Abstract
Multiangle laser light scattering and fluorescence anisotropy decay measurements clarified the oligomeric states of native and recombinant tear lipocalin (lipocalin-1, TL). Native TL is monomeric. Recombinant TL (5-68 μM) with or without the histidine tag shows less than 7% dimer formation that is not in equilibrium with the monomeric form. Fluorescence anisotropy decay showed a correlation time of 9-10 ns for TL (10 μM- 1mM). Hydrodynamic calculations based on the crystallographic structure of a monomeric TL mutant closely concur with the observed correlation time. The solution properties calculated with HYDROPRO and SOLPRO programs from the available crystallographic structure of a monomeric TL mutant concur closely with the observed fluorescence anisotropy decay. The resulting model shows that protein topology is the major determinant of rotational correlation time and accounts for deviation from the Stokes-Einstein relation. The data challenge previous gel filtration studies to show that native TL exists predominantly as a monomer in solution rather than as a dimer. Delipidation of TL results in a formation of a complex oligomeric state (up to 25%). These findings are important as the dynamic processes in the tear film are limited by diffusional, translational as well as rotational, properties of the protein.
Keywords: tear lipocalin, correlation time, oligomeric state, monomeric protein, multiangle laser light scattering, fluorescence anisotropy decay
1. Introduction
Tear lipocalin (TL) also known as LCN1 is part of the lipocalin superfamily of proteins that functions in binding and transport of hydrophobic molecules. TL is the principal lipid binding protein in tears. Ligands include a wide range of lipids including fatty acids, phospholipids, cholesterol, fatty alcohols and glycolipids [1]. In human tears, TL functions to scavenge fatty acids and phospholipids rapidly from the corneal surface [2] and probably to stabilize the tear film surface interface [3-5]. TL, as well as other proteins of the lipocalin family, exhibits magnesium dependent endonuclease activity [6]. TL has been invoked as a scavenger of toxic substances. The binding of fungal siderophores confers antimicrobial activity to TL [7]. TL's broad ligand affinity has led to the development of combinatorial loop modifications (anticalins) for enhancing the binding of an assortment of clinically useful ligands based on the structure of TL [8]. Both the solution structure of TL determined by site directed tryptophan fluorescence and the crystal structure of a TL mutant, assigned by independent laboratories, are concordant [9, 10]. The binding pocket of TL is large, leading to its promiscuity in binding. However, there exists some controversy about the oligomeric state of TL. For crystallization the TL mutant (C101S) was chosen to avoid the aggregation that may occur in lipocalins without this substitution [10]. This mutant also contains a C-terminal Strep-tag II that follows the β-strand I. β-lactoglobulin (BLG), which shows sequence homology (23%) to TL [9] and has a similar fold [10], forms a dimer at neutral pH via interaction with β-strand I [11]. Therefore, the C-terminal segment with a Strep-tag II theoretically could interfere with dimer formation. With these changes the mutant was monomeric [10]. Recombinant TL, which contained a polyhistidine tagged sequence was shown to be monomeric in the apo-form by analytic ultracentrifugation studies [12]. Native tear lipocalin, purified from tears contains 6 isoforms [13]. Based on size exclusion chromatography (SEC) some groups concluded independently that native TL elutes as a dimer with a predominant peak at 35-36 kDa [3] or at 45 kDa [14]. However, in some cases, a protein mass estimated by SEC may differ greatly from the actual value. The discrepancies are usually related to protein shape and possible matrix binding interactions. Mass spectroscopy excluded the possibility that native TL purified from tears contained a covalent dimer, because the “dimeric” MALDI signal, which accounted for less than 5% of the total, was not decreased with DTT [15]. Other investigators have found that after chemical cross linking holo-TL (histidine tagged recombinant TL saturated with cholesterol or fatty acids) showed up to 10% dimer formation by analytic ultracentrifugation but no significant dimer formation was apparent for apo-TL [12].
Overall rotational correlation time, related to the mass of a protein, has also been used to determine the oligomeric state of TL. The correlation time was determined by electron paramagnetic resonance method using a motionally restricted nitroxide side chain at position 99 [16]. The correlation times of 12.1* and 12.5* ns (*- recalculated for 298K) were found both in buffer and tears, respectively. We interpreted these data as evidence of at least a partial dimeric state because at that time BLG had been reported to have a correlation time of 8.4 ns as a monomer [17]. Furthermore, T4 lysozyme, a protein of about 18.7 kDa was estimated to have a correlation time of 6 ns associated with the monomeric state [18]. However, more recent data have indicated that the correct overall correlation time of BLG is 9.8* ns for the monomer and 22.4* ns for the dimer [19].
To clarify the oligomeric state of native TL and the major mutant forms used in various functional experiments, SEC with on-line multiangle laser light scattering (MALS) was employed on the native (contains 6 isoforms) as well as recombinant TL with and without an accompanying histidine tag. The SEC-MALS that use the DeBye plot method for the absolute molecular mass determination of a protein is superior to the conventional SEC method, which measures relative molecular mass [20].
