Abstract
We describe here a new component of the phosphatidylinositol 3-kinase/Akt signaling pathway that directly impacts mitochondria. Akt (protein kinase B) was shown for the first time to be localized in mitochondria, where it was found to reside in the matrix and the inner and outer membranes, and the level of mitochondrial Akt was very dynamically regulated. Stimulation of a variety of cell types with insulin-like growth factor-1, insulin, or stress (induced by heat shock), induced translocation of Akt to the mitochondria within only several minutes of stimulation, causing increases of nearly eight- to 12-fold, and the mitochondrial Akt was in its phosphorylated, active state. Two mitochondrial proteins were identified to be phosphorylated following stimulation of mitochondrial Akt, the β-subunit of ATP synthase and glycogen synthase kinase-3β. The finding that mitochondrial glycogen synthase kinase-3β was rapidly and substantially modified by Ser9 phosphorylation, which inhibits its activity, following translocation of Akt to the mitochondria is the first evidence for a regulatory mechanism affecting mitochondrial glycogen synthase kinase-3β. These results demonstrate that signals emanating from plasma membrane receptors or generated by stress rapidly modulate Akt and glycogen synthase kinase-3β in mitochondria.
Keywords: Akt, ATP synthase, glycogen synthase kinase-3β, insulin-like growth factor-1, mitochondria, phosphatidylinositol 3-kinase
The serine/threonine kinase Akt (protein kinase B) plays a vital role in many cellular processes such as proliferation and survival (Lawlor and Alessi 2001). Akt can be activated by many types of stimuli (Datta et al. 1999), including growth factors, such as insulin-like growth factor-1 (IGF-1), hormones, such as insulin, and stressors, such as heat shock. Akt is most widely associated with the phosphatidylinositol 3-kinase (PI3K) signaling pathway where activation of Akt commences after PI3K catalyzes the production of phosphatidylinositol 3,4-bisphosphate and phosphatidylinositol 3,4,5-trisphosphate (Vanhaesebroeck and Alessi 2000). These lipids recruit Akt from the cytosol to the plasma membrane to facilitate the phosphorylation of Akt on Thr308 and Ser473 by phosphoinositide-dependent kinases (Datta et al. 1999; Vanhaesebroeck and Alessi 2000; Lawlor and Alessi 2001). The subsequent release of activated Akt from the membrane allows it to phosphorylate numerous substrates in the cytosol, and activated Akt also translocates into the nucleus (Meier et al. 1997; Borgatti et al. 2000; Brami-Cherrier et al. 2002).
One of the first identified substrates of Akt was glycogen synthase kinase-3β (GSK3β) (Cross et al. 1995). GSK3β, like Akt, affects many fundamental cellular functions, such as metabolism, survival, gene expression, and cytoskeletal dynamics, because of its ability to phosphorylate key proteins governing these processes (Grimes and Jope 2001). GSK3β is generally considered to be a constitutively active enzyme that is predominantly maintained in the cytosol, but both its activity and its intracellular location are subject to dynamic regulation by signaling processes. The activity of GSK3β is regulated by phosphorylation, often achieved by signaling pathways that activate Akt, which phosphorylates Ser9 of GSK3β, inhibiting its activity (Cross et al. 1995). Reduced signaling through Akt can elevate the activity of GSK3β through loss of this inhibitory phosphorylation, with one of the consequences being increased susceptibility to apoptosis-mediated cell death (Pap and Cooper 1998; Bijur et al. 2000; Crowder and Freeman 2000; Hetman et al. 2000). In addition to phosphorylation, the actions of GSK3β also are regulated by its intracellular localization, which has a prominent role in determining the availability of substrates accessible for phosphorylation. Thus it is notable that dynamic changes in the level of GSK3β in the nucleus occur during the cell cycle (Diehl et al. 1998) and with changes in the activity of Akt, with increased levels of active GSK3β in the nucleus found in conditions of diminished Akt activity (Bijur and Jope 2001).
Although much is known about signaling systems that regulate Akt in the cytosol and nucleus, and subsequently GSK3β, nearly nothing is known about these enzymes in mitochondria. This is a surprising situation considering the importance of mitochondria in signaling that regulates cell proliferation and apoptosis. In addition to their classical role as the major site of energy production in aerobic cells, mitochondria harbor proteins that are central to processes that regulate cell survival and death. Apparently there have been no studies of Akt in mitochondria and only a single report which identified the presence of GSK3β in mitochondria (Hoshi et al. 1996). Consequently, it is unknown if Akt is present in mitochondria or if Akt regulates GSK3β in mitochondria.
Given the important functions of both Akt and GSK3β we examined if signals impacting Akt were directed to mitochondria. The results show that mitochondria contain a pool of Akt that is rapidly and robustly modulated by intracellular signaling activities, and that downstream of Akt the β-subunit of ATP synthase and GSK3β were phosphorylated by Akt in mitochondria.
Materials and methods
Cell culture and treatments
SH-SY5Y human neuroblastoma cells and HEK293 human embryonic kidney cells were grown in continuous culture RPMI media containing 10% horse serum, 5% Fetal Clone II (Hyclone, Logan, UT, USA), 2 mm l-glutamine, 100 units/mL penicillin, and 100 µg/mL streptomycin. 3T3L1 rat adipocytes were grown in Dulbecco’s modified Eagle’s medium containing 10% fetal bovine serum (Invitrogen, Carlsbad, CA, USA), 2 mm l-glutamine, 100 units/mL penicillin, and 100 µg/mL streptomycin. For serum withdrawal, adherent cells were rinsed twice with serum-free media supplemented with 2 mm l-glutamine, 100 units/mL penicillin, and 100 µg/mL streptomycin. SH-SY5Y cells were grown in serum-free media for 24 h and HEK293 cells for 48 h prior to treatment with 50 ng/mL IGF-1 (Serologicals, Purchase, NY, USA). 3T3L1 adipocytes were grown in serum-free media for 24 h prior to treatment with 100 nm insulin. For heat shock treatment, 100-mm tissue culture plates containing cells in serum-free media were sealed with Parafilm and floated in a 45°C water bath for 15 min with gentle agitation. Where indicated, cells were pre-treated with 40 nm wortmannin or 20 µm LY294002 (Alexis, San Diego, CA, USA) 30 min prior to IGF-1 treatment.
