Abstract
Cryptochromes (Cry) have been suggested to form the basis of light-dependent magnetic compass orientation in birds. However, to function as magnetic compass sensors, the cryptochromes of migratory birds must possess a number of key biophysical characteristics. Most importantly, absorption of blue light must produce radical pairs with lifetimes longer than about a microsecond. Cryptochrome 1a (gwCry1a) and the photolyase-homology-region of Cry1 (gwCry1-PHR) from the migratory garden warbler were recombinantly expressed and purified from a baculovirus/Sf9 cell expression system. Transient absorption measurements show that these flavoproteins are indeed excited by light in the blue spectral range leading to the formation of radicals with millisecond lifetimes. These biophysical characteristics suggest that gwCry1a is ideally suited as a primary light-mediated, radical-pair-based magnetic compass receptor.
Introduction
Each year millions of migratory birds travel thousands of kilometres between their breeding and wintering grounds. A magnetic compass sense has been shown to play a key role in enabling them to find their way [1]–[3]. But how do migratory birds detect the reference compass direction provided by the Earth's magnetic field? This remains one of the most fascinating unresolved mysteries of sensory biology. From behavioural experiments, we know that the ability of night-migrating birds to perform magnetic compass orientation is affected by the wavelengths of the ambient light [4], [5] and that pinealectomised birds [6] are still able to use their magnetic compass to orient in the laboratory. This led to the suggestion that photochemical reactions in the birds' eyes could produce spin-correlated radical pairs as the primary sensor in a magnetic field-dependent signalling cascade [7], [8], based on the fact that the recombination reactions of spin-correlated radical pairs are known to be magnetic field-sensitive under favourable conditions [9], [10].
Spin-correlated radical pairs are typically the result of a photochemical reaction (e.g. electron transfer) that creates radicals from a molecular precursor that is in either an electronic singlet (S) or triplet (T) state. Under the influence of intramolecular electron-nuclear hyperfine interactions, the radical pair can oscillate between the S and T states, a process known as S↔T interconversion or S↔T mixing. External static magnetic fields can affect the extent and frequency of this process and hence alter the yields of the respective reaction products formed from the S and T radical pairs [10], [11]. In order for a magnetic field to have a substantial effect on the S↔T mixing process, the radical pair needs to be sufficiently long-lived (for an Earth-strength magnetic field, the radical pair lifetime should probably exceed ∼1 µs; [12]). Despite encouraging theoretical considerations [8] and supporting calculations [12], [13], there is no experimental proof as yet that the recombination yields of such radical pair reactions are sensitive to the orientation of the pair with respect to the external magnetic field (or indeed that any radical pair reaction responds significantly to a field as weak as the Earth's (∼50 µT)). This directional information is of course of pivotal importance in the discussion of magnetoreception in migratory birds.
Magnetic fields oscillating with frequencies in the MHz range are also known to affect the S↔T interconversion in spin-correlated radical pairs, even in the absence of a static external magnetic field [8], [14]–[16]. The hypothesis that the avian magnetic compass is based on a radical pair mechanism is strongly supported by the observation that magnetic compass orientation of migratory birds can indeed be disturbed by weak radiofrequency magnetic fields with frequencies in the MHz range [17], [18]. However, for this radical-pair hypothesis to be realistic it must be shown that molecules with the required biophysical characteristics actually exist in the eyes of migratory birds. Cryptochromes [19], a class of proteins that exhibit a close homology to photolyases (flavoproteins involved in the repair of UV light-induced DNA damage), were first proposed by Ritz et al. [8] as the host molecules for the crucial radical pair cofactors that putatively act as a primary magnetoreceptor. It has recently been demonstrated, using UV/visible and EPR (electron paramagnetic resonance) spectroscopies [20]–[22], that photolyases are able to form long-lived radical-pairs including a flavin radical and a radical derived from a redox-active amino acid. So far it has been shown that cryptochromes exist in the eyes of migratory birds [23], [24], and that at least some cryptochrome-containing cells within the retina are active at night when the birds perform magnetic orientation in the laboratory [23]. Furthermore, a distinct part of the forebrain which primarily processes input from the eye is highly active at night in night-migratory garden warblers (Sylvia borin) and European robins (Erithacus rubecula) [25], [26]. All these findings are consistent with the hypothesis that magnetic compass detection in migratory birds takes place in the eye and that cryptochromes could be a primary magnetoreceptors. Despite this mounting body of indirect evidence, it has still be to demonstrated convincingly that cryptochrome is directly involved in the avian magnetic compass sense. For instance, several fundamental biophysical characteristics including the absorption spectrum and the formation of long-lived radical pairs have not been experimentally validated for any vertebrate cryptochrome [27]. The aim of the present study is to experimentally explore the biophysical properties of cryptochromes from migratory garden warblers.
