotaped testing session. The data shown is the average freezing across all of the tone trials in a given test.
2.9. Statistical Analysis
All behavioral tests were performed blind. Wild-type versus PAR1−/− comparisons for each behavioral test were performed using unpaired Student’s t-test, with p-values ≤ 0.05 considered significant. All values shown are mean ± SEM. In the passive avoidance experimentation, 3 of 39 animals tested were excluded from analysis because they fell greater than two standard deviations from the mean.
3. Results
3.1. Locomotor Activity
Age-matched C57Bl/6 male wild-type (n=7) and PAR1−/− (n=6) mice were tested to assess differences in locomotion and exploratory behavior in an open field. There were no significant genotype differences in total distance traveled (Fig. 1A; p>0.05 at each time point) or in ratio of center distance to total distance (Fig. 1B; p>0.05 at each time point). In addition, wild-type and PAR1−/− mice displayed similar vertical activity counts (data not shown). These data suggest that male PAR1−/− mice show normal exploratory and locomotor behavior.
Figure 1. Wild-type and PAR1−/− mice display similar locomotor activity and exploratory behavior.

Behavior was assessed during a one hour exposure to the activity monitoring box. Age-matched wild-type and PAR1−/−mice derived from the same initial breedings traveled similar distances in the open field (A), and spent similar amounts of time in the center as measured by the ratio of center to total distance (B). Each data point represents the mean ± SEM (WT n=7; PAR1−/− n=6).
3.2. Elevated Plus Maze
To investigate potential differences in anxiety-like behavior, male wild-type (n=11) and PAR1 −/− (n=10) mice were tested in the elevated plus maze. Both PAR1−/− and wild-type mice spent similar amounts of time in the open arms (Fig. 2A; p>0.05) and made similar numbers of entries (Fig. 2B; p>0.05). The total distances traveled in the closed arms by the PAR1−/− and wild-type mice was not significantly different (Fig. 2C; p>0.05), consistent with the open field locomotor activity (Fig 1). These data indicate that PAR1 deletion had no effect on anxiety-like behavior.
Figure 2. Wild-type and PAR1−/− mice display similar performances in the elevated plus-maze.
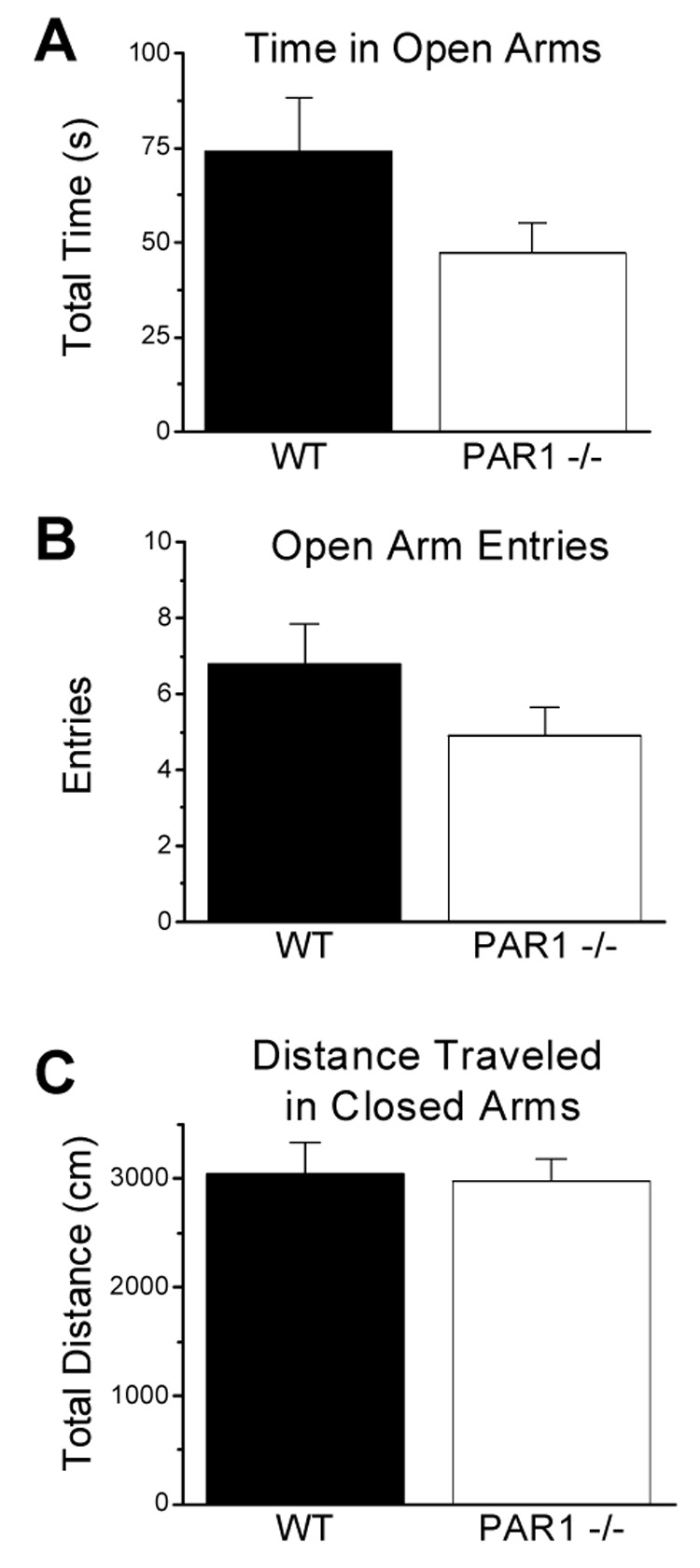
Mice were allowed to freely explore an elevated plus-maze for 10 min. There were no genotype differences in total time spent in the open arms (A), number of open arm entries (B), or total distance traveled in the closed arms (C). Data are presented as mean ± SEM (WT n=11; PAR1−/− n=10).