Isolation and purification of mitochondria
All steps were carried out at 0–4°C and are modifications of previously published procedures (Gottlieb and Adachi 2000). Mitochondria were isolated from cells cultured in 100-mm plates by washing the adherent cells with phosphate-buffered saline (PBS) and adding 1 mL of cavitation buffer (5 mm HEPES, pH 7.4, 3 mm MgCl2, 1 mm EGTA, 250 mm sucrose) containing protease and phosphatase inhibitors (1 mm sodium orthovanadate, 50 mm sodium fluoride, 100 µm phenylmethylsulfonyl fluoride, 10 µg/mL leupeptin, 10 µg/mL aprotinin, 5 µg/mL pepstatin A, and 1 nm okadaic acid). The adherent cells were harvested and homogenized by nitrogen cavitation (200 p.s.i., for 5 min) in a cell disruption bomb (Parr Instrument Co., Moline, IL, USA). The cell homogenate was centrifuged twice at 500 g for 5 min to remove unbroken cells and nuclei. The crude mitochondria-containing supernatant was layered over a 1 m/1.5 m discontinuous sucrose gradient containing protease and phosphatase inhibitors and centrifuged at 100 000 g (Beckman SW50.1 rotor) for 1 h. A 100-µL aliquot from the uppermost layer of the supernatant was removed and saved as the cytosolic fraction. Mitochondria in a diffuse white band between the 1 m and 1.5 m sucrose layers were transferred to a 1.5-mL microcentrifuge tube and diluted 1 : 1 (v/v) in dilution buffer (5 mm HEPES, pH 7.4, 3 mm MgCl2, 1 mm EGTA, containing protease and phosphatase inhibitors). After gentle mixing the mitochondria were centrifuged at 20 800 g for 20 min. The supernatant was discarded and the mitochondrial pellet was gently washed three times (20 800 g for 5 min) in cavitation buffer. Mitochondrial extracts were obtained by incubating mitochondria for 30 min at 4°C in lysis buffer (20 mm Tris, pH 7.5, 0.2% Nonidet P-40, 150 mm NaCl, 2 mm EDTA, 2 mm EGTA, and protease and phosphatase inhibitors). The mitochondrial extract was clarified by centrifugation at 20 800 g for 10 min. Protein concentrations were determined by the bicinchoninic acid method (Pierce).
For trypsin digestion, purified cytosol and mitochondria (100 µg protein) were treated with 200 µg/mL trypsin in 200 µL of cavitation buffer for 20 min at 4°C. To stop the reaction a cocktail of protease inhibitors, which also contained 500 µg/mL soybean trypsin inhibitor, was added to the reaction mixture. The mitochondria were centrifuged at 20 800 g for 5 min and mitochondrial extracts were obtained by incubating mitochondria for 30 min at 4°C in lysis buffer containing protease and phosphatase inhibitors.
Subfractionation of mitochondria
Mitochondria were subfractionated by using a phosphate swelling-shrinking method described previously (Hovius et al. 1990; Milon et al. 2000), with minor modifications. Briefiy, purified mitochondria were subjected to swelling by the addition of swelling buffer (10 mm KH2PO4, pH 7.4, containing protease and phosphatase inhibitors) at a concentration of 253 µg mitochondrial protein/mL, and incubated for 20 min at 4°C with gentle mixing. An equal volume of shrinking buffer (10 mm KH2PO4, pH 7.4, 32% sucrose, 30% glycerol, 10 mm MgCl2, and protease and phosphatase inhibitors) was added and samples were incubated for an additional 20 min at 4°C. The suspension was centrifuged at 10 000 g for 10 min, yielding a supernatant containing outer membrane and the intermembrane-space fractions, which was saved as mixture 1, and a pellet comprised of mitoplasts. The mitoplasts were washed three times in the 1 : 1 mixture of swelling and shrinking buffer, then resuspended in 500 µL of swelling buffer and sonicated twice at 40 watts for 15 s with a 1-min interval. This suspension containing the inner membrane and matrix fractions was saved as mixture 2. Mixtures 1 and 2 were centrifuged at 150 000 g (Beckman 50Ti rotor) for 1 h. The supernatant from mixture 1 was saved as the intermembrane space fraction and the supernatant from mixture 2 was saved as the matrix fraction. Proteins from the intermembrane space fraction were concentrated using a Centriplus 10 filter concentrator (Amicon) and proteins from the matrix fraction were concentrated using a refrigerated centrifugal concentrator (Savant). The outer membrane pellet in mixture 1 and the inner membrane pellet in mixture 2 were washed once in swelling buffer and centrifuged at 150 000 g for 1 h. The outer and inner membrane pellets were solubilized in RIPA buffer [25 mm Tris, pH 8.2, 50 mm NaCl, 0.5% NP40, 0.5% deoxycholate, 0.1% sodium dodecyl sulfate (SDS), and protease and phosphatase inhibitors]. Protein concentrations were determined by the bicinchoninic acid method.
Immunoblotting
Extracts were mixed with Laemmli sample buffer (2% SDS), placed in a boiling water bath for 5 min, and proteins were resolved in SDS-polyacrylamide gels [8% for tubulin, Akt, GSK-3β, and ATP synthase β-subunit immunoblots, 10% for histone, Mn-superoxide dismutase, and porin immunoblots, 12% for cytochrome oxidase, cytochrome c, and Tom20 immunoblots, and 4–15% gradient gel for phospho-Akt substrate (PAS) antibody immunoblots], transferred to nitrocellulose, and incubated with GSK3β, Tom20, cytochrome c (BD-PharMingen/Transduction Laboratories, San Diego, CA, USA); phospho-Ser9-GSK3β, phospho-Ser473-Akt, phospho-Thr308-Akt, total Akt, PAS (Cell Signaling, Beverly, MA, USA); porin (Oncogene, San Diego, CA, USA); cytochrome oxidase subunit IV, ATP synthase β-subunit (Molecular Probes, Eugene, OR, USA); Mn-superoxide dismutase (Calbiochem, San Diego, CA, USA); histone protein (Chemicon, Temecula, CA, USA); or β-tubulin III (Sigma, St. Louis, MO) antibodies. Immunoblots were developed using horseradish peroxidase-conjugated goat antimouse, or goat anti-rabbit IgG, followed by detection with enhanced chemiluminescence, and the protein bands were quantitated with a densitometer.
Matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS)
Proteins immunoprecipitated with the PAS antibody from purified mitochondria were analyzed by mass spectrometry. Purified mitochondrial extract (3.4 mg protein) prepared from SH-SY5Y cells treated with 50 ng/mL IGF-1 for 15 min was pre-cleared with 100 µL of protein A sepharose for 2 h at 4°C. The extract was centrifuged and the supernatant was incubated with 14.5 µg of PAS antibody for 18 h at 4°C, followed by incubation with 100 µL protein A-sepharose for 2 h at 4°C. The immobilized immune complexes were washed three times with lysis buffer, mixed with 60 µL of Laemmli sample buffer, and placed in a boiling waterbath for 5 min. Proteins were separated in a 4–15% gradient SDS—polyacrylamide gel. The gel was stained with colloidal Coomassie Blue (Invitrogen). The bands were excised and proteins were digested overnight with trypsin. Extracted peptides were mixed with the matrix α-cyano-4-hydroxycinammic acid (Aldrich), spotted onto a MALDI plate, and analyzed with a Voyager DePro mass spectrometer (Applied Biosystems) and the NCBI database was searched to identify proteins.