Materials and Methods
Cloning
For detection and cloning of garden warbler (gw) Cry1 transcripts, total RNA was extracted from the retina of a garden warbler performing magnetic orientation behaviour in an orientation cage [28] in the laboratory during the migratory season as described in [23]. The retina was collected immediately after the bird had shown at least 1 h of consistent migratory-restlessness behavior. cDNA synthesis was performed from 450 ng of RNA with Revert AID H−First-Strand cDNA synthesis kit (MBI Fermentas) according to the manufacturer's protocol.
Hot start PCR amplification reactions were performed in 25 µl total reaction volume containing 1 µl of the cDNA synthesis reaction as template, 1× reaction buffer (SAWADY/Amersham-Pharmacia, depending on the DNA polymerase used), 0.25 mM dNTPs (Pharmacia Biotech), of each primer 1.25 pmol/µl PCR volume, 1 unit of Pwo polymerase (SAWADY) or 2 units of Taq polymerase (Amersham-Pharmacia). The PCR reaction was carried out in a MWG Primus25 thermocycler. The temperature profile for all hot-start PCR reactions was as follows: first denaturation 3 min 94°C; 45 cycles of 45 s at 94°C, 45 s annealing at 54°C and 40 s extension at 72°C (the duration of extension phase depended on the expected size of the amplified fragment and the enzyme used; the extension step comprised 1 min/kb DNA for amplification with Taq polymerase and 2 min/kb DNA for Pwo amplification); the last cycle was extended to 5 min at 72°C to ensure completion of the final extension. The full length sequence of gwCry1a (GenBank accession no. AJ632120) was reconstructed as described in Mouritsen et al. [23].
To probe for potential influences of the C-terminal extension of gwCry1a in the reaction process, we additionally expressed the photolyase homology region (510 aa) of gwCry1a (Cry1-PHR). The PHR lies within the N-terminal domain, which is identical for both gwCry1a and gwCry1b (GenBank accession no. DQ838738), is similar to photolyases and highly conserved in all members of the photolyase/cryptochrome superfamily [29], [30]. For expression of gwCry1-PHR the N-terminal 510 amino acids were chosen based on the structural analysis of A. thaliana Cry1-PHR [29] and the lack of homology between garden warbler and A. thaliana Cry1 beyond amino acid residue 510. A C-terminal fragment for gwCry1-PHR was PCR-amplified from a cloned gwCry1a template with the primer pair: DSP1PHR (p741), 5′-TAGTTCCTTCTTTCAGCAGTTT-3′; USP1PHR (p742), 5′-AGCAAGCGGCCGCTTATCAA GTTGCAAGAAGACCCAGTCCT-3′. The amplified fragment (320 bp) was digested with BstXI/NotI (resulting in a 107 bp fragment) and cloned with a 1408 bp N-terminal fragment isolated from a full-length gwCry1a plasmid by NcoI/partial BstXI digest of the full length gwCry1a plasmid.
Coding regions for gwCry1a and gwCry1-PHR proteins were subcloned into a pVL1392-derived baculovirus transfervector to allow for subsequent recombinant expression of the target proteins in a Sf9/baculovirus expression system. For selective purification by means of immobilised metal affinity chromatography (IMAC) matrices, we expressed the genes as fusions with a vector-encoded N-terminal (His)6-tag. The full length sequence of gwCry1a (1866 bp) was subcloned into the polylinker region of the pDIA92B-His transfervector by SmaI/NotI digest. The PHR region of gwCry1 (1536 bp) was subcloned into the polylinker region via NcoI/NotI digest. Recombinant baculoviruses were generated by cotransfection of transfer vectors and baculoviral genomic DNA and clonal isolation of gwCry1 protein-expressing baculoviruses according to standard methods [31], [32].