3.3. Rotarod
Motor coordination, motor learning, and balance in wild-type and PAR1−/− mice were assessed using the rotarod task. Male mice were trained for two days before testing (see Methods), and tested on two consecutive trials using an accelerating rod with a five minute rest period. There were no significant differences observed in task acquisition during initial exposure to the rotarod apparatus (Fig. 3A; p>0.05, n=16 for each genotype). There were also no significant differences in motor learning as wild-type and PAR1−/− mice showed similar fall latencies when tested on day three after training (Fig. 3B; p>0.05). These data suggest that PAR1−/− mice do not show overt differences from wild-type mice in either motor learning or balance.
Figure 3. Wild-type and PAR1−/− mice show similar rotarod task acquisition or motor learning.
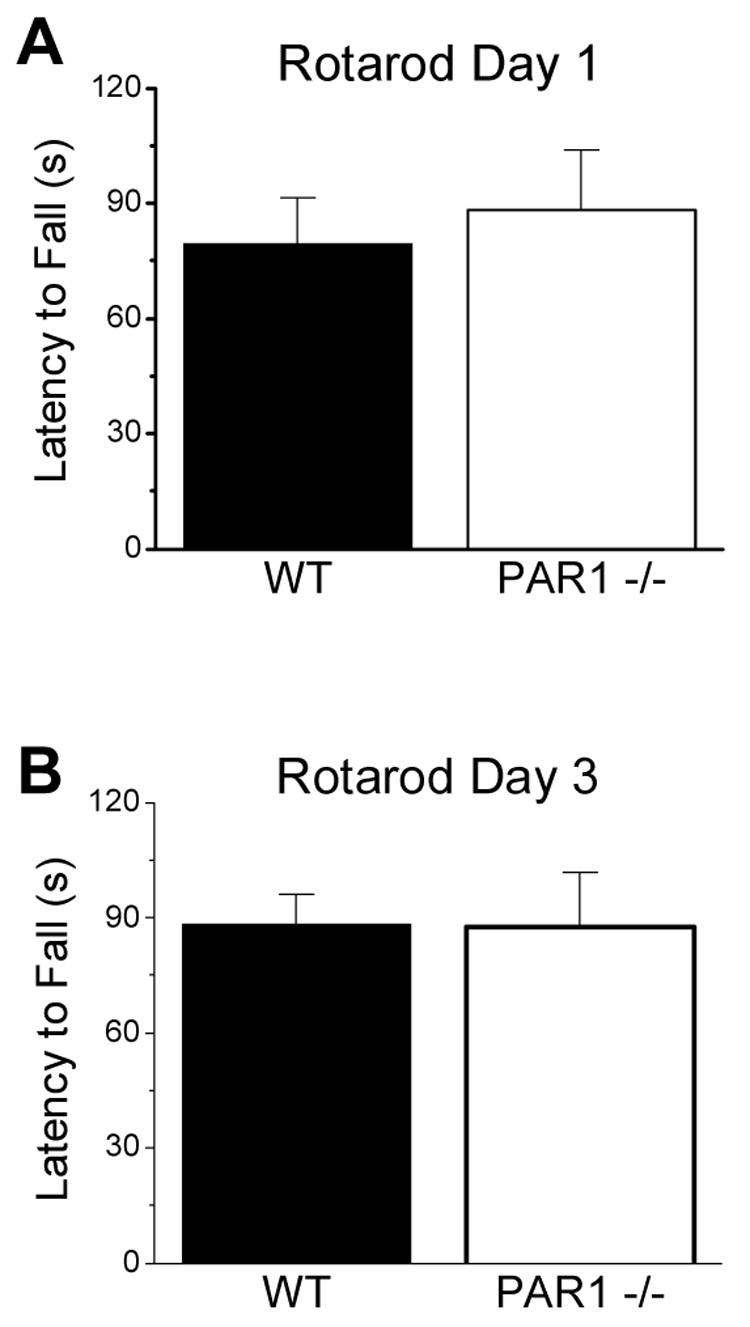
Mice were tested for motor coordination, balance, and motor learning on a rotarod apparatus. No significant genotype differences in rotarod performance were observed in the initial exposure to the rotarod (A), nor when tested again 3 days later (B). Data are presented as mean ± SEM (n=16 per genotype).
3.4. Nociceptive Responses
To evaluate whether PAR1−/− mice showed altered nociceptive responses, we tested the sensitivity of male wild-type and PAR1 −/− mice to the hot plate and shock threshold (Fig. 4). Stimulus intensity-response latency curves generated for each genotype revealed consistently longer latencies for the response of PAR1−/− mice to thermal stimulus across the temperatures tested, however differences were not statistically significant (data not shown). At 52°C, the highest temperature tested, there was no significant difference in response latency between wild-type (n=11) and PAR1−/− mice (n=12; Fig. 4A; p>0.05). In the shock threshold test, male wild-type and PAR1−/− (Fig. 5B; n=12 for each genotype) displayed similar sensitivities to stimulus intensities required to elicit flinching, jumping, and vocalization (p>0.05 for each response). Even though PAR1 is expressed in sensory neurons (Vergnolle et al., 2003; Zhu et al., 2005), these data suggest that PAR1−/− animals do not show detectable differences in nociceptive thresholds for thermal stimulation or electrical shock.
Figure 4. PAR-1 −/− mice show no deficits in nociceptive responses to heat or electrical shock.

(A) Both wild-type (n=11) and PAR1−/− (n=12) mice exhibited similar latencies to hind-paw licking at 52 °C using conventional hot plate test. (B) Wild-type and PAR1−/− mice also displayed similar thresholds for flinching, jumping and vocalizing in tests of the electrical shock threshold (n=12 per genotype). Data are presented as mean ± SEM.