Immunoprecipitation
To immunoprecipitate ATP synthase β-subunit with the PAS antibody, 100 µg protein from purified mitochondria (1 µg/µL) was pre-cleared with 25 µL 50% protein A-sepharose for 2 h at 4°C. The extract was centrifuged, the supernatant was incubated with 0.43 µg of PAS antibody for 18 h at 4°C, and then incubated with 25 µL protein A-sepharose for 2 h at 4°C. The immobilized immune complex was washed three times with lysis buffer, mixed with 30 µL of Laemmli sample buffer, and placed in a boiling waterbath for 5 min. The proteins were separated in an 8% SDS—polyacrylamide gel, transferred to nitrocellulose, and the membrane was incubated with ATP synthase β-subunit antibody. The immunoblot was developed using horseradish peroxidase-conjugated goat antimouse light chain followed by detection with enhanced chemiluminescence.
Results
Akt is localized in mitochondria
To determine if mitochondria contain Akt under normal growth conditions, purified mitochondrial, cytosolic, and nuclear fractions were prepared from SH-SY5Y cells maintained in serum-containing media. The level of Akt in the mitochondria was compared to levels in the cytosol and nucleus, compartments previously reported to contain Akt (Meier et al. 1997; Borgatti et al. 2000; Brami-Cherrier et al. 2002). Immunoblots of equal amounts of protein from each fraction revealed that Akt was predominantly cytosolic (Fig. 1a), but Akt also was detected in the mitochondria. Nuclear Akt levels were very low. Complete separation of the cytosolic, nuclear, and mitochondrial preparations was confirmed by immunoblotting each fraction for tubulin, a cytosolic protein, histone, a nuclear protein, and cytochrome oxidase subunit IV, a mitochondrial protein. Tubulin and histone were not detected in the mitochondrial fraction (Fig. 1b), verifying purification of mitochondria from the cytosolic and nuclear fractions.
Fig. 1.
Distribution of Akt in the cytosol, nucleus and mitochondria. SH-SY5Y cells maintained in serum-containing media were fractionated into cytosolic (C), nuclear (N), and mitochondrial (M) fractions as described in ‘Materials and methods’. (a) Fractions (5 µg protein) were immunoblotted for Akt. (b) To verify separation of the cytosolic, nuclear, and mitochondrial fractions, extracts from each fraction were immunoblotted for tubulin, histone, and subunit IV of cytochrome oxidase (COX IV) and over-exposed immunoblots are shown to verify separation of each fraction. (c) Mitochondria were subfractionated into the outer membrane (OM), intermembrane space (IMS), inner membrane (IM) and matrix (Mx) fractions, and these were immunoblotted for the compartment-specific proteins porin, cytochrome c, COX IV, and Mn-superoxide dismutase (MnSOD), respectively, and for Akt. The non-specific band in the porin immunoblot provides verification of equal loading of protein in each lane and immunoblots of marker proteins were overexposed to verify fractionation procedures. (d) Purified intact mitochondria or cytosolic preparations were subjected to trypsin digestion as described in ‘Materials and methods’. Mitochondrial extracts were immunoblotted for Akt and Tom20, then stripped and reprobed with COX IV antibody. The cytosol fraction was immunoblotted for Akt to verify that this procedure was adequate to completely proteolyze Akt.
To identify the compartmentalization of Akt within mitochondria, purified mitochondria were subfractionated to obtain preparations containing proteins from the outer membrane, intermembrane space, inner membrane, and matrix. Separation of each mitochondrial compartment was verified by immunoblotting the subfractions for mitochondrial compartment-specific proteins, as described by Milon et al. (2000), using antibodies for porin, a protein found in the outer membrane and at outer membrane-inner membrane contact sites, cytochrome c, a intermembrane space protein, cytochrome oxidase subunit IV, a inner membrane protein, and Mn-superoxide dismutase, a matrix protein. Akt was found predominantly in the mitochondrial membrane fractions and to a lesser degree in the matrix (Fig. 1c).
Trypsin digestion was used to further verify that Akt was located within mitochondria and not attached to the external mitochondrial surface. Proteins on the outer surface of mitochondria are accessible to trypsin digestion, whereas proteins embedded within mitochondria are not proteolyzed. Trypsin treatment did not decrease the amount of Akt in the mitochondrial fraction (Fig. 1d), whereas Akt in the cytosolic fraction was completely proteolyzed by the same treatment. Treatment of mitochondria with trypsin completely proteolyzed Tom20, a mitochondrial import receptor which resides on the outer surface of the mitochondria (Gotow et al. 2000), but did not proteolyze the inner mitochondrial membrane-associated protein cytochrome oxidase subunit IV. This data indicates that Akt is located within mitochondria and is not merely attached to the outer surface.
Activated Akt accumulates in the mitochondria
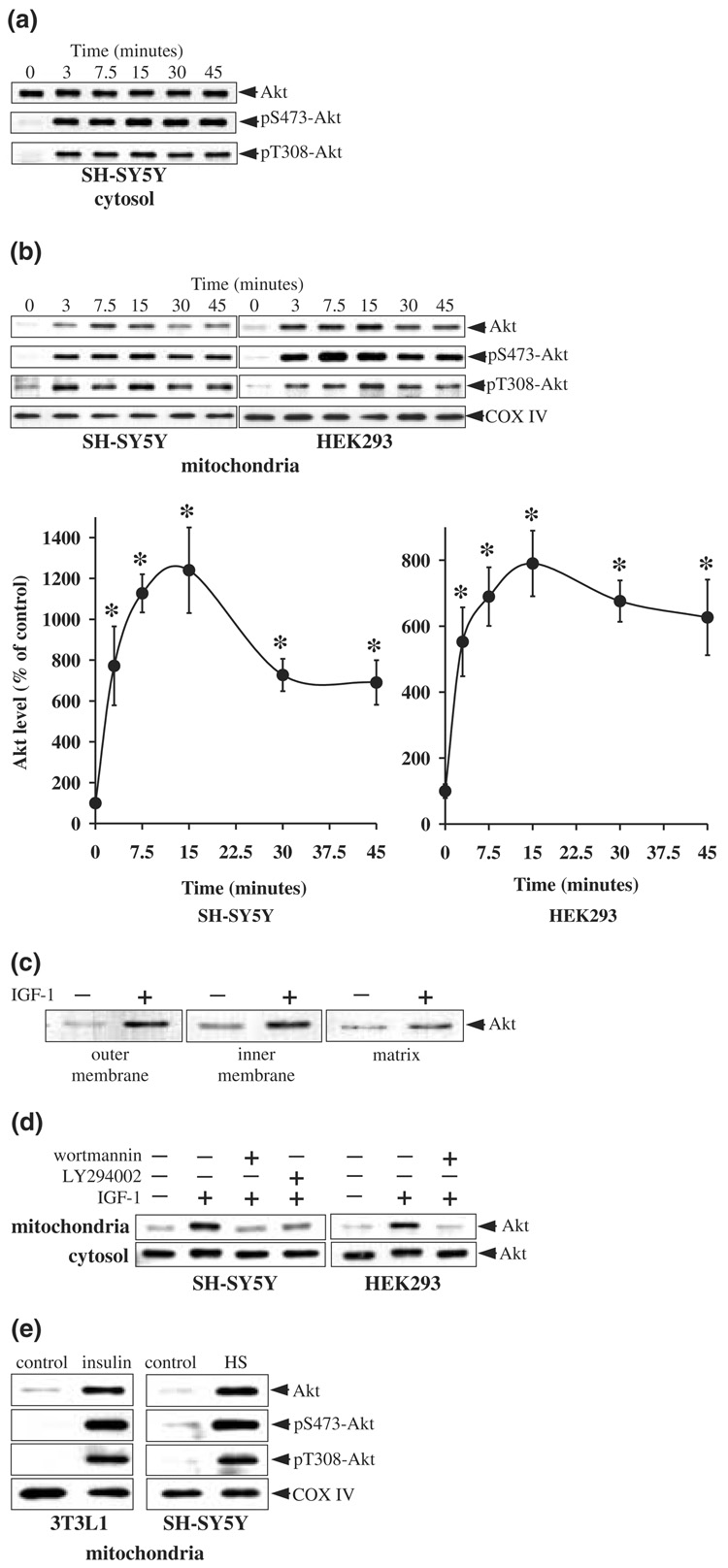
IGF-1 stimulates receptors coupled to activation of PI3K and Akt in SH-SY5Y cells. To test if this treatment has some influence on the intracellular distribution of Akt, cells were treated with IGF-1 (50 ng/mL) and the purified cytosolic and mitochondrial fractions were immunoblotted for phosphorylated and total Akt. IGF-1 treatment resulted in a rapid increase in the phosphorylation of Akt in the cytosol on Ser473 and Thr308 (Fig. 2a). Phosphorylation of Akt in the cytosol was evident within 3 min of treatment with IGF-1, and was maintained for 45 min.