Recombinant expression
To characterise the molecular properties of gwCry1a and gwCry1-PHR proteins, we expressed the proteins in Sf9 cells transfected with the corresponding recombinant baculovirus. Infected cells were incubated in ExCell420 culture medium (JRH) supplemented with 25 µM each of FAD and FMN (Sigma) in spinner flasks (0.6 l culture volume) for 66 h (±4 h) at 27°C. Cell pellets were harvested, washed in PBS and quick-frozen in liquid nitrogen.
Purification
gwCry1a (628 aa; ∼62 kDa) and gwCry1-PHR (510 aa; ∼56 kDa) were expressed as soluble fusion proteins and purified to near homogeneity by immobilized metal affinity chromatography IMAC) in 20 mM HEPES buffer (pH 7.4) containing 500 mM NaCl and 50 µM FAD. Recombinant gwCry1a was bound to the Chelating Sepharose matrix and unspecifically bound contaminants were washed away with 20 mM HEPES buffer containing 500 mM NaCl and 50 µM FAD (pH 7.4). The column-bound protein was denatured by a linear gradient (10 column volumes) of 0 M to 6 M urea in 20 mM HEPES buffer containing 50 µM FAD, renatured by a “reversed” linear gradient from 6 M to 0 M urea in 20 mM HEPES 50 µM FAD buffer, and finally eluted by increasing imidazole concentration. Samples were concentrated in Vivaspin contractor columns (membrane: 30,000 MWCO) (Sartorius). Excess unbound (free) flavin was eliminated by a final desalting step using HiTrap 5 ml columns (Amersham Bioscience). Samples were stored in 20% glycerol at a final protein concentration of 15–25 µM, shock frozen in liquid nitrogen and stored at −80°C.
The quality of the purified proteins was analysed by SDS-PAGE and Western blot analyses using standard procedures. On PVDF membrane (Pall) Western blots, gwCry1a protein was probed by goat polyclonal antibody α(anti)-Cry1 (A-20) sc-5953 (Santa Cruz Biotechnology), and gwCry1-PHR was detected by a commercially available mouse monoclonal α-His tag antibody (PentaHis antibody, Qiagen); the primary antibodies were detected by appropriate alkaline phosphatase-conjugated secondary antibodies and nitroblue tetrazolium/bromo-chloro-indoxyl phosphate staining on the membrane.
Samples were concentrated by ultrafiltration through 30 kDa microconcentrators (Millipore, Billerica, MA, USA) at 4°C. The flowthrough was periodically monitored spectroscopically: concentrations of free flavin were below the detection limit of 1 µM.
UV/visible spectral analysis
Purified gwCry1a and gwCry1-PHR, both containing FAD in its fully oxidised redox state, were supplemented with 5 mM DTT, deoxygenated and illuminated at 290 K with blue light (Halolux 30HL, Streppel, Wermelskirchen-Tente, Germany) selected with a 420–470 nm band filter (Schott, Mainz). Reoxidation was performed under aerobic conditions in the dark. The concentration of protein-bound flavin was estimated based on its absorbance at 450 nm (ε450 = 11.3×104 M−1 cm−1). The concentrations of gwCry1-PHR and gwCry1a holoproteins, with FAD bound, were 7 µM and 4 µM respectively.
Transient absorption experiments
Solutions of proteins (concentrations in the range 15–25 µM) in 20 mM HEPES buffer and 20% glycerol at 270 K were photoexcited at 355 nm using a Nd-YAG laser (5 ns, 5 mJ/pulse). The monitoring beam was provided by a tungsten lamp (150 W). Light from the sample was fed to a monochromator (Newport model 77250) before entering a photomultiplier tube (Hamamatsu R-928). The time-resolution of the measurement was 20 µs. The signals presented in Figure 1 are averages of 2 measurements.
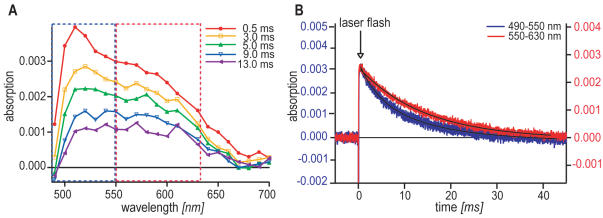
Figure 1. Transient absorption spectra for gwCry1a.