Fluorescence anisotropy decay measurements were performed to estimate molecular mass of TL in concentrations that exceeded the range possible with the SEC-MALS method. Determination of the oligomeric state of TL is critical to understanding the structural data, possible ligand binding sites, as well as processes, in which diffusional (translational and rotational) properties of the protein are limiting factors. Here we present evidence from multiple methodologies that holo-TL, native as well as recombinant TL with and without histidine tag, exists mainly in monomeric form in solution. Delipidation of the protein, in contrast to finding of a previous study [12], resulted in a formation of dimeric and higher oligomeric forms.
Hydrodynamic and solution properties of TL calculated with HYDROPRO and SOLPRO programs from the available crystallographic structure of the monomeric TL mutant (C101S) show that the protein size and also topology are the major determinants of rotational correlation time (i.e., diffusion related property) and a reason for deviation from the Stokes-Einstein relationship.
2. Materials and Methods
2.1. Preparation of native TL
TL was purified from pooled human tear samples by size exclusion and ion-exchange column chromatographies as previously described. [1, 13]
2.2. Preparation of recombinant TL and cysteine mutants
Recombinant TL identical to native TL except an initiating methionine was previously constructed in PCR II (Invitrogen, San Diego, CA [21, 22]), to clone the TL gene spanning bases 115-592 of the previously published cDNA sequence [14] into pET 20b (Novagen). Flanking restriction sites for NdeI and BamHI were added to produce the native protein sequence of the major TL isoform as found in tears including the native cysteine at position 101 [13]. Cysteine mutant, F99C was prepared from the template of the previously well characterized TL mutant, C101L. Amino acid 1 corresponds to His, bases 115-118 according to published data [14].
The histidine tagged lipocalin, expressed TL with the additional C-terminal residues SRSHHHHHH, was prepared as previously described [23].
2.3. Expression of recombinant TL
The TL recombinant plasmid identical to the major isoform except for an initiating methionine, was transformed in E. Coli, BL 21 (DE3) and cells were cultured and proteins were expressed, purified, and analyzed as previously described [16].
Histidine tagged TL was expressed in E. coli M15 and purified by affinity chromatography on NiNTA resin (Qiagen Inc., USA) as previously described [23]. This mutant also contains C101.
2.4. Preparation of apo-TL
TL purified from tears and expressed in E. Coli were considered as the holo-form. Recombinant TL is rich with palmitic acid but in contrast to native TL does not contain cholesterol [1, 24].
To generate apo-TL, delipidation of recombinant TL was performed by a rigorous chloroform/methanol extraction and verified by thin layer chromatography as previously described [1].
2.5. Fluorescence labeling of TL
Previously it has been shown that side chain Phe99 is buried deeply inside of the TL cavity and its motion is restricted [16]. Therefore, F99C mutant of TL is a good candidate to study the overall rotational correlation time of the protein. TL mutant containing cysteine at position 99 (F99C) was labeled with a 5x molar excess of the fluorescent label MTS-dansyl (TRC Inc., North York, Ontario, Canada) in buffer 10 mM NaP, pH 6.8, at 4 °C overnight. Free label was separated from the labeled protein by gel filtration on a desalting column (Pharmacia Biotech HiTrap, 5 mL). Concentration of the label was calculated by absorbance (spectrophotometer Shimadzu UV-2400PC) using the extinction coefficient ε335 = 4100 M−1 cm−1 for MTS-dansyl. The protein concentration was determined by the biuret method. The efficiency of labeling was about 0.98.
2.6. Size exclusion chromatography - multiangle laser light scattering
The native proteins were analyzed by using SEC with online MALS, absorbance, and refractive index detectors [20, 25]. A Superose 6HR 10/30 column (Amersham Biosciences) was connected in line to a UV detector (Amersham Biosciences UV-900), a Dawn-EOS (Wyatt Technology Corp.) multiangle laser light scattering detector, and an Optilab-DSP (Wyatt Technology Corp.), refractive index detector. Native, recombinant TL and apo-TL (100 μl) were loaded onto the column at a concentration of 1.5-14.8 mg/ml using a buffer 10 mM sodium phosphate, 100 mM NaCl or buffer with a composition similar to tears [2] pH 7.2. Light scattering data were analyzed by equation that based on the Zimm formalism:
where R(θ) is the excess intensity of scattering light at DAWN angle θ, c is the sample concentration (g/mol), MW is the weight-average molecular weight (molar mass), A2 is the second virial coefficient (mL · mol / g2), K* is an optical parameter equal to , n is the solvent refractive index and dn / dc is the refractive index increment, NA is Avogadro's number, λ0 is the wavelength of the scattered light in vacuum (cm). P(θ) describes the angular dependence of the scattered light, which is related to the root mean square radius of the protein. Data were fitted by using instrument software. The fit degree was 1. The second virial coefficient term (2A2c) can be neglected when 2A2cMW << 1. A typical value of A2 = 10−5 mL · mol / g2 (for globular proteins) used for the highest concentration of TL did not change the results to any appreciable degree. Therefore, (2A2c) was neglected for samples in the fitting procedure.