Fig. 2.
Stimulation of PI3K signaling induces accumulation of Akt in mitochondria. Cells in serum-free media were treated with IGF-1 (50 ng/mL) for 0, 3, 7.5, 15, 30, and 45 min, and cytosolic and mitochondrial fractions were prepared. (a) Cytosolic fractions from SH-SY5Y cells were immunoblotted for phospho-Ser473-Akt, phospho-Thr308-Akt, and total Akt. (b) Mitochondrial fractions from SH-SY5Y cells and HEK293 cells were immunoblotted for total Akt, phospho-Ser473-Akt, phospho-Thr308-Akt, and cytochrome oxidase (COX IV). Akt bands were analyzed by densitometry. Means ± SE, n = 3 experiments; *p < 0.05 (anova) compared to values from untreated cells. (c) Mitochondrial outer membrane, inner membrane, and matrix fractions from control and IGF-1 treated SH-SY5Y cells were immunoblotted for Akt. (d) Cells were pre-incubated for 30 min without and with wortmannin (40 nm) or LY294002 (20 µm) prior to treatment with IGF-1 (50 ng/mL for 15 min). Mitochondrial and cytosolic extracts were immunoblotted for Akt. (e) 3T3L1 adipocytes were treated with insulin (100 nm for 15 min) and SH-SY5Y cells were subjected to an elevated temperature (45°C) ‘heat shock’ (HS) for 15 min. Mitochondrial extracts were immunoblotted for total Akt, phospho-Ser473-Akt, phospho-Thr308-Akt, and cytochrome oxidase (COX IV).
Treatment with IGF-1 also had a rapid regulatory effect on the level of Akt in mitochondria. Following IGF-1 treatment, the level of Akt in mitochondria increased sharply within 3 min and remained elevated for 45 min, reaching a maximal level after 15 min that was more than 1200% of the basal level (Fig. 2b). IGF-1-induced increases in the levels of phospho-Ser473-Akt and phospho-Thr308-Akt in mitochondria paralleled the accumulation of Akt, indicating that the accumulated Akt in mitochondria was predominantly in the active state. Cytochrome oxidase subunit IV levels were unchanged after IGF-1 treatment. Akt accumulation was also examined in the mitochondrial subfractions. Mitochondrial subfractions prepared from cells maintained in serum-free media had low levels of Akt within all three mitochondrial compartments (Fig. 2c). Treatment with IGF-1 for 15 min resulted in relatively large accumulations of Akt in the membrane fractions and to a lesser degree in the matrix (Fig. 2c), consistent with the submitochondrial distribution of Akt in cells maintained in serum media (Fig. 1c).
To test if this large increase in accumulation of Akt in mitochondria caused by IGF-1 stimulation was cell-type specific, experiments were carried out in HEK293 cells. As was found with SH-SY5Y cells, treatment with IGF-1 caused a rapid and large increase in accumulation of Akt in mitochondria of HEK293 cells (Fig. 2b), reaching a maximal level after 15 min of treatment that was nearly 800% of the basal level. As in SH-SY5Y cells, the accumulation of Akt in mitochondria of HEK293 cells after exposure to IGF-1 was predominantly in the phosphorylated, active form, as increases in the levels of phospho-Ser473-Akt and phospho-Thr308-Akt in mitochondria paralleled the increase in total Akt in mitochondria.
To verify that the IGF-1-induced accumulation of Akt in mitochondria is linked to the PI3K signaling pathway and to test if the activation of Akt is obligatory for its accumulation in the mitochondria, cells were pretreated with two structurally distinct inhibitors of PI3K, wortmannin and LY294002, prior to stimulation with IGF-1. Both wortmannin and LY294002 inhibited the IGF-1-induced accumulation of Akt in mitochondria (Fig. 2d). Taken together, these results show in two cell types that activation of a receptor coupled to the PI3K/Akt signaling pathway results in a marked accumulation of active Akt in the mitochondria.
Akt also can be activated by a variety of other signaling molecules and stressors, such as insulin and heat shock. To test if these distinctly different stimuli also induced accumulation of Akt in mitochondria, 3T3L1 cells were treated with insulin (100 nm for 15 min) and SH-SY5Y cells were subjected to heat shock (45°C for 15 min). Both stimuli induced robust increases in Akt levels in mitochondria (Fig. 2e) with corresponding increases in phospho-Ser473-Akt and phospho-Thr308-Akt levels. Thus, activation of the Akt signaling pathway caused rapid increases in active Akt levels in mitochondria in three types of cells and with three different treatment paradigms that activate Akt.
The membrane potential facilitates accumulation of Akt in mitochondria
The transport of proteins into mitochondria can be dependent, in part, on the membrane potential across the mitochondrial inner membrane (Neupert and Brunner 2002). The protonophore carbonyl cyanide m-chlorophenylhydrazone (CCCP) disrupts the mitochondrial membrane potential, thus selectively inhibiting the membrane potential-dependent component of protein import (Geissler et al. 2000) and the mitochondrial import of specific proteins in mammalian cells (Valgardsdottir et al. 2001). To examine whether this mechanism influences accumulation of Akt in mitochondria, and to confirm that the Akt detected in the mitochondrial fraction was due to the uptake of Akt by mitochondria specifically, cells were treated with 50 µm CCCP 30 min prior to IGF-1 treatment. Figure 3 shows that CCCP treatment blocked the IGF-1-induced accumulation of total and phosphorylated Akt in mitochondria, but did not inhibit the IGF-1-induced phosphorylation of Akt in the cytosol, indicating that the CCCP-induced blockade of the accumulation of Akt in the mitochondria was not due to impaired IGF-1-mediated signaling. CCCP treatment did not result in the general loss of proteins in mitochondria, as treatment with CCCP did not alter the level of cytochrome c in the mitochondria. This result indicates that disruption of the mitochondrial membrane potential impedes the influx of Akt into mitochondria.