A Transient absorption spectra for gwCry1a as a function of wavelength (monitoring beam) proves that radical pairs are produced in cryptochromes of a migratory bird. The five graphs reflect the optical properties of the sample at different times, t delay, after the laser flash. The amplitude of the signals depends on the protein concentration. The spectra were obtained by averaging two time profiles (cf. Fig. 1B) centred at t delay using a time window of 500 µs. The variation of the absorption signal is about 5%, which is estimated from the data at 0.5 ms from 500 to 600 nm. For a detailed analysis of the spectra, see main text. B Transient absorption time profiles for gwCry1a. A laser flash (indicated by the arrow) is given at time = 0. The transient signal is obtained by averaging 7 time-profiles for the 490–550 nm wavelength region (corresponding to the optical features framed in blue in Fig. 1A) in 10 nm steps (blue curve, left scale) and 9 time-profiles for the 550–630 nm region (corresponding to the optical features framed in red in Fig. 1A) in 10 nm steps (red, graph, right scale), respectively. Solid lines show the fitted single or double exponential decays. The laser flash leads to radical formation as reflected by positive changes in the absorption signal. Significant radical concentrations are detectable up to ∼25 ms. For further discussion, see main text.
NMR spectroscopy
600 MHz 1H NMR spectra of the ∼20 µM gwCry1a samples used for the transient absorption experiments were recorded on a Varian Inova 600 (14.1 T) NMR spectrometer to confirm the absence of significant amounts of free flavin (see Supporting Information (Text S1, Fig. S1)).
Animal husbandry as well as all experiments and animal procedures were approved by the Animal Care and Use Committees of the Bezirksregierung Weser-Ems (Oldenburg, Germany).
Results
Garden warbler cryptochrome (gwCry1a) absorbs blue light
To determine the visible absorption spectra of garden warbler cryptochromes, we recombinantly expressed and purified Cry1a and the photolyase homology region of the migratory warbler (shown for gwCry1a in Fig. 2). The absorption spectra of purified gwCry1a and gwCry1-PHR proteins are shown in Fig. 3. FAD (flavin adenine dinucleotide) cofactors show characteristic optical absorption properties in all three of their biologically relevant redox states: fully reduced (FADH− or FADH2), semiquinone radical (FAD•− or FADH•), and fully oxidised (FADox). Both cryptochrome constructs were isolated in a yellow form characteristic of a flavoprotein with a fully oxidised cofactor, FADox. gwCry1a and gwCry1-PHR with bound FADox both have absorption maxima at 450 and 366 nm representing respectively the S0→S1 and S0→S2 transitions of the flavin cofactor. Members of the photolyase family often bind a pterin or deazaflavin in addition to the FAD [for reviews see 30], [33], [34]: there was no evidence of a second chromophore in either of the samples. The spectra resemble those of other flavoproteins [35] except that the vibrational structure of the 450 nm band is not pronounced; we return to this point below.
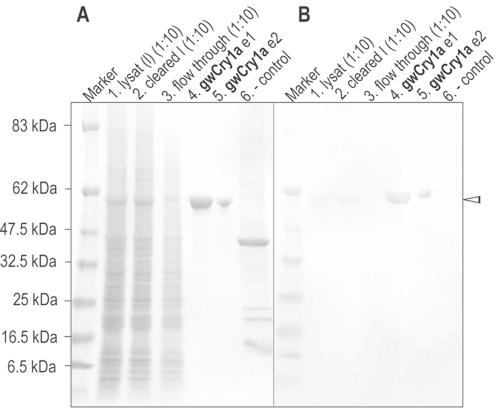
Figure 2. SDS-PAGE of recombinantly expressed garden warbler cryptochrome (shown for gwCry1a protein).
A Coomassie stained SDS gel, and B Western-blot (with α-Cry1 antiserum from immunised goat), both showing identical fractions of the IMAC purification process. gwCry1a (628 aa) is recognisable as a distinct band at ∼62 kDa in both the coomassie stained SDS gel and on the Western-blot (indicated by the arrowhead to the right). 1: gwCry1a lysate (protein extract from insect cell pellet, 1∶10 dilution); 2: gwCry1a cleared lysate (centrifuged and filtered lysate, 1∶10 dilution); 3: gwCry1a flow through (after sample loading on column, 1∶10 dilution); 4: gwCry1a elution step1 (e1) [75% imidazole]; 5: gwCry1a elution step2 (e2) [100% imidazole] protein quantity for e2 is 5 µg; 6: negative control (recombinantly expressed human La/SSB autoantigen; ∼48 kDa) confirms the specificity of the antibody.