Figure 5. PAR1−/− mice show significant deficits in the passive avoidance test.
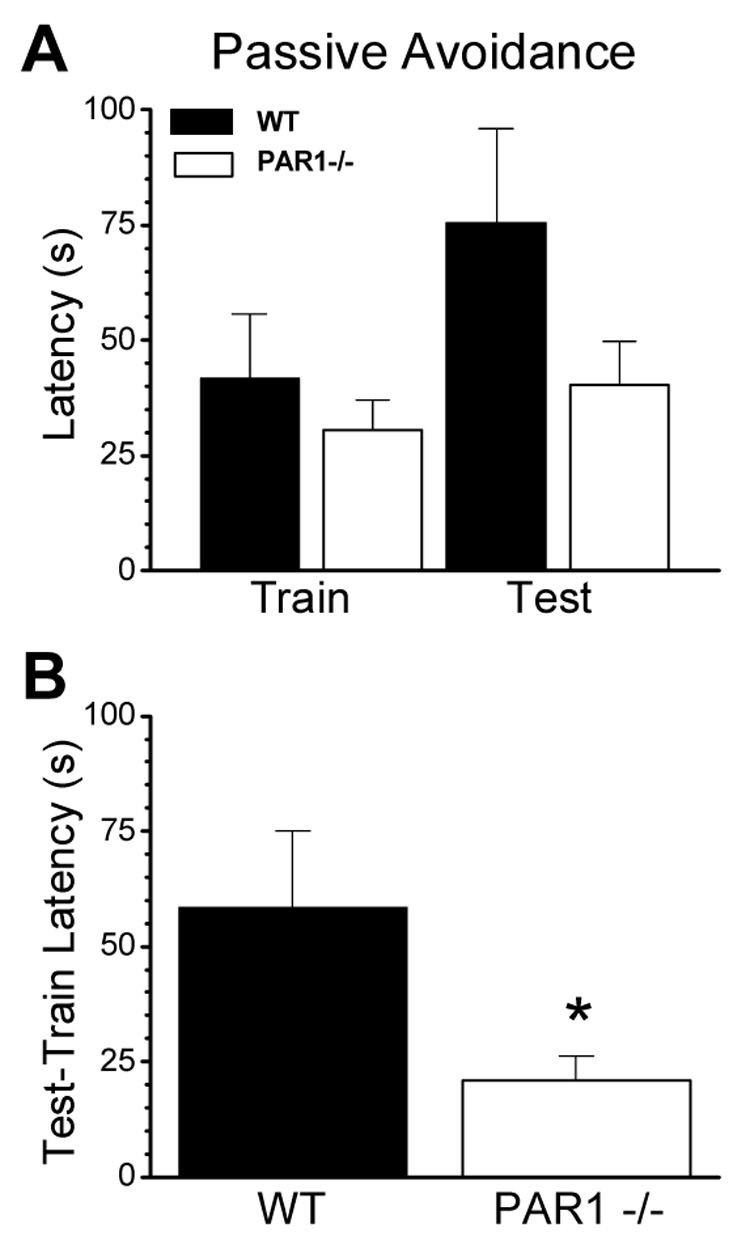
(A) No significant differences were observed between wild-type (n=17) and PAR1−/− (n=19) mice in escape latencies during the training session for passive avoidance. (B) When tested 24 h later, PAR1−/− mice entered the dark compartment much earlier than their wild-type counterparts, as measured as a difference in train and test latencies (p=0.03, unpaired t-test). Data are presented as mean ± SEM.
3.5. Passive Avoidance
A passive avoidance paradigm was used as a test of learning and memory in age-matched adult male wild-type and PAR1−/− mice. Mice were trained in a dual chamber apparatus with an electrified grid underlying the darkened portion of the cage. A remotely operated door controlled access, and animals were trained to avoid the dark side of the apparatus by receiving an electrical shock 7 secs after they entered the dark side of the cage, which triggered door closing (see Methods). The latency to entering the dark side of the testing apparatus was measured 1 day after the training session. There were no significant differences observed for intial latencies between wild-type (n=17) and PAR1−/− mice (n=19; Fig. 5A; p>0.05). When tested one day later, PAR1−/− mice exhibited a passive avoidance deficit as measured by a reduction in latency to enter the dark side between first training day and the second test day (Fig. 5B; t(34)=2.26; p=0.03).
3.6. Pre-pulse inhibition and conditioned freezing
Pre-pulse inhibition is a measure of sensorimotor gating, which is in place to prevent sensory overload. Pre-pulse inhibition to acoustic startle is modulated by several brain regions including the hippocampus, the prefrontal cortex, and the amygdala (Geyer, 1996; Geyer et al., 2002). Age-matched male wild-type and PAR1−/− mice showed no difference in this model at any of the pulses tested (Fig. 6A; p>0.05 at each prepulse stimulus intensity). Further, there was also no significant difference between wild-type or PAR1−/− mice in their response to pulse alone, indicating their baseline startle is the same (p>0.05; n=6 mice per group).
Figure 6. PAR1−/− male mice show significant deficits in fear-conditioned freezing.