2.7. Time dependent anisotropy decay
The fluorescence anisotropy decays of the dansyl-labeled mutant of TL were measured using a PTI Time Master fluorescence lifetime instrument, which consists of a nitrogen laser (GL-3300) -pumped dye laser (GL 302), frequency doubler (GL 303) and a stroboscopic detector. DCM (Exciton, Inc, Dayton, Ohio, USA) solution was used to emit 670 nm light from the dye laser. 335 nm pulses (fwhm ∼ 1.5ns) from frequency doubled light were used for excitation of dansyl labeled TL mutant. The emission wavelength was 490 nm with a slit of 6 nm. The temperature was maintained at 25 °C with a thermojacketed cuvette holder. The instrument response function (IRF) was determined by measuring light scatter from a solution of glycogen. The exciting light (335nm) was vertically polarized by Glan-Thompson polarizers. The emission polarizer was set at 0° and 90° for lifetime experiments to measure IVV (t) and IVH (t), respectively. A DPU-15 optical depolarizer (Optics for Research, Caldwell, NJ) was placed before the emission monochromator to eliminate polarization dependence of the detection train, so that G= 1 for anisotropy experiments. It was confirmed by measuring ratio of IHV / IHH. Each data point on a lifetime decay curve represents the average of 20-25 laser flashes, and each decay represents 200 of these data points evenly spaced over the collection time interval. Actual intensity values at the end of decays for IVV(t) and IVH(t) vary slightly, which can account for residual anisotropy. To minimize this shortcoming of multiple runs, one run of IVV(t) and IVH(t) decay experiments were conducted in temporal proximity to each other and then intensities of averaged decays of IVV(t) and IVH(t) were normalized to that of the first run. Anisotropy decay curves were constructed as:
In this study deconvolution of emission intensities is not necessary because observed excited fluorescence life times (20.5 ns) and rotational correlation time (9-10 ns) are sufficiently long compared to the instrumental response time (1.5 ns).
To examine non-covalent dimer formation, anisotropy decay experiments were performed with 10 μM of dansyl labeled F99C (F99Dansyl) alone and compared to that incubated with various concentrations of recombinant TL. Measurements were also taken of F99Dansyl in tears.
Anisotropy decay data were analyzed as:
where roi are fractional anisotropies which decays with correlation time θi. The expected correlation time for the TL monomer was calculated from Stokes-Einstein relationship:
where DR is the rotational diffusion coefficient, v̄ is the specific volume of the protein and h is the hydration, T is the temperature, R is the gas constant, and η is viscosity.
The intensity decay data were analyzed in terms of the following multiexponential decay law:
where αi and τi are the normalized preexponential factor and decay time, respectively. Data were analyzed using the PTI software FeliX32.
2.8. Calculation of hydrodynamic and solution properties of monomeric TL by HYDROPRO and SOLPRO programs
To estimate the rotational correlation time of monomeric TL that would take into account size and topology of the protein HYDROPRO program was employed. Harmonic mean rotational correlation time of monomeric TL was calculated from the atomic-level structure (PDB: 1XKI) using the bead-modeling methodologies described in detail [26-28]. Briefly, the procedure starts from building a primary hydrodynamic model by replacing non-hydrogen atoms with spherical elements of fixed size. The resulting primary hydrodynamic particle, consisting of overlapping spheres, then is used to construct a shell model (Fig. 1). For TL the atomic element radius was taken as 2.6 Å, based on similar sized proteins lysozyme and BLG. This shell of overlapping spheres is filled by smaller spheres that act as point sources of hydrodynamic friction, and the radius of the small spheres are then extrapolated to zero. The model building and calculation were carried out with HYDROPRO public domain software [27]. In this calculation, we used a bulk solvent viscosity of η0 = 0.89 cP as for H2O at 298 K. The atomic coordinates of TL were taken from the PDB site (1XKI).
Figure 1.

Representations of TL from crystal structure (PDB: 1XKI). (A) ribbon diagram, (B) surface representation generated from SwissPdb Viewer [40], (C) bead-model of TL used in HYDROPRO and SOLPRO programs.
The result from HYDROPRO was used to run the SOLPRO [29] program to predict solution properties, i.e. the fluorescence anisotropy decay of monomeric TL. The r0 value of calculated fluorescence anisotropy was normalized to that of experimental data for suitable comparison.