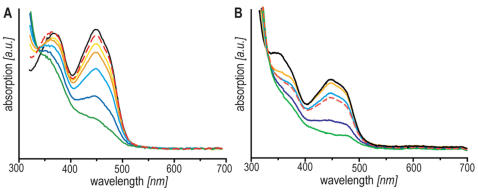
Figure 3. Optical spectroscopy of cryptochrome holoprotein from migratory garden warblers.
A gwCry1-PHR, illumination time: 0 s (black), 40 s (yellow), 80 s (orange), 130 s (light blue), 190 s (blue) and 340 s (green), respectively. B gwCry1a, illumination time: 0 s (black), 90 s (yellow), 150 s (orange), 240 s (light blue), 360 s (blue) and 540 s (green), respectively. Both samples were illuminated under anaerobic conditions as indicated in the Methods section. Reoxidation was achieved by bubbling air into the optical cuvette (red dashed lines).
Illumination of gwCry1a and gwCry1-PHR with blue light (420<λ<470 nm) attenuates both bands (Fig. 3). This behaviour is characteristic of the photolyase/cryptochrome superfamily [e.g. 36], [37], [38]. After illumination in the presence of dithiothreitol (DTT; as an exogenous electron donor), the fully (two-electron) reduced FADH− form of the flavin was formed (Fig. 3), exhibiting a broad absorption band around 360 nm. In contrast to other members of the photolyase/cryptochrome family, however, flavin radicals (exhibiting absorption maxima at around 580 and 620 nm) do not accumulate to a significant extent in gwCry1a and gwCry-PHR. Moreover, no significant radical formation is observed while reoxidising the proteins under aerobic conditions. The reason could be either a small energy difference between the radical and the fully reduced form of the FAD, or, more likely, that radical disproportionation is occurring on a fast timescale [20].
Garden warbler cryptochrome forms long-lived radical-pairs
To test whether gwCry1a forms radical-pairs upon photoexcitation and to investigate whether they have sufficient lifetimes for the putative geomagnetic field effects to take place, we recorded transient absorption spectra for gwCry1a protein using laser flash photolysis at 355 nm. The identity of the transient photo-intermediates formed from gwCry1a was investigated via their time-resolved absorption characteristics (Fig. 1). Figure 1A shows the transient absorption spectra obtained for gwCry1a as a function of both the time delay between the laser flash and the 500 µs monitoring window (t delay = 0.5, 3, 5, 9, 13 ms) and the wavelength of the monitoring light (horizontal axis). The spectra show that garden warbler cryptochrome forms long-lived radical pairs and that this radical-pair formation is strongly dependent on both wavelength and time, with a pronounced absorption peak around 510 nm at early times (t delay = 0.5 ms). It is clear from a comparison of the five spectra, that this peak (blue dotted frame) has a shorter lifetime than the features at longer wavelengths (red dotted frame). Figure 1B shows this behaviour in a more quantitative fashion. Here, the absorption of the sample is detected as a function of time after the laser flash for the two wavelength regions (blue curve: 490–550 nm; red curve: 550–630 nm). The shorter wavelength decay can only be modelled satisfactorily as a double exponential decay with lifetimes of 4 ms and 14 ms, while the longer wavelength decay can be accurately modelled as a single exponential with a lifetime of 14 ms.
The absence of pronounced vibrational structure around 450 nm in Fig. 3 may, at first glance, suggest a free flavin molecule rather than a flavin cofactor bound to a photolyase or a cryptochrome. The protein purification protocol used (see Material and Methods and Supporting Information (Text S1, Fig. S1, Fig. S2)) makes it is very unlikely that there were any significant amounts of free flavin left in our samples. Nevertheless, considering the form of the recorded spectra, it is important to demonstrate unequivocally that the transient absorption data (Fig. 1) cannot originate from radicals formed from free flavin molecules. We therefore performed a number of control experiments including NMR spectroscopy and transient absorption measurements on solutions of FAD and FMN (flavin mononucleotide) with either tryptophan or hen lysozyme, a ∼14 kDa protein containing two solvent-accessible tryptophan residues that are known to be reactive with photoexcited flavins [39], [40]. As described in detail in the Supporting Information (Text S1, Fig. S1, Fig. S2), these control experiments include NMR spectra that show no detectable amount of free flavin in the samples; additional ultra-filtration steps after protein purification that yield no detectable amount of free flavin; transient absorption measurements showing that free flavin results in signals that are one to two orders of magnitude weaker than the cryptochrome signals presented here and that free flavin radicals are about two orders of magnitude shorter lived than those observed for garden warbler cryptochrome under the same conditions. These control experiments unequivocally establish that the data presented here do indeed result from photo-induced intra-protein electron transfer between garden warbler cryptochrome and its bound flavin cofactor.