Fig. 3.
CCCP treatment blocks IGF-1-induced accumulation of Akt in mitochondria. SH-SY5Y cells were pre-incubated with CCCP (50 µm for 30 min) prior to treatment with IGF-1 (50 ng/mL, 15 min). Mitochondrial extracts were immunoblotted for Akt, phospho-Ser473-Akt, phospho-Thr308-Akt and cytochrome c. Cytosolic extracts were immunoblotted for Akt, phospho-Ser473-Akt, and phospho-Thr308-Akt.
Phosphorylation of proteins in the mitochondria by Akt
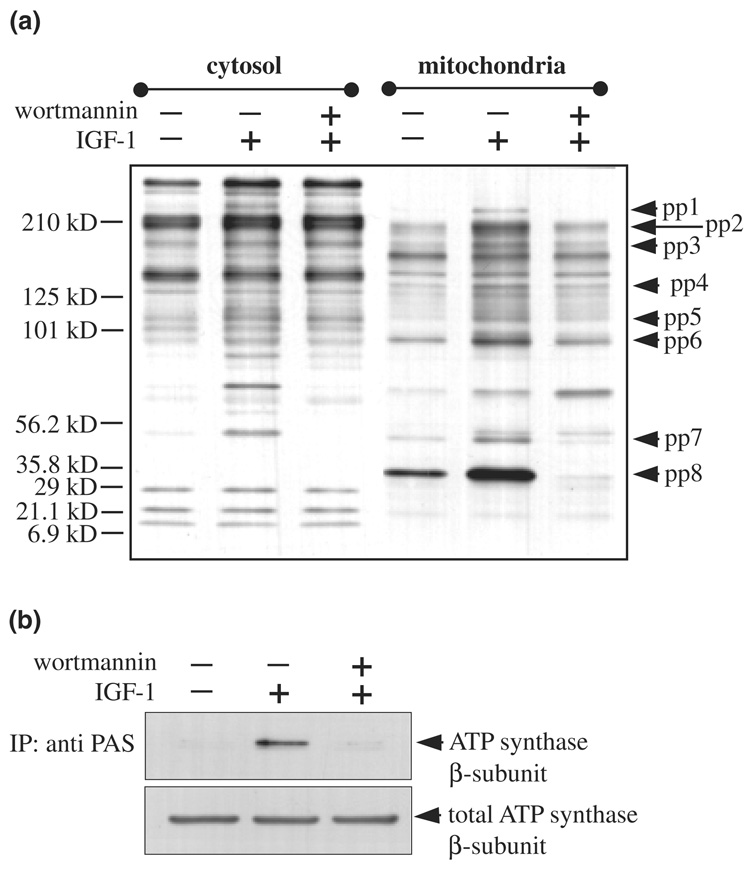
The findings that Akt is present in mitochondria and rapidly accumulates in the mitochondria following its activation, raised the question of what are the substrates phosphorylated by Akt in mitochondria. To address this issue, we used the recently developed phospho-Akt substrate (PAS) antibody (Berwick et al. 2002; Kane et al. 2002; Zhang et al. 2002) in combination with mass spectrometry. The PAS antibody, which was developed to identify phosphorylated substrates of Akt (Berwick et al. 2002; Kane et al. 2002; Zhang et al. 2002), recognizes the consensus Akt substrate motif RXRXXT*/S* (where X represents any amino acid, and T* and S* represent phosphorylated threonine and serine, respectively) and variations of this motif (Zhang et al. 2002). We first compared PAS immunoreactivity in cytosol and mitochondria from SH-SY5Y cells. The basal patterns of PAS immunoreactivity in the two fractions were completely different, raising the possibility that each fraction contained different Akt substrates (Fig. 4a). Furthermore, stimulation with IGF-1 (50 ng/mL, 15 min), and inhibition of PI3K with wortmannin in conjunction with IGF-1 stimulation, revealed PI3K-dependent increases in PAS immunoreactivity in several bands, with many marked differences between the cytosolic and mitochondrial fractions. Notably, there were several distinct bands in the mitochondria (pp1–pp8) that showed PI3K-dependent increases in PAS immunoreactivity following IGF-1 treatment.
Fig. 4.
PAS antibody-immunoreactive proteins in mitochondria. SH-SY5Y cells were pre-incubated without and with wortmannin (40 nm, 30 min) prior to IGF-1 treatment (50 ng/mL, 15 min). (a) Purified cytosolic and mitochondrial proteins were separated in a 4–15% gradient SDS-polyacrylamide gel and immunoblotted with a phospho-Akt substrate (PAS) antibody. (b) Mitochondrial extracts from control, IGF-1, and wortmannin plus IGF-1 treated cells were immunoprecipitated with the PAS antibody and immunoblotted with ATP synthase β-subunit antibody and mitochondrial extracts were also immunoblotted for total levels of ATP synthase β-subunit.
To identify proteins in mitochondria that were phosphorylated by Akt in mitochondria, mitochondrial extracts from IGF-1 treated cells were immunoprecipitated with the PAS antibody, proteins were separated by SDS-PAGE, and analyzed by MALDI-TOF MS. This method identified the β-subunit of ATP synthase, a well-known enzyme in mitochondria critical for energy metabolism. Immunoblots of the ATP synthase β-subunit were used to verify the mass spectrometry results. The PAS antibody was used to immunoprecipitate phosphorylated proteins from mitochondria and the amount of the β-subunit of ATP synthase was determined by immunoblotting (Fig. 4b). In the absence of stimulation, a very low level of ATP synthase β-subunit was detected in PAS immunoprecipitants. Following stimulation with IGF-1, increased ATP synthase β-subunit was present in the PAS immunoprecipitant, and this increase was blocked by inhibition of PI3K with wortmannin. In the same samples, none of these treatments changed the total level of the β-subunit of ATP synthase in the mitochondria, indicating that only its phosphorylation status was altered. This data confirms the findings obtained in the mass spectrometric analysis, and together these results show that the β-subunit of ATP synthase is phosphorylated following accumulation of Akt in mitochondria.