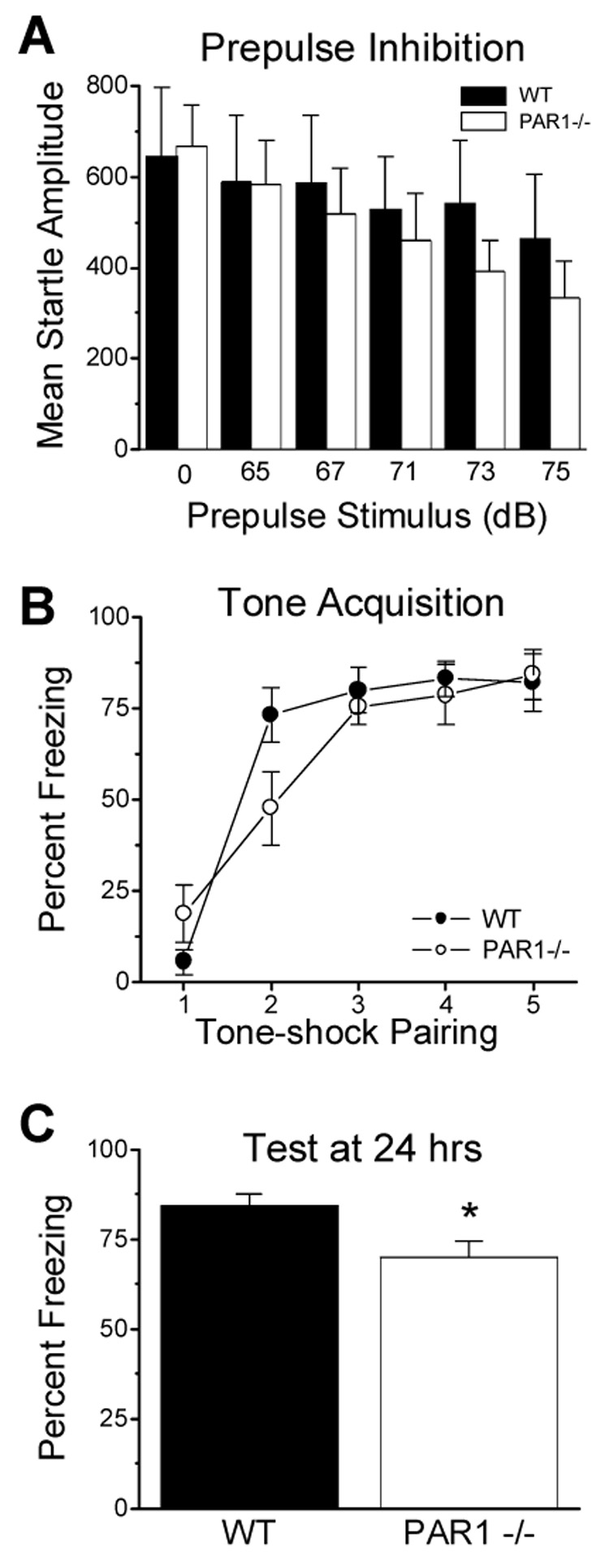
(A) Wild-type and PAR1−/− mice (n=6 per genotype) showed similar baseline startle and prepulse inhibition responses (n=6 per genotype; p>0.05 for each prepulse stimulus, unpaired t-test). (B), Wild-type and PAR1−/− mice also acquired similar freezing responses by the end of training. (C) When tested 1 day after training, PAR1−/− mice displayed significantly reduced freezing compared to wild-type mice (p=0.02; unpaired t-test; WT n=11, PAR1−/− n=12). Data are presented as mean ± SEM.
For conditioned freezing, mice were trained to associate a 6 kHz tone with a mild foot shock (see Methods). During initial training, PAR1−/− mice were slower to associate the tone with the impending shock (Fig. 6B). However, by the third tone-shock pairing, PAR1−/− mice and wild-type mice showed the same level of freezing in response to the tone. Wild-type mice showed 80±6.3% pre-shock freezing, and PAR1−/− mice showed 76±4.9% pre-shock freezing, which continued through rest of the training, indicating that both genotypes acquired similar freezing responses by the end of training. At 24 hours following conditioning, the wild-type mice continued to exhibit considerable freezing in response to the tone as measured by percent freezing during tone (Fig. 6C, 85±3.2%, n=11). The PAR1−/− mice, however, showed significantly reduced freezing (70 ± 4.4%; t(21)=2.61; p=0.017; n=12).
4. Discussion
The most important finding of this study is that PAR1−/− animals showed significant deficits in two tests of behavioral learning, passive avoidance and conditioned fear learning. Notably, although both of these measures are emotionally-motivated, we found a more robust effect with the passive avoidance measure of fear compared to the cued-freezing measure of fear. In tests of baseline behavior, PAR1−/− mice did not appear to show detectable deficits in normal exploratory behavior, anxiety levels, locomotion, or motor coordination. PAR1−/− mice also were not different than their wild-type counterparts in their ability to acquire the rotarod task, and displayed similar nociceptive responses to heat and electrical shock. Furthermore, the baseline startle in the pre-pulse inhibition was the same between the genotypes. Together, these data suggest that there is not a global difference in pain perception but that there are more specific deficits in learning and memory. Additionally, since neither wild-type nor PAR1−/− mice showed freezing at the full 100% level during any of the testing periods, the relative freezing deficit in the PAR1−/− mice does not appear to be due to a potential ceiling effect of over-learning in the wild-type animals. Finally, the animals’ similar pain thresholds, startle responses, baseline anxiety, and locomotor activity would suggest that these non-associative processes do not account for the cued-freezing and passive-avoidance deficits. Thus, we interpret the data showing that PAR1−/− mice have deficits in tests of emotionally-motivated learning to suggest that PAR1 has a direct role in aversive memory formation in mice. This conclusion supports that idea that PAR1 has important functions in the formation of memories in addition to its previously suggested roles in CNS neurodegeneration following insult or blood-brain barrier breakdown (Gingrich & Traynelis, 2000; Xi et al., 2003).