Discussion
We have experimentally verified several key assumptions crucial for the theoretical concept of light-mediated magnetoreception in birds [7], [8]. We succeeded in expressing gwCry1a, which occurs in the retinae of magnetically orienting night-migratory garden warblers [23], and its highly conserved N-terminal PHR domain as holoproteins (with their flavin cofactor bound). The absorption spectra of flavoproteins are dependent on the redox status of the bound flavin cofactor, which in turn is influenced both by the apoprotein and by the redox environment [41]. gwCry1a and gwCry1-PHR purified from insect cells both contained the flavin cofactor in the fully oxidised state (Fig. 3), as was also found for isolated AtCry1 from the plant Arabidopsis thaliana [36]. In isolated photolyases, however, the flavin cofactor is usually bound in a mixture of reduced and semiquinone states [e.g. 42], [43]. The absorption spectrum of gwCry1a fits with the observations that songbirds, including garden warblers, are able to orient well using only their magnetic compass under blue and turquoise light, whereas they seem challenged doing this under yellow and red light [2], [4], [5]. The fact that songbirds are well-oriented under green light (565 nm) is not consistent with the absorption spectra of the fully oxidised form of garden warbler cryptochrome. However, a second longer-wavelength receptor could well be involved [e.g. 5], [44,45].
Using a laser flash photolysis experiment, we were able to prove that illumination of gwCry1a (at 355 nm) produces radical pair species with lifetimes of the order of milliseconds. This is a crucial finding as the evolution of a magnetic field effect relies on the existence of radical pairs that live long enough for the field to affect the S↔T mixing processes. For a magnetic field as weak as that of the Earth, the lifetime should exceed at least a microsecond; a condition clearly fulfilled by the radical species produced in the cryptochromes investigated here which live about five orders of magnitude longer than the minimum required lifetime. Although radical pairs with appropriate lifetimes have been detected in AtCry1 [37], and now in the migratory garden warbler, it has still to be demonstrated directly that magnetic fields as weak as the Earth's (∼50 µT) can affect cryptochrome signalling in any species.
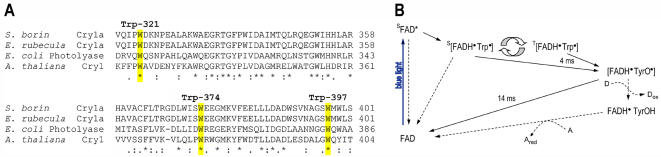
The three members of the Trp-triad electron transfer chain in AtCry1 (Trp-400, Trp-377, Trp-324) that are involved in signalling through photoreduction [37], [46] are highly conserved amongst members of the photolyase/cryptochrome family and clearly present (Trp-397, Trp-374, Trp-321) in the Cry1a sequences of both migratory garden warbler and European robin (Erithacus rubecula) cryptochromes, (Fig. 4A). On this basis, and by analogy with the recently proposed reaction scheme for AtCry1 [37] (Fig. 4B) we interpret the results as follows. Illumination of the sample by the laser pulse is followed by rapid formation of a primary radical pair consisting of a flavin radical, FADH• (broad absorption from 500 to 650 nm, 5 ms half-life in AtCry1, 14 ms lifetime in gwCry1a) and a tryptophanyl radical, Trp• (narrow component from 500 to 550 nm, 1 ms half-life in AtCry1, 4 ms lifetime in gwCry1a). This process may be followed by an electron transfer from a tyrosine residue to the Trp• to produce a tyrosyl radical, TyrO•, accompanied by a pronounced positive contribution to the transient absorption spectrum in the 460–560 nm range. Giovani et al. [37] also observe two slow components (half-lives of 5 ms and > 100 ms, respectively) corresponding to the FADH•→TyrO• back electron transfer. The similarities in the two time constants (for the TyrOH→Trp• and FADH•→TyrO• electron transfers) strongly indicates that gwCry1a and AtCry1 have very similar responses to illumination. The differences in the time constants are probably due to the different nature of the two cryptochromes, and hence the environments of the cofactors, and to the different temperatures of the two measurements (270 K here, 285 K in [37]). The pronounced feature at 510 nm in the transient absorption experiments at t delay = 0.5 ms (red trace in Fig. 1A) is most likely attributable to the TyrOH→Trp• electron transfer, again in accordance with the findings of Giovani et al. [37].