3. Results and Discussion
3.1. Multiangle laser light scattering
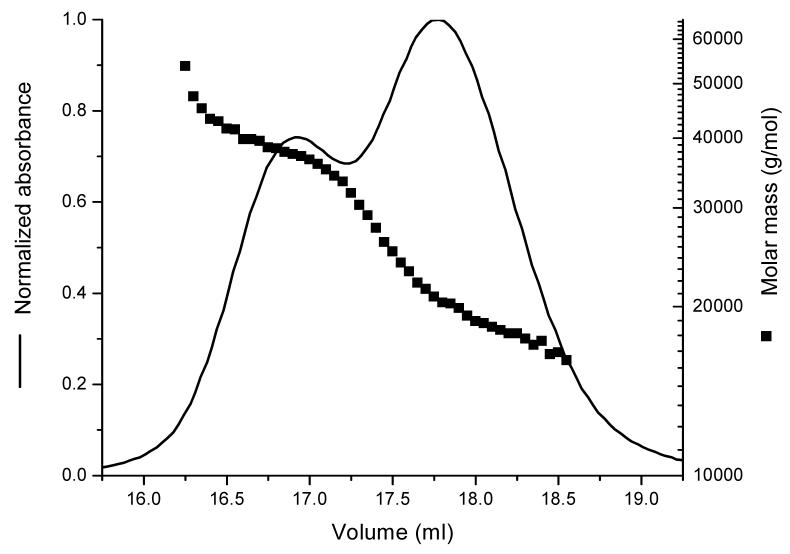
The Debye plot for native TL purified from tears is shown in Figure 2. The y-intercept of the weighted regression line yields a molecular mass for TL of 17.64 kDa; the measured mass by electrospray mass spectrometry for the major TL isoform is 17.45 kDa in tears [15]. The molar mass distribution graph is shown in Figure 2 for MALS experiments. Both native and recombinant TL show a single major elution peak at 17.7 ml elution volume with a corresponding molecular mass of about 17.6 kDa and 18.8 kDa, respectively. The higher than expected value for recombinant monomeric TL (18.8 kDa) may arise from the overlap of the elution peak shoulder of the dimeric form of TL with the monomeric peak. For native TL there is no peak corresponding to a dimer or higher oligomeric form. The molecular mass for TL engineered with a histidine tag (HT-TL) is about 20.7 kDa, which concurs with the predicted mass. In contrast, the recombinant TL forms a small shoulder (∼7%) at 16.7 ml elution volume, which corresponds to a molecular mass of 36 kDa, the dimeric form. The fractions corresponding to this small peak were collected and applied to the column (Fig. 3). The resulting molar mass distribution indicates that the proportion of the dimeric form is relatively increased, excluding the possibility of reequilibration of the monomer-dimer TL. Gel electrophoresis (data not shown) run in reduced and non-reduced conditions of a number of lots of recombinant TL demonstrate variable amounts (up to 7%) of dimer that disappears with reduction, presumably due to disulfide scrambling.
Figure 2.
Molar mass determination of TL. (A) Molar mass distribution of TL by SEC coupled to MALS. (B) Debye plots for native- and recombinant (with and without histidine tag) TL corresponding to the center of the elution peaks. Buffer: 10mM sodium phosphate, 100mM NaCl, pH 7.3.
Figure 3.
Molar mass distribution of dimer fraction of recombinant TL by SEC coupled to MALS. Buffer: 10mM sodium phosphate, 100mM NaCl, pH 7.3.
In order to examine the possible non-covalent dimer formation, MALS experiments were performed at various concentrations of the proteins. A monomer-dimer equilibrium could not be identified in the concentration-molar mass distribution plots for native and recombinant forms of TL (Fig. 4). Delipidation of TL with organic solvents resulted in the appearance of oligomers, mainly dimers and hexamers (Fig. 5). This is contrast to published data [12] that showed apo-TL, expressed with a histidine tag, was mainly in monomeric form. One possible explanation is that the delipidation methods used by our laboratories differ [1, 12]. In our experiments native and recombinant TL required more extensive treatment with the chloroform/methanol mixture to achieve thorough delipidation. In any case our results for holo-TL (native TL or recombinant TL) are quite concordant.
Figure 4.
Assessment of the possible non-covalent dimer formation for TL. Molar mass of native-and recombinant (with and without histidine tag) TL at various concentrations.
Figure 5.
Molar mass determination of apo-TL. (A) Molar mass distribution of apo-TL by SEC coupled to MALS. (a), (b), (c) denote the elution peaks for monomer, dimer and hexamer, respectively. (B) Debye plots for apo-TL corresponding to the center of the elution peaks shown at (A). Buffer: 10mM sodium phosphate, 100mM NaCl, pH 7.3.