One important action of PAR1 in the CNS involves its ability to enhance synaptic NMDA receptor function (Gingrich et al., 2000; Lee et al., 2007; Mannaioni et al., 2007). Because NMDA receptors are Ca2+ permeable and are well known to be involved in cellular processes underlying learning and memory (Lisman, 2003; Nicoll, 2003), we propose that the ability of PAR1 to regulate NMDA receptor function could be linked to changes in learning and memory. NMDA receptor activation has been shown to be critical in both of the described learning paradigms (for review see Walker and Davis, 2002). Administration of NMDA receptor antagonists during passive avoidance training inhibits the ability of mice to learn to associate the dark side of the box with a shock (Venable and Kelly, 1990; Danysz, 1991; Sharma and Kulkarni, 1991). Similarly, NMDA antagonists inhibit associative learning to a conditioned cue (i.e. a tone or a light) with an unconditioned aversive stimulus such as a shock (Gewirtz and Davis, 1997; Lee and Kim, 1998; Schauz and Koch, 2000; Fendt, 2001; Savenko et al., 2003). One interpretation of the data described here is that the effects observed in PAR1−/− mice are due to decreased NMDA receptor signaling. However, since NMDA receptor function has been shown to be normal in PAR1 −/− animals (Gingrich et al., 2000; Lee et al., 2007), another interpretation of the data is that potentiation of NMDA receptors by activation of PAR1 is important for learning and memory. Furthermore, both PAR1 (Striggow et al., 2001), and NMDA receptor subunits (Ishii et al., 1993) are highly expressed in brain regions involved with emotional learning, including hippocampus and amygdala. These results it well with suggestions that serine proteases can impact learning (Baranes et al., 1998; Pawlak et al., 2002; Nagai et al., 2004, 2006; Pang et al., 2004; Pawlak et al., 2005), and may suggest that PAR1 is a substrate at which serine proteases such as plasmin exert their actions.
One important caveat is the use of a PAR1−/− mouse in these studies, which lacks PAR1 throughout its life. Although no neurological phenotype has been previously described for this animal, it is possible that the lack of PAR1 throughout development could result in subtle changes in neuronal connections or processing that lead to changes in learning and memory. Several reports have hinted that PAR1 may have roles in CNS development (Gurwitz and Cunningham, 1988; Debeir et al., 1998), although compelling examples of PAR1 driven neuronal development do not exist. Alternatively, it is possible that the effects of PAR1 in adult learning and memory are actually more robust than is seen in these experiments. Although we find significant deficits in passive avoidance and conditioned freezing measures of fear learning, there is not a complete behavioral deficit. It is possible that the developmental loss of PAR1 has allowed for compensatory molecular alterations which prevent the full expression of PAR1−/− deficits on adult learning and memory. A wide range of genes might potentially compensate for a PAR1 deficit, including other protease receptors as well as modifiers of downstream signaling. Future studies of the role of PAR1 in learning and memory will need to utilize pharmacological means of blocking PAR1 or an inducible PAR1 −/− mouse.
Combined with previously described effects of PAR1 on glutamatergic transmission, the most parsimonious interpretation of our current results is that PAR1 is important in memory formation. These data show a very important, yet somewhat specific, role of PAR1 in normal brain function. Moreover, these data provide a working hypothesis for the role of PAR1 in the CNS that yields a number of testable predictions. In particular, experimentation on the cellular and molecular basis of learning and memory should provide further clarification for the hypothesized role of protease activated receptors in behavior.
Acknowledgements
This work was supported by NIH-NINDS (NS39419 SFT, NS530062 CEH), NARSAD (SFT), NIMH (MH069884 KJR, MH070218 JPC, MH 57014 JDS). We thank Dr. Scott Heldt for his help on the pre-pulse inhibition experiments, Kimberly Haustein for excellent technical assistance, and Dr. Stephen G. Holtzman for use of his laboratory space and equipment during completion of these studies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anagnostaras SG, Josselyn SA, Frankland PW, Silva AJ. Computer-assisted behavioral assessment of Pavlovian fear conditioning in mice. Learning and Memory. 2000;7:58–72. doi: 10.1101/lm.7.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranes D, Lederfein D, Huang YY, Chen M, Bailey CH, Kandel ER. Tissue plasminogen activator contributes to the late phase of LTP and to synaptic growth in the hippocampal mossy fiber pathway. Neuron. 1998;21:813–825. doi: 10.1016/s0896-6273(00)80597-8. [DOI] [PubMed] [Google Scholar]
- Chhatwal J, Stanek-Rattiner L, Davis M, Ressler KJ. Amygdala BDNF signaling is required for consolidation but not encoding of extinction. Nat Neurosci. 2006;9:870–872. doi: 10.1038/nn1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng T, Liu D, Griffin JH, Fernandez JA, Castellino F, Rosen ED, Fukudome K, Zlokovic BV. Activated protein C blocks p53-mediated apoptosis in ischemic human brain endothelium and is neuroprotective. Nat. Med. 2003;9:338–342. doi: 10.1038/nm826. [DOI] [PubMed] [Google Scholar]