Figure 4. Sequence alignment between different members and species within the cryptochrome/photolyase protein family.
A Sequence alignment of Cry1a of two migratory songbird species Sylvia borin (Garden warbler; GenBank accession no. AJ632120), Erithacus rubecula (European robin; AY585716), Cry1 of Arabidopsis thaliana (mouse-ear cress; Q43125) and DNA photolyase of Escherichia coli (P00914). The alignment shows that all three members of the Trp-triad electron transfer chain known to be involved in intraprotein electron transfer during signalling through photoreduction in both E. coli photolyse and AtCry1 are clearly present in the Cry1a sequences of both migratory bird species (Trp-397, Trp-374, Trp-321). B Reaction scheme for gwCry1a (adapted from a very similar scheme for AtCry1 proposed by Giovani et al. [36]). Processes directly observed here are represented by solid arrows. Other steps are indicated by dashed arrows [36]. The excited state of the FAD is assumed here to be a singlet; an almost identical scheme would result if, instead, triplet FAD were assumed to be the photoactive excited state. Singlet-triplet interconversion in the [FADH• Trp•] radical pair is indicated by the curved block arrows. D and A are respectively an external electron donor and acceptor.
The methods used to purify cryptochrome of the migratory garden warbler together with the NMR measurements and transient absorption control experiments (see Supporting Information, (Text S1, Fig. S1, Fig. S2)) all indicate strongly that the spectroscopic data presented here are due to photo-induced electron transfer in garden warbler cryptochrome. Nevertheless, the flavin absorption band of our sample does bear a closer resemblance to that of free flavin than that of previously published members of the photolyase/cryptochrome superfamily. Given that there is no significant amount of free flavin present, this suggests that either the flavin is incorrectly bound to the apo-protein, e.g. in a non-native binding site, or that it is in the native binding pocket but, perhaps, rather loosely bound. If the former were true, then it is highly unlikely that there would be aromatic amino acid residues close enough to the flavin to undergo photoinduced electron transfer to give long-lived radicals in high yield, as observed. There could be a nearby tryptophan or tyrosine residue that might donate an electron to the flavin excited state, but such a radical pair would almost certainly be as short-lived with respect to back electron transfer as to be undetectable in our experiments. To get long-lived radicals by intramolecular electron transfer, there must be the possibility of sequential electron transfers as occurs with the Trp-triad in photolyase [47], [48]. It seems highly unlikely that a non-natively bound flavin would, by chance, be close to a series of electron donors at just the right separations and orientations to allow efficient charge separation and energy stabilization and to result in lifetimes so similar to those measured by Giovani et al. [37]. By elimination, therefore, we are left with the conclusion that the flavin is bound in the correct site and with the correct orientation to undergo sequential electron transfer reactions with aromatic amino acid sidechains in the protein. The absence of more pronounced vibrational structure in the absorption spectrum seems to be a characteristic of the holo-form of gwCry1. Why it has this unusual property will be a subject of future work.
Crucially, the work presented here proves that cryptochromes of a migratory bird absorb light leading, in gwCry1a, to formation of radical species that live about five orders of magnitude longer than the ∼1 µs theoretically required to elicit significant Earth-strength magnetic field effects. It is important to realise, however, that the radical pairs do not only have to be long lived, but their spin-correlation has also to persist for at least ∼1 µs. This issue can be addressed by time-resolved EPR and by measurements of the magnetic field-sensitivity of the radical pair lifetime; both experiments are challenging because of the low quantum yield of radical pair formation [37]. So far, no animal cryptochrome has been expressed and isolated as a holoprotein in sufficiently high concentration to perform these additional analyses. Furthermore, the cryptochromes have to be restricted in their motion in order for any potential magnetic field effect to encode directional information. In any case, our observation of very long-lived photoinduced radical pairs in a migratory bird cryprochrome considerably strengthens the hypothesis of cryptochromes as the primary magnetoreceptor molecules in the avian magnetic compass [8], [49].