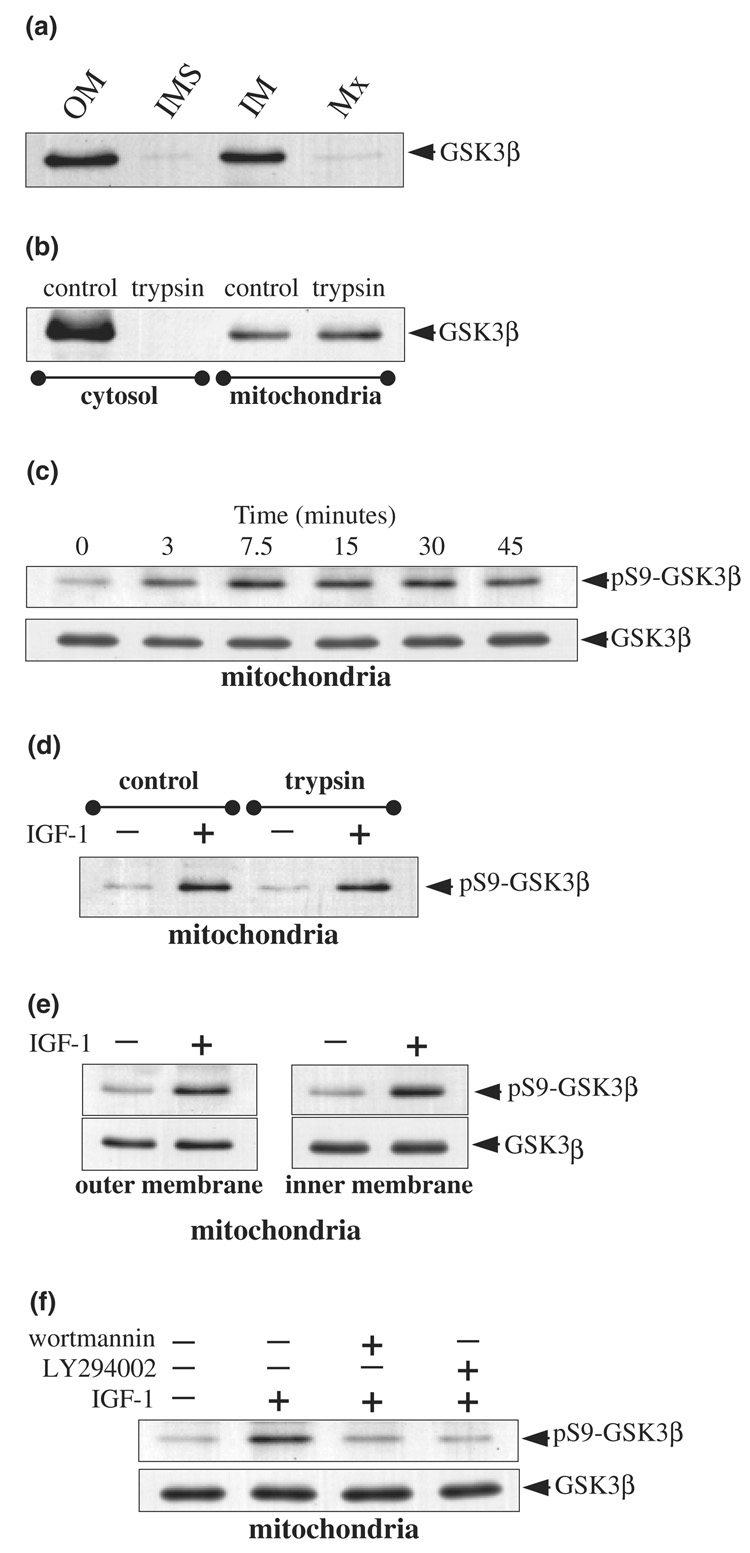
Further examination revealed that GSK3β in mitochondria also was phosphorylated following accumulation of active Akt in mitochondria. GSK3β in the cytosol is one of the most well-documented substrates of Akt, which Akt inhibits by phosphorylating Ser9 of GSK3β (Cross et al. 1995). We first investigated if GSK3β was detectable in purified mitochondria and examined the submitochondrial localization of GSK3β to test if it colocalized with Akt in mitochondria, which would be necessary for it to be a substrate of Akt in mitochondria. Immunoblots of submitochondrial fractions prepared from SH-SY5Y cells revealed that GSK3β was primarily associated with mitochondrial membranes (Fig. 5a). Furthermore, GSK3β was found to be sequestered within the mitochondria, as trypsin treatment of purified mitochondria did not result in any proteolysis of GSK3β in mitochondria (Fig. 5b). In comparison, GSK3β in the cytosol was completely proteolyzed by trypsin. Together, these results demonstrate that GSK3β colocalizes with, and thus is accessible to, Akt in mitochondria.
Fig. 5.
IGF-1 treatment induces serine-9 phosphorylation of GSK3β in mitochondria. (a) Mitochondrial subfractions from SH-SY5Y cells were immunoblotted for GSK3β. (b) Cytosol and purified intact mitochondria were treated with trypsin (200 µg/mL for 20 min at 4°C) and immunoblotted for GSK3β. (c) SH-SY5Y cells were treated with IGF-1 (50 ng/mL) for 0, 3, 7.5, 15, 30, and 45 min and mitochondrial extracts were immunoblotted for phospho-Ser9-GSK3β and total GSK3β. (d) Purified intact mitochondria isolated from unstimulated and IGF-1 (50 ng/mL, 15 min) stimulated cells were incubated with trypsin (200 µg/mL for 20 min at 4°C) and mitochondrial extracts were immunoblotted for phospho-Ser9-GSK3β. (e) Mitochondrial outer membrane and inner membrane fractions from control and IGF-1 treated SH-SY5Y cells were immunoblotted for phospho-Ser9-GSK3β and total GSK3β. (f) Cells were pre-incubated for 30 min without or with wortmannin (40 nm) or LY294002 (20 µm) prior to treatment with IGF-1 (50 ng/mL, 15 min). Mitochondrial extracts were immunoblotted for phospho-Ser9-GSK3β and total GSK3β.
We next tested if signaling stimulated by IGF-1 extended to GSK3β in mitochondria. IGF-1 treatment resulted in a time-dependent increase in phospho-Ser9-GSK3β in mitochondria (Fig. 5c), which mirrored the time-dependent accumulation of active Akt in mitochondria. The increase in phospho-Ser9-GSK3β was not due to changes in levels of total GSK3β in mitochondria, as these did not change following IGF-1 treatment. The IGF-1-induced increase in the level of phospho-Ser9-GSK3β in mitochondria was unaffected by trypsin treatment of isolated mitochondria (Fig. 5d), and the increase in phospho-Ser9-GSK3β levels occurred within the mitochondrial membrane fractions (Fig. 5e). These results indicate that the Akt-mediated phosphorylation of GSK3β occurs within the mitochondria. Furthermore, treatment with the PI3K inhibitors wortmannin and LY294004 inhibited the IGF-1-induced phosphorylation of Ser9 of GSK3β in mitochondria (Fig. 5f). Taken together, these results demonstrate that IGF-1-induced signaling increases the level of active Akt in mitochondria and this phosphorylates GSK3β in mitochondria.