- Connolly AJ, Ishihara H, Kahn ML, Farese RV, Coughlin SR. Role of the thrombin receptor in development and evidence for a second receptor. Nature. 1997;381:516–519. doi: 10.1038/381516a0. [DOI] [PubMed] [Google Scholar]
- Coughlin SR. Thrombin signalling and protease-activated receptors. Nature. 2000;407:258–264. doi: 10.1038/35025229. [DOI] [PubMed] [Google Scholar]
- Crawley JN. Behavioral phenotyping of transgenic and knockout mice: experimental design and evaluation of general health, sensory functions, motor abilities, and specific behavioral tests. Brain Res. 1999;835:18–26. doi: 10.1016/s0006-8993(98)01258-x. [DOI] [PubMed] [Google Scholar]
- Danysz W. Metaphit fails to antagonize PCP-induced passive avoidance deficit. Pharmacol. Biochem. Behav. 1991;38:231–233. doi: 10.1016/0091-3057(91)90618-c. [DOI] [PubMed] [Google Scholar]
- Debeir T, Benavides J, Vige X. Involvement of proteinase activated receptor-1 in the in vitro development of mesencephalic dopaminergic neurons. Neuroscience. 1996;82:739–752. doi: 10.1016/s0306-4522(97)00317-5. [DOI] [PubMed] [Google Scholar]
- D'Hooge R, Lullmann-Rauch R, Beckers T, Balschun D, Schwake M, Reiss K, von Figura K, Saftig P. Neurocognitive and psychotiform behavioral alterations and enhanced hippocampal long-term potentiation in transgenic mice displaying neuropathological features of human alpha-mannosidosis. J.Neurosci. 2005;25:6539–6549. doi: 10.1523/JNEUROSCI.0283-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fendt M. Injections of the NMDA receptor antagonist aminophosphonopentanoic acid into the lateral nucleus of the amygdala block the expression of fear-potentiated startle and freezing. J. Neurosci. 2001;21:4111–4115. doi: 10.1523/JNEUROSCI.21-11-04111.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gewirtz JC, Davis M. Second-order fear conditioning prevented by blocking NMDA receptors in amygdala. Nature. 1997;388:471–474. doi: 10.1038/41325. [DOI] [PubMed] [Google Scholar]
- Geyer MA. Serotonergic functions in arousal and motor activity. Behav. Brain Res. 1996;73:31–35. doi: 10.1016/0166-4328(96)00065-4. [DOI] [PubMed] [Google Scholar]
- Geyer MA, McIlwain KL, Paylor R. Mouse genetic models for prepulse inhibition: an early review. Mol. Psychiatry. 2002;7:1039–1053. doi: 10.1038/sj.mp.4001159. [DOI] [PubMed] [Google Scholar]
- Gingrich MB, Traynelis SF. Serine proteases and brain damage -- is there a link? Tr. Neurosci. 2000;23:399–407. doi: 10.1016/s0166-2236(00)01617-9. [DOI] [PubMed] [Google Scholar]
- Gingrich MB, Junge CE, Lyuboslavsky P, Traynelis SF. Potentiation of NMDA receptor function by the serine protease thrombin. J. Neurosci. 2000;20:4582–4595. doi: 10.1523/JNEUROSCI.20-12-04582.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Liu D, Gelbard H, Cheng T, Insalaco R, Fernandez JA, Griffin JH, Zlokovic BV. Activated protein C prevents neuronal apoptosis via protease activated receptors 1 and 3. Neuron. 2004;41:563–572. doi: 10.1016/s0896-6273(04)00019-4. [DOI] [PubMed] [Google Scholar]
- Gurwitz D, Cunningham DD. Thrombin modulates and reverses neuroblastoma neurite outgrowth. Proc. Natl. Acad. Sci. USA. 1988;85:3440–3444. doi: 10.1073/pnas.85.10.3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill CE, Goldshmidt A, Nicole O, McKeon RJ, Brat DJ, Traynelis SF. Glial reactivity after damage: Implication for scar formation and neuronal recovery. Clinical Neurosurgery. 2005;52:29–44. [PubMed] [Google Scholar]
- Harro J. Measurement of exploratory behavior in rodents. In: Conn PM, editor. Paradigms for the Measurement of Behavior. San Diego: Academic Press; 1993. pp. 359–377. [Google Scholar]
- Heldt S, Green A, Ressler KJ. Prepulse inhibition deficits in GAD65 knockout mice and the effect of antipsychotic treatment. Neuropsychopharmacology. 2004;29:1610–1619. doi: 10.1038/sj.npp.1300468. [DOI] [PubMed] [Google Scholar]
- Heldt SA, Stanek L, Chhatwal JP, Ressler KJ. Hippocampus-specific deletion of BDNF in adult mice impairs spatial memory and extinction of aversive memories. Mol Psychiatry. 2007 doi: 10.1038/sj.mp.4001957. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenberg MD, Mokashi S, Leblond L, DiMaio J. Synergistic actions of a thrombin-derived synthetic peptide and a thrombin receptor-activating peptide in stimulating fibroblast mitogenesis. J. Cell. Physiol. 1996;169:491–496. doi: 10.1002/(SICI)1097-4652(199612)169:3<491::AID-JCP9>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Hollenberg MD, Saifeddine M, al-Ani B, Kawabata A. Proteinase-activated receptors: structural requirements for activity, receptor cross-reactivity, and receptor selectivity of receptor-activating peptides. Can. J. Physiol. Pharmacol. 1997;75:832–841. [PubMed] [Google Scholar]
- Holmes A, Parmigiani S, Ferrari PF, Palanza P, Rodgers RJ. Behavioral profile of wild mice in the elevated plus-maze test for anxiety. Physiol. Behav. 2000;71:509–516. doi: 10.1016/s0031-9384(00)00373-5. [DOI] [PubMed] [Google Scholar]