3.2. Fluorescence anisotropy decay: Hydrodynamic and solution properties of TL
The technical limitations of column chromatography with MALS preclude the study of monomer-dimer equilibrium states for TL at high protein concentrations. Fluorescence anisotropy decay experiments on dansyl labeled F99C (F99Dansyl) permitted us to achieve concentrations up to 1mM. The data revealed an overall correlation time for TL of 9 ns (Fig. 6). The experimental anisotropy decay fit a single exponential decay function and shows residual anisotropy of 0.013, which is about 4% of r0. Fitting of the experimental anisotropy decay with a double exponential decay function, with a fixed r∞ = 0 value, gives a very high value for r2, which likely reflects error in intensity values rather than TL aggregates. The correlation time is only slightly increased with concentrations of TL even up to 1mM (Fig. 7). Assuming that the dimeric form of TL has a correlation time 17.8 ns, the roughly estimated Kd for dimer formation is about 5 mM. These data exclude the possibility of an equilibrium state of non-covalent TL oligomers with monomers. The correlation time was independent of the concentration of sodium chloride. The correlation time of TL in tears is higher than in sodium chloride, which may reflect the viscosity of tears and/or also weak interactions with lysozyme and lactoferrin [30]. The data from the anisotropy decay experiment can be compared to the data calculated from the Stokes-Einstein relationship. For TL with molecular weight of ∼17.5 kDa expected correlation times for anhydrous (0.73 ml/g) and hydrated (h = 0.23 g (H2O)/g) spheres would be 4.6 ns and 6.0 ns, respectively. The discrepancy between the experimental and predicted values for the rotational correlation times of proteins have been recognized for a long time. The early approach to resolve these discrepancies was to assign different hydration values to proteins, i.e. change the size of the putative solvation layer of water molecules. Values between 0.2-0.35 for h have been used as “typical” for proteins, although ∼0.5 has been suggested as more representative [31]. The program PERKINS using amino acid content of TL predicts v¯ and h to be 0.736 ml/g and 0.43 g (H2O)/g (Protein), respectively [32]. Adjusting for the specific volume and hydration, the estimated overall rotational correlation time for TL is 7.3 ns. The experimentally determined correlation time for TL is significantly greater than this value and beyond the range of experimental error. The discrepancy between the experimental and calculated correlations times could not be explained by protein shapes, such as sphere and ellipsoid, which are widely used for estimation hydrodynamic properties of globular proteins (e.g. DR). The prolate spheroid model of aspect ratio of 1.5, as in the case of hen egg-white lysozyme (HEWL), reduces DR only about 6% from that of the sphere model.
Figure 6.
Comparison fluorescence anisotropy decay of TL to that generated from SOLPRO for monomeric TL. Time depend fluorescence anisotropy decay for C99Dansyl (10 μM) at 298 K. Square and triangle symbols are residuals for the exponential fit and SOLPRO prediction, respectively.
Figure 7.
Assessment of the possible non-covalent dimer formation for TL by fluorescence anisotropy decay experiments. The correlation times for recombinant TL at various concentrations and conditions at 298K. The solid line represents the linear fit of rotational correlation time data with 100 mM NaCl and the dashed line is the average of all rotational correlation time data.
The model based on the concept that the effective radius of the proteins and corresponding rotational correlation time is increased by a hydration shell is known to be flawed. The studies on protein hydration dynamics by the nuclear magnetic relaxation dispersion of 1H, 2H and 17O relaxation rate have shown that the mean residence time of water molecules at the protein surface is too small (few hundreds of picoseconds or shorter) compared with the overall correlation time of proteins (e.g., about 13 ns for a protein with molecular weight of 25 kDa) [33, 34]. The number of long-lived, bound, water molecules is very small (e.g., 4± 1 molecules for cytochrome C [34]).
The discrepancy is much better explained by surface topology. Irregular contours on the protein surface retard the rotation of the protein [27, 33]. Recent advances in methods developed for computing the hydrodynamic friction tensors of rigid biomolecular structures described in atomic detail [27] brings the theoretical calculation much closer to the experimental result. This treatment reveals that protein topology is the major determinant of rotational correlation time and a reason for deviation from the Stokes-Einstein relation. It has been shown that DR is not influenced by the finer details of protein shape [35]. The large-scale shape irregularities, such as the binding cleft in hen egg-white lysozyme (HEWL), reduces DR because it displaces a larger number of solvent molecules than would a compact protein of the same volume (molecular mass). The shape of proteins can be estimated from the principal components of the rotational diffusion tensor as A= 2D11/(D22+D33). HYDROPRO estimates A= 0.97 which is very close to ideal spherical shape (A=1). The similar value, 0.93, was obtained for BLG [36] which structurally resembles TL. Interestingly, there was no deviation between calculated and experimentally determined values of rotational diffusion coefficient for BLG [36]. The mean rotational correlation time for TL calculated with HYDROPRO is 9.2 ns. The calculated overall rotational correlation time for TL is very close to that obtained from fluorescence anisotropy decay experiments (Fig. 6). The close agreement between calculated and experimental values for lipocalins reflects similar surface topology and the lack of extended polypeptide segments in crystal structures. As pointed out, [36] hydrodynamic calculation treats these extended tails (obviously flexible) as rigid. As a result, this treatment produces rotational correlation times of proteins that are much higher than one would expect from the contribution of this segment to molecular mass.
The current data for correlation times can be compared to values obtained previously. The correlation time of about 12.1* ns as measured by electron resonance paramagnetic resonance was published for a spin labeled TL mutant C99 that incorporated a motionally restricted nitroxide side chain [16]. At the time of our publication, 15.4 ns was the correlation time given for dimeric BLG [17]. Taking into consideration that TL is a smaller molecule than BLG, and the lack of any concentration dependent experiments, we erroneously concluded that 12.1* ns for TL represented a dimeric state. More recent studies suggest that the measured correlation time of 9.8 ns and 22 ns corresponds to the monomeric and dimeric states of BLG, respectively [19, 37, 38].