Discussion
In the short period of time since its discovery, Akt has achieved a justifiably prominent position in studies of signaling systems, as discussed in many recent excellent reviews (Datta et al. 1999; Brazil and Hemmings 2001; Lawlor and Alessi 2001). This attention is due to the central role of Akt in several intensely studied subjects, such as insulin signaling, apoptosis, proliferation, as well as many others. Thus, it is surprising that Akt signaling in mitochondria has remained unexplored until now. In this report we show signaling pathways that activate Akt extend directly to the mitochondria where, when activated, Akt is rapidly imported. This optimally positions Akt for modulating mitochondrial functions.
The magnitude and rapidity of the increases in levels of Akt in mitochondria following stimulation were especially notable. Within only 3 min of treatment with IGF-1, Akt levels in mitochondria increased over fivefold in both SH-SY5Y cells and HEK293 cells. Furthermore, the maximal increases in Akt levels in mitochondria were large, reaching approximately 10-fold accumulations, and these were not cell-type specific. Equivalently robust increases in Akt levels in mitochondria occurred shortly after exposure to insulin and to heat shock. The rapid accumulation of Akt in mitochondria was equivalent to the rate of activation of cytosolic Akt following IGF-1 treatment. The accumulation of mitochondrial Akt contrasts with the nuclear import of Akt, which is considered to be an important event after Akt activation, where more delayed (20–30 min) increases in nuclear levels of Akt were observed following IGF-1 stimulation of HEK293 cells (Andjelkovic et al. 1997; Meier et al. 1997). The mitochondrial import and export mechanisms for Akt have yet to be investigated. However, the close correlation between phosphorylation of cytosolic Akt, the accumulation of Akt in mitochondria, and the rapid phosphorylation of mitochondrial GSK3β suggests that these events are linked, possibly through a Akt phosphorylation-dependent uptake, or retention, mechanism. Furthermore, the blockade of accumulation of active Akt in mitochondria by the protonophore CCCP indicates the requirement of a proton-gradient across the mitochondrial inner membrane to facilitate the mitochondrial import of active Akt. Considering the diverse stimuli that both activated Akt and also increased levels of Akt in the mitochondria, it is possible that a number of other stimuli also will have this effect. A few years ago a review by Datta et al. (1999) listed over 40 stimuli that induced increased Akt activity. It seems likely that these also activate the mitochondrial import of Akt since three different stimuli were found to do so in this study.
Once in the mitochondria, the ramifications of Akt’s actions largely remain to be explored. Notably, Akt was distributed almost throughout mitochondria, as it was detected in fractions containing the outer membranes, the inner membranes, and the matrix, the central core of mitochondria. This places Akt in a position to interact with substrates throughout mitochondria. The detection of several changes in PAS immunoreactivity in mitochondria after stimulation of Akt raise the possibility that there are several substrates of mitochondrial Akt. One of these was identified as the β-subunit of ATP synthase. ATP synthase resides in the inner mitochondrial membrane and harnesses the proton gradient across the mitochondrial membrane for the synthesis of ATP (reviewed in Boyer 1997). The β-subunit is a component of a multiprotein complex and is the catalytic site for ATP synthesis. Another substrate of mitochondrial Akt was found to be GSK3β. The levels of GSK3β in mitochondria were unaffected by stimuli that activate Akt, unlike nuclear GSK3β where the level is subject to regulation (Diehl et al. 1998; Bijur and Jope 2001). GSK3β in mitochondria colocalized with Akt in both the outer membrane and inner membrane fractions. Furthermore, rapid increases in phospho-Ser9-GSK3β in mitochondria, evident within 3 min of IGF-1 treatment, mirrored the accumulation of active Akt in mitochondria. Phosphorylation of serine-9 of GSK3β is well known to inhibit its activity (Cross et al. 1995). Thus, one consequence of the action of Akt in mitochondria is to inhibit GSK3β in mitochondria. Only one function of GSK3β in mitochondria has been reported, the phosphorylation of pyruvate dehydrogenase (Hoshi et al. 1996), which inhibits its activity. Also, since cellular GSK3β facilitates apoptotic signaling (Pap and Cooper 1998; Bijur et al. 2000; Crowder and Freeman 2000; Hetman et al. 2000), if mitochondrial GSK3β contributes to apoptosis, its inhibition by Akt would serve to promote cell survival, a well-known outcome of Akt activity. In addition to these two substrates, it is highly likely that other mitochondrial proteins also are Akt substrates based on the PAS immunoreactivity measurements. Furthermore, since these measurements were carried out in unstressed cells, additional proteins in mitochondria may be phosphorylated by Akt following stress.
In summary, this study demonstrates the existence of a previously unrecognized component of Akt signaling. Akt is present in mitochondria, and the level of Akt in mitochondria is dynamically regulated by cellular signaling activities. Within the mitochondria, Akt phosphorylates the β-subunit of ATP synthase, GSK3β, and other unidentified proteins. Further exploration of the actions of Akt within mitochondria should provide new information about its regulation of mitochondrial function.
Acknowledgements
We thank Dr Stuart Frank for providing the 3T3L1 cells. This research was supported by grants from the National Institutes of Health. The University of Alabama at Birmingham Mass Spectrometry Shared Facility is supported by the National Center for Research Resources Grant S10 RR11329 and the UAB Comprehensive Cancer Center Core Support Grant P30 CA-13148.