- Ishii T, Moriyoshi K, Sugihara H, Sakurada K, Kadotani H, Yokoi M, Akazawa C, Shigemoto R, Mizuno N, Masu M, Nakanishi S. Molecular characterization of the family of the N-methyl-D-aspartate receptor subunits. J Biol Chem. 1993;268(4):2836–2843. [PubMed] [Google Scholar]
- Jones S, Heldt S, Davis M, Ressler KJ. Olfactory-mediated fear conditioning in mice: Simultaneous measurements of fear-potentiated startle and freezing. Behavioral Neuroscience. 2005;119:329–335. doi: 10.1037/0735-7044.119.1.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junge CE, Sugawara T, Mannaioni G, Alagarsamy S, Conn PJ, Brat DJ, Chan PH, Traynelis SF. The contribution of protease-activated receptor 1 to neuronal damage caused by transient focal cerebral ischemia. Proc. Natl. Acad. Sci. USA. 2003;100:13019–13024. doi: 10.1073/pnas.2235594100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junge CE, Lee CJ, Hubbard KB, Zhang Z, Olson JJ, Hepler JR, Brat DJ, Traynelis SF. Protease-activated receptor-1 in human brain: localization and functional expression in astrocytes. Exp. Neurol. 2004;188:94–103. doi: 10.1016/j.expneurol.2004.02.018. [DOI] [PubMed] [Google Scholar]
- Kalinichev M, Easterling KW, Holtzman SG. Periodic postpartum separation from the offspring results in long- lasting changes in anxiety-related behaviors and sensitivity to morphine in Long-Evans mother rats. Psychopharmacology (Berl) 2000;152:431–439. doi: 10.1007/s002130000556. [DOI] [PubMed] [Google Scholar]
- Kawao N, Ikeda H, Kitano T, Kuroda R, Sekiguchi F, Kataoka K, Kamanaka Y, Kawabata A. Modulation of capsaicin-evoked visceral pain and referred hyperalgesia by protease-activated receptors 1 and 2. J. Pharmacol. Sci. 2004;94:277–285. doi: 10.1254/jphs.94.277. [DOI] [PubMed] [Google Scholar]
- Lee CJ, Mannaioni G, Yuan H, Woo DH, Gingrich MB, Hamill CE, Traynelis SF. Astrocytic control of synaptic NMDA receptors. J. Physiology. 2007 doi: 10.1113/jphysiol.2007.130377. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Kim JJ. Amygdalar NMDA receptors are critical for new fear learning in previously fear-conditioned rats. J. Neurosci. 1998;18:8444–8454. doi: 10.1523/JNEUROSCI.18-20-08444.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J. Long-term potentiation: outstanding questions and attempted synthesis. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2003;358:829–842. doi: 10.1098/rstb.2002.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macfarlane SR, Seatter MJ, Kanke T, Hunter GD, Plevin R. Proteinase-activated receptors. Pharmacol. Rev. 2001;53:245–282. [PubMed] [Google Scholar]
- Mannaioni G, Goldshmidt A, Hamill CE, Yuan H, Hubbard KB, Junge CE, Lee CJ, Yepes M, Hepler JR, Traynelis SF. Plasmin potentiates synaptic NMDA receptor function in hippocampal neurons through activation of PAR1. 2007 doi: 10.1074/jbc.M803015200. Manuscript submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai T, Yamada K, Yoshimura M, Ishikawa K, Miyamoto Y, Hashimoto K, Noda Y, Nitta A, Nabeshima T. The tissue plasminogen activator-plasmin system participates in the rewarding effect of morphine by regulating dopamine release. Proc. Natl. Acad. Sci. USA. 2004;101:3650–3655. doi: 10.1073/pnas.0306587101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai T, Ito M, Nakamichi N, Mizoguchi H, Kamei H, Fukakusa A, Nabeshima T, Takuma K, Yamada K. The rewards of nicotine: regulation by tissue plasminogen activator-plasmin system through protease activated receptor-1. J. Neurosci. 2006;26:12374–12383. doi: 10.1523/JNEUROSCI.3139-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan S, Riexinger D, Peluso M, Seiler SM. 'Tethered ligand' derived pentapeptide agonists of thrombin receptor: a study of side chain requirements for human platelet activation and GTPase stimulation. Int. J. Pept. Protein Res. 1995;45:145–151. doi: 10.1111/j.1399-3011.1995.tb01033.x. [DOI] [PubMed] [Google Scholar]
- Niclou SP, Suidan HS, Pavlik A, Vejsada R, Monard D. Changes in the expression of protease-activated receptor 1 and protease nexin-1 mRNA during rat nervous system development and after nerve lesion. Eur. J. Neurosci. 1998;10:1590–1607. doi: 10.1046/j.1460-9568.1998.00183.x. [DOI] [PubMed] [Google Scholar]
- Nicole O, Goldshmidt A, Hamill CE, Sorensen SD, Sastre A, Lyuboslavsky P, Hepler JR, McKeon RJ, Traynelis SF. Activation of protease-activated receptor-1 triggers astrogliosis after brain injury. J. Neurosci. 2005;25:4319–4329. doi: 10.1523/JNEUROSCI.5200-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll RA. Expression mechanisms underlying long-term potentiation: a postsynaptic view. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2003;358:721–726. doi: 10.1098/rstb.2002.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson EE, Lyuboslavsky P, Traynelis SF, McKeon RJ. PAR-1 deficiency protects against neuronal damage and neurologic deficits after unilateral cerebral hypoxia/ischemia. J. Cereb. Blood Flow Metab. 2004;24:964–971. doi: 10.1097/01.WCB.0000128266.87474.BF. [DOI] [PubMed] [Google Scholar]
- Pang PT, Teng HK, Zaitsev E, Woo NT, Sakata K, Zhen S, Teng KK, Yung WH, Hempstead BL, Lu B. Cleavage of proBDNF by tPA/plasmin is essential for long-term hippocampal plasticity. Science. 2004;306:487–491. doi: 10.1126/science.1100135. [DOI] [PubMed] [Google Scholar]