In this study the rotational correlation time of F99Dansyl calculated with fluorescence anisotropy was between 9-10.5 ns (Fig. 7) suggestive of a monomeric state for TL and corroborating the MALS data. The use of this method permitted increasing the protein concentration, in a range sensitive to detect equilibrium of monomer-dimer complexes. Since the correlation time was unchanged in increasing protein concentrations, as well as in various salt concentrations and tears, the possibility of TL homo or hetero-oligomer complexes is excluded. Expressed forms of TL, with or without a histidine tag, are also mainly monomeric. No increase in correlation time was detected with increasing protein concentration of these expressed forms.
4. Conclusion
The existence of TL in a monomeric state in tears has implications for its function as a lipid scavenger from the corneal surface and as a stabilizer of the tear film. The processes involved in stabilization of the tear film are unknown but will require different phases including translational diffusion of the protein-ligand complexes and its subsequent reorientation for enhanced interaction with the lipid layer of the tear film. The translational diffusion coefficient for monomeric TL is estimated to be 1.16 x 10−6 cm2/s from HYDROPRO. Given that the tear thickness is about 4 x 10−3cm and the ocular surface temperature is 308 K, the calculated average time required for monomeric TL to move the length of 40 micron is 5.4 sec. (Dt = 1.47 x 10−6 cm2/s, T=308K). The monomer of BLG (PDB:1b8e) happens to possess a mass that is quite similar to TL due to the unresolved C-terminus. The structure is almost superimposable with that of TL and amenable to comparison. The calculated correlation time is similar to that of TL, 9.1ns. Dimeric BLG (PDB: 1beb), with a molecular weight of 35.2 kDa has a calculated correlation time of 20.8 ns (Dt= 1.13 x 10−6 cm2/s, T=308 K) and is probably a reasonable estimate for dimeric TL. The dimeric form would take 7.1 seconds to diffuse the same distance. The data corroborate with previous findings. Surface pressure generation in the Langmuir trough experiment demonstrated that apo-TL compared to holo-TL has a longer induction phase [3]. The reorientation (rotational diffusion) time of the monomeric and dimeric forms of TL would differ about 2 times. Although oversimplified, these considerations may have relevance because tear break-up time, an accepted measure of tear film instability, occurs in the same general time frame. Furthermore, dry eye patients have unstable tear films with shorter tear breakup times. A lower concentration of TL (34-102 μM) correlates with a shorter tear break-up time (2-12 seconds) in dry eye patients compared to normal controls [39]. Although the mechanisms of insertion of tear lipocalin into the aqueous-lipid interface of tears are not known, future studies will need to account for monomeric tear lipocalin in the aqueous phase.
These experiments have implications for functional studies utilizing various forms of tear lipocalin. The complex mixture of isoforms of native TL as well as recombinant TL with and without the histidine tag exist as monomers. This is true for holo forms that are purified with the the naturally bound lipids from tears or the culture media (recombinant protein). However, apoTL is produced by rigorous multiple extractions with organic solvents. Although necessary for complete removal of bound lipids, this process results in oligomeric forms. These findings will be useful for future experiments in which interactions with other biologic components are tested.
Acknowledgments
Supported by U.S. Public Health Service Grants NIH EY-11224 and EY00331 as well as the Edith and Lew Wasserman Endowed Professorship in Ophthalmology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Glasgow BJ, Abduragimov AR, Farahbakhsh ZT, Faull KF, Hubbell WL. Tear lipocalins bind a broad array of lipid ligands. Curr Eye Res. 1995;14:363–372. doi: 10.3109/02713689508999934. [DOI] [PubMed] [Google Scholar]
- 2.Gasymov OK, Abduragimov AR, Prasher P, Yusifov TN, Glasgow BJ. Tear lipocalin: evidence for a scavenging function to remove lipids from the human corneal surface. Invest Ophthalmol Vis Sci. 2005;46:3589–3596. doi: 10.1167/iovs.05-0569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glasgow BJ, Marshall G, Gasymov OK, Abduragimov AR, Yusifov TN, Knobler CM. Tear lipocalins: potential lipid scavengers for the corneal surface. Invest Ophthalmol Vis Sci. 1999;40:3100–3107. [PubMed] [Google Scholar]