Abbreviations used
- CCCP
carbonyl cyanide m-chlorophenylhydrazone
- COX
cytochrome oxidase
- GSK3β
glycogen synthase kinase-3β
- IGF-1
insulin-like growth factor-1
- MALDI-TOF MS
matrix-assisted laser desorption ionization time-of-flight mass spectrometry
- PAS
phospho-Akt substrate
- PI3K
phosphatidylinositol 3-kinase
- SDS
sodium dodecyl sulfate
References
- Andjelkovic M, Alessi DR, Meier R, Fernandez A, Lamb NJ, Frech M, Cron P, Cohen P, Lucocq JM, Hemmings BA. Role of translocation in the activation and function of protein kinase B. J. Biol. Chem. 1997;272:31515–31524. doi: 10.1074/jbc.272.50.31515. [DOI] [PubMed] [Google Scholar]
- Berwick DC, Hers I, Heesom KJ, Moule SK, Tavare JM. The identification of ATP-citrate lyase as a protein kinase B (Akt) substrate in primary adipocytes. J. Biol. Chem. 2002;277:33895–33900. doi: 10.1074/jbc.M204681200. [DOI] [PubMed] [Google Scholar]
- Bijur GN, Jope RS. Proapoptotic stimuli induce nuclear accumulation of glycogen synthase kinase-3β. J. Biol. Chem. 2001;276:37436–37442. doi: 10.1074/jbc.M105725200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijur GN, De Sarno P, Jope RS. Glycogen synthase kinase-3β facilitates staurosporine- and heat shock-induced apoptosis. Protection by lithium. J. Biol. Chem. 2000;275:7583–7590. doi: 10.1074/jbc.275.11.7583. [DOI] [PubMed] [Google Scholar]
- Borgatti P, Martelli AM, Bellacosa A, Casto R, Massari L, Capitani S, Neri LM. Translocation of Akt/PKB to the nucleus of osteoblast-like MC3T3—E1 cells exposed to proliferative growth factors. FEBS Lett. 2000;477:27–32. doi: 10.1016/s0014-5793(00)01758-0. [DOI] [PubMed] [Google Scholar]
- Boyer PD. The ATP synthase — a splendid molecular machine. Annu. Rev. Biochem. 1997;66:717–749. doi: 10.1146/annurev.biochem.66.1.717. [DOI] [PubMed] [Google Scholar]
- Brami-Cherrier K, Valjent E, Garcia M, Pages C, Hipskind RA, Caboche J. Dopamine induces a PI3-kinase-independent activation of Akt in striatal neurons: a new route to cAMP response element-binding protein phosphorylation. J. Neurosci. 2002;22:8911–8921. doi: 10.1523/JNEUROSCI.22-20-08911.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brazil DP, Hemmings BA. Ten years of protein kinase B signalling: a hard Akt to follow. Trends Biochem. Sci. 2001;26:657–664. doi: 10.1016/s0968-0004(01)01958-2. [DOI] [PubMed] [Google Scholar]
- Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- Crowder RJ, Freeman RS. Glycogen synthase kinase-3β activity is critical for neuronal death caused by inhibiting phosphatidylinositol 3-kinase or Akt but not for death caused by nerve growth factor withdrawal. J. Biol. Chem. 2000;275:34266–34271. doi: 10.1074/jbc.M006160200. [DOI] [PubMed] [Google Scholar]
- Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999;13:2905–2927. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- Diehl JA, Cheng M, Roussel MF, Sherr CJ. Glycogen synthase kinase-3β regulates cyclin D1 proteolysis and subcellular localization. Genes Dev. 1998;12:3499–3511. doi: 10.1101/gad.12.22.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geissler A, Krimmer T, Bomer U, Guiard B, Rassow J, Pfanner N. Membrane potential-driven protein import into mitochondria: the sorting sequence of cytochrome b(2) modulates the deltapsi-dependence of translocation of the matrix-targeting sequence. Mol. Biol. Cell. 2000;11:3977–3991. doi: 10.1091/mbc.11.11.3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotow T, Shibata M, Kanamori S, Tokuno O, Ohsawa Y, Sato N, Isahara K, Yayoi Y, Watanabe T, Leterrier JF, et al. Selective localization of Bcl-2 to the inner mitochondrial and smooth endoplasmic reticulum membranes in mammalian cells. Cell Death Differ. 2000;7:666–674. doi: 10.1038/sj.cdd.4400694. [DOI] [PubMed] [Google Scholar]
- Gottlieb RA, Adachi S. Nitrogen cavitation for cell disruption to obtain mitochondria from cultured cells. Methods Enzymol. 2000;322:213–221. doi: 10.1016/s0076-6879(00)22022-3. [DOI] [PubMed] [Google Scholar]
- Grimes CA, Jope RS. The multifaceted roles of glycogen synthase kinase-3β in cellular signaling. Prog. Neurobiol. 2001;65:391–426. doi: 10.1016/s0301-0082(01)00011-9. [DOI] [PubMed] [Google Scholar]
- Hetman M, Cavanaugh JE, Kimelman D, Xia Z. Role of glycogen synthase kinase-3β in neuronal apoptosis induced by trophic withdrawal. J. Neurosci. 2000;20:2567–2574. doi: 10.1523/JNEUROSCI.20-07-02567.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi M, Takashima A, Noguchi K, Murayama M, Sato M, Kondo S, Saitoh Y, Ishiguro K, Hoshino T, Imahori K. Regulation of mitochondrial pyruvate dehydrogenase activity by tau protein kinase I/glycogen synthase kinase 3β in brain. Proc. Natl Acad. Sci. USA. 1996;93:2719–2723. doi: 10.1073/pnas.93.7.2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovius R, Lambrechts H, Nicolay K, de Kruijff B. Improved methods to isolate and subfractionate rat liver mitochondria. Lipid composition of the inner and outer membrane. Biochim. Biophys. Acta. 1990;1021:217–226. doi: 10.1016/0005-2736(90)90036-n. [DOI] [PubMed] [Google Scholar]
- Kane S, Sano H, Liu SC, Asara JM, Lane WS, Garner CC, Lienhard GE. A method to identify serine kinase substrates: Akt phosphorylates a novel adipocyte protein with a Rab GTPase-activating protein (GAP) domain. J. Biol. Chem. 2002;277:22115–22118. doi: 10.1074/jbc.C200198200. [DOI] [PubMed] [Google Scholar]
- Lawlor MA, Alessi DR. PKB/Akt: a key mediator of cell proliferation, survival and insulin responses? J. Cell. Sci. 2001;114:2903–2910. doi: 10.1242/jcs.114.16.2903. [DOI] [PubMed] [Google Scholar]
- Meier R, Alessi DR, Cron P, Andjelkovic M, Hemmings BA. Mitogenic activation, phosphorylation, and nuclear translocation of protein kinase Bβ. J. Biol. Chem. 1997;272:30491–30497. doi: 10.1074/jbc.272.48.30491. [DOI] [PubMed] [Google Scholar]
- Milon L, Meyer P, Chiadmi M, Munier A, Johansson M, Karlsson A, Lascu I, Capeau J, Janin J, Lacombe ML. The human nm23—H4 gene product is a mitochondrial nucleoside diphosphate kinase. J. Biol. Chem. 2000;275:14264–14272. doi: 10.1074/jbc.275.19.14264. [DOI] [PubMed] [Google Scholar]
- Neupert W, Brunner M. The protein import motor of mitochondria. Nat. Rev. Mol. Cell Biol. 2002;3:555–565. doi: 10.1038/nrm878. [DOI] [PubMed] [Google Scholar]
- Pap M, Cooper GM. Role of glycogen synthase kinase-3 in the phosphatidylinositol 3-kinase/Akt cell survival pathway. J. Biol. Chem. 1998;273:19929–19932. doi: 10.1074/jbc.273.32.19929. [DOI] [PubMed] [Google Scholar]
- Valgardsdottir R, Brede G, Eide LG, Frengen E, Prydz H. Cloning and characterization of MDDX28, a putative dead-box helicase with mitochondrial and nuclear localization. J. Biol. Chem. 2001;276:32056–32063. doi: 10.1074/jbc.M011629200. [DOI] [PubMed] [Google Scholar]
- Vanhaesebroeck B, Alessi DR. The PI3K-PDK1 connection: more than just a road to PKB. Biochem. J. 2000;346:561–576. [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Zha X, Tan Y, Hornbeck PV, Mastrangelo AJ, Alessi DR, Polakiewicz RD, Comb MJ. Phosphoprotein analysis using antibodies broadly reactive against phosphorylated motifs. J. Biol. Chem. 2002;277:39379–39387. doi: 10.1074/jbc.M206399200. [DOI] [PubMed] [Google Scholar]