- Pawlak R, Nagai N, Urano T, Napiorkowska-Pawlak D, Ihara H, Takada Y, Collen D, Takada A. Rapid, specific and active site-catalyzed effect of tissue plasminogen activator on hippocampus-dependent learning in mice. Neuroscience. 2002;113:995–1001. doi: 10.1016/s0306-4522(02)00166-5. [DOI] [PubMed] [Google Scholar]
- Pawlak R, Rao BS, Melchor JP, Chattarji S, McEwen B, Strickland S. Tissue plasminogen activator and plasminogen mediate stress-induced decline of neuronal and cognitive functions in the mouse hippocampus. Proc. Natl. Acad. Sci. USA. 2005;102:18201–18206. doi: 10.1073/pnas.0509232102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattiner LS, Davis M, French CT, Ressler KJ. BDNF and the TrkB Receptor are Involved in Amygdala-Dependent Learning. J Neuroscience. 2004a;24:4796–4806. doi: 10.1523/JNEUROSCI.5654-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattiner LS, Davis M, Ressler KJ. Differential Regulation of Brain-Derived Neurotrophic Factor Transcripts during the Consolidation of Fear Learning. Learning and Memory. 2004b;11:727–731. doi: 10.1101/lm.83304. [DOI] [PubMed] [Google Scholar]
- Riewald M, Petrovan RJ, Donner A, Mueller BM, Ruf W. Activation of endothelial cell protease activated receptor 1 by the protein C pathway. Science. 2002;296:1880–1882. doi: 10.1126/science.1071699. [DOI] [PubMed] [Google Scholar]
- Savonenko A, Werka T, Nikolaev E, Zielinski K, Kaczmarek L. Complex effects of NMDA receptor antagonist APV in the basolateral amygdala on acquisition of two way avoidance reaction and long-term fear memory. Learning and Memory. 2003;10:293–303. doi: 10.1101/lm.58803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauz C, Koch M. Blockade of NMDA receptors in the amygdala prevents latent inhibition of fear-conditioning. Learning and Memory. 2000;7:393–399. doi: 10.1101/lm.33800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma AC, Kulkarni SK. Effects of MK-801 and ketamine on short-term memory deficits in passive avoidance step-down task paradigm in mice. Methods Find. Exp. Clin. Pharmacol. 1991;13:155–159. [PubMed] [Google Scholar]
- Shibata M, Kumar SR, Amar A, Fernandez JA, Hofman F, Griffin JH, Zlokovic BV. Anti-inflammatory, antithrombotic, and neuroprotective effects of activated protein C in a murine model of focal ischemic stroke. Circulation. 2001;103:1799–1805. doi: 10.1161/01.cir.103.13.1799. [DOI] [PubMed] [Google Scholar]
- Striggow F, Riek-Burchardt M, Kiesel A, Schmidt W, Henrich-Noack P, Breder J, Krug M, Reymann KG, Reiser G. Four different types of protease-activated receptors are widely expressed in the brain and are up-regulated in hippocampus by severe ischemia. Eur J Neurosci. 2001;14(4):595–608. doi: 10.1046/j.0953-816x.2001.01676.x. [DOI] [PubMed] [Google Scholar]
- Suo Z, Wu M, Ameenuddin S, Anderson HE, Zoloty JE, Citron BA, Andrade-Gordon P, Festoff BW. Participation of protease-activated receptor-1 in thrombin-induced microglial activation. J. Neurochem. 2002;80:655–666. doi: 10.1046/j.0022-3042.2001.00745.x. [DOI] [PubMed] [Google Scholar]
- Venable N, Kelly PH. Effects of NMDA receptor antagonists on passive avoidance learning and retrieval in rats and mice. Psychopharmacology (Berl) 1990;100:215–221. doi: 10.1007/BF02244409. [DOI] [PubMed] [Google Scholar]
- Vergnolle N, Ferazzini M, D’Andrea MR, Buddenkotte J, Steinhoff M. Proteinase-activated receptors: novel signals for peripheral nerves. Tr Neurosci. 2003;26:496–500. doi: 10.1016/S0166-2236(03)00208-X. [DOI] [PubMed] [Google Scholar]
- Walker DL, Davis M. The role of amygdala glutamate receptors in fear learning, fear-potentiated startle, and extinction. Pharmacol. Biochem. Behav. 2002;71:379–392. doi: 10.1016/s0091-3057(01)00698-0. [DOI] [PubMed] [Google Scholar]
- Weeber EJ, Atkins CM, Selcher JC, Varga AW, Mirnikjoo B, Paylor R, Leitges M, Sweatt JD. A role for the β isoform of protein kinase C in fear conditioning. J. Neurosci. 2000;20:5906–5914. doi: 10.1523/JNEUROSCI.20-16-05906.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein JR, Gold SJ, Cunningham DD, Gall CM. Cellular localization of thrombin receptor mRNA in rat brain: expression by mesencephalic dopaminergic neurons and codistribution with prothrombin mRNA. J. Neurosci. 1995;15:2906–2919. doi: 10.1523/JNEUROSCI.15-04-02906.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White DA, Kalinichev M, Holtzman SG. Individual differences in locomotor reactivity to a novel environment and sensitivity to opioid drugs in the rat. II. Agonist-induced antinociception and antagonist-induced suppression of fluid consumption. Psychopharmacology (Berl) 2004;177:68–78. doi: 10.1007/s00213-004-1921-8. [DOI] [PubMed] [Google Scholar]
- Xi G, Reiser G, Keep RF. The role of thrombin and thrombin receptors in ischemic, hemorrhagic and traumatic brain injury: deletrious or protective? J. Neurochem. 2003;84:3–9. doi: 10.1046/j.1471-4159.2003.01268.x. [DOI] [PubMed] [Google Scholar]
- Zhu W-J, Yamanaka H, Obata K, Dai Y, Kobayashi K, Kozai T, Tokunaga A, Noguchi K. Expression of mRNA for four subtypes of the proteinase-activated receptor in rat dorsal root ganglia. Brain Research. 2005;1041:205–211. doi: 10.1016/j.brainres.2005.02.018. [DOI] [PubMed] [Google Scholar]