- 4.Nagyova B, Tiffany JM. Components responsible for the surface tension of human tears. Curr Eye Res. 1999;19:4–11. doi: 10.1076/ceyr.19.1.4.5341. [DOI] [PubMed] [Google Scholar]
- 5.Tragoulias ST, Anderton PJ, Dennis GR, Miano F, Millar TJ. Surface pressure measurements of human tears and individual tear film components indicate that proteins are major contributors to the surface pressure. Cornea. 2005;24:189–200. doi: 10.1097/01.ico.0000138837.52694.37. [DOI] [PubMed] [Google Scholar]
- 6.Yusifov TN, Abduragimov AR, Gasymov OK, Glasgow BJ. Endonuclease activity in lipocalins. Biochem J. 2000;347(Pt 3):815–819. [PMC free article] [PubMed] [Google Scholar]
- 7.Fluckinger M, Haas H, Merschak P, Glasgow BJ, Redl B. Human tear lipocalin exhibits antimicrobial activity by scavenging microbial siderophores. Antimicrob Agents Chemother. 2004;48:3367–3372. doi: 10.1128/AAC.48.9.3367-3372.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Skerra A. 'Anticalins': a new class of engineered ligand-binding proteins with antibody-like properties. J Biotechnol. 2001;74:257–275. doi: 10.1016/s1389-0352(01)00020-4. [DOI] [PubMed] [Google Scholar]
- 9.Gasymov OK, Abduragimov AR, Yusifov TN, Glasgow BJ. Site-directed tryptophan fluorescence reveals the solution structure of tear lipocalin: evidence for features that confer promiscuity in ligand binding. Biochemistry. 2001;40:14754–14762. doi: 10.1021/bi0110342. [DOI] [PubMed] [Google Scholar]
- 10.Breustedt DA, Korndorfer IP, Redl B, Skerra A. The 1.8-A crystal structure of human tear lipocalin reveals an extended branched cavity with capacity for multiple ligands. J Biol Chem. 2005;280:484–493. doi: 10.1074/jbc.M410466200. [DOI] [PubMed] [Google Scholar]
- 11.Brownlow S, Morais Cabral JH, Cooper R, Flower DR, Yewdall SJ, Polikarpov I, North AC, Sawyer L. Bovine beta-lactoglobulin at 1.8 A resolution--still an enigmatic lipocalin. Structure. 1997;5:481–495. doi: 10.1016/s0969-2126(97)00205-0. [DOI] [PubMed] [Google Scholar]
- 12.Gouveia SM, Tiffany JM. Human tear viscosity: an interactive role for proteins and lipids. Biochim Biophys Acta. 2005;1753:155–163. doi: 10.1016/j.bbapap.2005.08.023. [DOI] [PubMed] [Google Scholar]
- 13.Glasgow BJ. Tissue expression of lipocalins in human lacrimal and von Ebner's glands: colocalization with lysozyme. Graefes Arch Clin Exp Ophthalmol. 1995;233:513–522. doi: 10.1007/BF00183433. [DOI] [PubMed] [Google Scholar]
- 14.Redl B, Holzfeind P, Lottspeich F. cDNA cloning and sequencing reveals human tear prealbumin to be a member of the lipophilic-ligand carrier protein superfamily. J Biol Chem. 1992;267:20282–20287. [PubMed] [Google Scholar]
- 15.Glasgow BJ, Abduragimov AR, Yusifov TN, Gasymov OK, Horwitz J, Hubbell WL, Faull KF. A conserved disulfide motif in human tear lipocalins influences ligand binding. Biochemistry. 1998;37:2215–2225. doi: 10.1021/bi9720888. [DOI] [PubMed] [Google Scholar]
- 16.Glasgow BJ, Gasymov OK, Abduragimov AR, Yusifov TN, Altenbach C, Hubbell WL. Side chain mobility and ligand interactions of the G strand of tear lipocalins by site-directed spin labeling. Biochemistry. 1999;38:13707–13716. doi: 10.1021/bi9913449. [DOI] [PubMed] [Google Scholar]
- 17.Eftink M. Quenching-resolved emission anisotropy studies with single and multitryptophan-containing proteins. Biophys J. 1983;43:323–334. doi: 10.1016/S0006-3495(83)84356-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Voss J, Salwinski L, Kaback HR, Hubbell WL. A method for distance determination in proteins using a designed metal ion binding site and site-directed spin labeling: evaluation with T4 lysozyme. Proc Natl Acad Sci U S A. 1995;92:12295–12299. doi: 10.1073/pnas.92.26.12295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uhrinova S, Smith MH, Jameson GB, Uhrin D, Sawyer L, Barlow PN. Structural changes accompanying pH-induced dissociation of the beta-lactoglobulin dimer. Biochemistry. 2000;39:3565–3574. doi: 10.1021/bi992629o. [DOI] [PubMed] [Google Scholar]
- 20.Wen J, Arakawa T, Philo JS. Size-exclusion chromatography with on-line light-scattering, absorbance, and refractive index detectors for studying proteins and their interactions. Anal Biochem. 1996;240:155–166. doi: 10.1006/abio.1996.0345. [DOI] [PubMed] [Google Scholar]
- 21.Glasgow BJ, Heinzmann C, Kojis T, Sparkes RS, Mohandas T, Bateman JB. Assignment of tear lipocalin gene to human chromosome 9q34-9qter. Curr Eye Res. 1993;12:1019–1023. doi: 10.3109/02713689309029229. [DOI] [PubMed] [Google Scholar]
- 22.Gasymov OK, Abduragimov AR, Yusifov TN, Glasgow BJ. Resolution of ligand positions by site-directed tryptophan fluorescence in tear lipocalin. Protein Sci. 2000;9:325–331. doi: 10.1110/ps.9.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holzfeind P, Merschak P, Rogatsch H, Culig Z, Feichtinger H, Klocker H, Redl B. Expression of the gene for tear lipocalin/von Ebner's gland protein in human prostate. FEBS Lett. 1996;395:95–98. doi: 10.1016/0014-5793(96)01008-3. [DOI] [PubMed] [Google Scholar]
- 24.Gasymov OK, Abduragimov AR, Yusifov TN, Glasgow BJ. Binding studies of tear lipocalin: the role of the conserved tryptophan in maintaining structure, stability and ligand affinity. Biochim. Biophys. Acta. 1999;1433:307–320. doi: 10.1016/s0167-4838(99)00133-8. [DOI] [PubMed] [Google Scholar]
- 25.Wyatt PJ. Light scattering and the absolute characterization of macromolecules. Anal.Chim. Acta. 1993;272:1–40. [Google Scholar]
- 26.Carrasco B, Harding SE, Garcia de la Torre J. Bead modeling using HYDRO and SOLPRO of the conformation of multisubunit proteins: sunflower and rape-seed 11S globulins. Biophys Chem. 1998;74:127–133. doi: 10.1016/s0301-4622(98)00170-7. [DOI] [PubMed] [Google Scholar]
- 27.Garcia De La Torre J, Huertas ML, Carrasco B. Calculation of hydrodynamic properties of globular proteins from their atomic-level structure. Biophys J. 2000;78:719–730. doi: 10.1016/S0006-3495(00)76630-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carrasco B, de la Torre JG, Byron O, King D, Walters C, Jones S, Harding SE. Novel size-independent modeling of the dilute solution conformation of the immunoglobulin IgG Fab' domain using SOLPRO and ELLIPS. Biophys J. 1999;77:2902–2910. doi: 10.1016/S0006-3495(99)77123-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garcia de la Torre J, Carrasco B, Harding SE. SOLPRO: theory and computer program for the prediction of SOLution PROperties of rigid macromolecules and bioparticles. Eur Biophys J. 1997;25:361–372. doi: 10.1007/s002490050049. [DOI] [PubMed] [Google Scholar]
- 30.Gasymov OK, Abduragimov AR, Yusifov TN, Glasgow BJ. Interaction of tear lipocalin with lysozyme and lactoferrin. Biochem Biophys Res Commun. 1999;265:322–325. doi: 10.1006/bbrc.1999.1668. [DOI] [PubMed] [Google Scholar]
- 31.Squire PG, Himmel ME. Hydrodynamics and protein hydration. Arch Biochem Biophys. 1979;196:165–177. doi: 10.1016/0003-9861(79)90563-0. [DOI] [PubMed] [Google Scholar]
- 32.Perkins SJ. Protein volumes and hydration effects. The calculations of partial specific volumes, neutron scattering matchpoints and 280-nm absorption coefficients for proteins and glycoproteins from amino acid sequences. Eur J Biochem. 1986;157:169–180. doi: 10.1111/j.1432-1033.1986.tb09653.x. [DOI] [PubMed] [Google Scholar]
- 33.Denisov VP, Halle B. Protein hydration dynamics in aqueous solution. Faraday Discuss. 1996:227–244. doi: 10.1039/fd9960300227. [DOI] [PubMed] [Google Scholar]
- 34.Van-Quynh A, Willson S, Bryant RG. Protein reorientation and bound water molecules measured by 1H magnetic spin-lattice relaxation. Biophys J. 2003;84:558–563. doi: 10.1016/S0006-3495(03)74875-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao H, Pearlstein AJ. Stokes-flow computation of the diffusion coefficient and rotational diffusion tensor of lysozyme, a globular protein. Phys. Fluids. 2002;14:2376–2387. [Google Scholar]
- 36.Halle B, Davidovic M. Biomolecular hydration: from water dynamics to hydrodynamics. Proc Natl Acad Sci U S A. 2003;100:12135–12140. doi: 10.1073/pnas.2033320100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Collini M, Leo B, Baldini G, Monaco HL, Galliano M. Probing protein aggregation by time-resolved fluorescence during beta-lactoglobulin crystal growth. Eur Biophys J. 2002;31:111–117. doi: 10.1007/s00249-002-0208-4. [DOI] [PubMed] [Google Scholar]
- 38.Gottschalk M, Nilsson H, Roos H, Halle B. Protein self-association in solution: the bovine beta -lactoglobulin dimer and octamer. Protein Sci. 2003;12:2404–2411. doi: 10.1110/ps.0305903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamada M, Mochizuki H, Kawai M, Tsubota K, Bryce TJ. Decreased tear lipocalin concentration in patients with meibomian gland dysfunction. Br J Ophthalmol. 2005;89:803–805. doi: 10.1136/bjo.2004.055822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guex N, Peitsch MC. SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modeling. Electrophoresis. 1997;18:2714–2723. doi: 10.1002/elps.1150181505. [DOI] [PubMed] [Google Scholar]