Supporting Information
NMR measurements and transient absorption control experiments.
(0.04 MB DOC)
Aromatic region of the 600 MHz 1H NMR spectrum of gwCRY1a protein. Aromatic region of the 600 MHz 1H NMR spectrum of the CRY1a sample used for the transient absorption experiments before (blue) and after (red) addition of 20 µM FAD. Note the additional signals from FAD in the latter spectrum and their absence in the former.
(3.63 MB TIF)
Transient absorption signals at 550 nm for garden warbler cryptochrome and equimolar mixtures of FAD with hen lysozyme. Transient absorption signals at 550 nm recorded for aqueous solutions of 20 µM cryptochrome (purple) and for equimolar mixtures (20, 40 and 80 µM) of FAD with hen lysozyme (blue, orange and red, respectively). The abscissa is time (µs); the ordinate is absorbance. Note the much stronger and much longer lived signal from flavin radicals in the case of the cryptochrome compared to that observed for reactions of free flavin with lysozyme.
(4.19 MB TIF)
Acknowledgments
We are very grateful to Professor Thorsten Ritz who originated the collaboration between the Oldenburg, Oxford and Berlin groups. We further thank DIARECT AG, Freiburg for generously providing access to their equipment and facilities.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by the VolkswagenStiftung (Nachwuchsgruppe and Lichtenberg-Professorship grants to HM), the Deutsche Forschungsgemeinschaft (FOR 701 TP6 and MO 1408/1-2 grants to HM; FOR 526 TP6 to ES), the University of Oldenburg (HM), the EPSRC (PJH and CRT), the Human Frontier Science Programme (CRT and KM), the EMF Biological Research Trust (PJH, CRT and KH), and the Royal Society (University Research Fellowship to CRT). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Wiltschko W, Wiltschko R. Magnetic compass of Europena robins. Science. 1972;176:62–64. doi: 10.1126/science.176.4030.62. [DOI] [PubMed] [Google Scholar]
- 2.Wiltschko W, Wiltschko R. Migratory orientation of European robins is affected by the wavelength of light as well as by a magnetic pulse. J Comp Physiol. 1995;177:363–369. [Google Scholar]
- 3.Cochran B, Mouritsen H, Wikelski M. Free-flying songbirds recalibrate their magnetic compass daily from sunset cues. Science. 2004;304:405–408. doi: 10.1126/science.1095844. [DOI] [PubMed] [Google Scholar]
- 4.Wiltschko W, Munro U, Ford H, Wiltschko R. Red light disrupts magnetic orientation of migratory birds. Nature. 1993;364:525–527. [Google Scholar]
- 5.Wiltschko W, Möller A, Gesson M, Noll C, Wiltschko R. Light-dependent magnetoreception in birds: analysis of the behaviour under red light after pre-exposure to red light. J Exp Biol. 2004;207:1193–1202. doi: 10.1242/jeb.00873. [DOI] [PubMed] [Google Scholar]
- 6.Schneider T, Thalau HP, Semm P, Wiltschko W. Melatonin is crucial for the migratory orientation of Pied flycatchers (Ficedula hypoleuca). J Exp Biol. 1994;194:255–262. doi: 10.1242/jeb.194.1.255. [DOI] [PubMed] [Google Scholar]
- 7.Schulten K, Swenberg C, Weller A. A biomagnetic sensory mechanism based on magnetic field modulated coherent electron spin motion. Z Phys Chem. 1978;NF111:1–5. [Google Scholar]
- 8.Ritz T, Adem S, Schulten K. A model for photoreceptor-based magnetoreception in birds. Biophys J. 2000;78:707–718. doi: 10.1016/S0006-3495(00)76629-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salikhov K, Molin Y, Sagdeev R, Buchanenko A. Spin polarization and magnetic field effects in radical reactions. 22, Budapest: Elsevier; 1984. [Google Scholar]
- 10.Steiner U, Ulrich T. Magnetic field effects in chemical kinetics and related phenomena. Chem Rev. 1989;89:51–147. [Google Scholar]
- 11.Brocklehurst B. Magnetic fields and radical reactions: recent developments and their role in nature. Chem Soc Rev. 2002;31:301–311. doi: 10.1039/b107250c. [DOI] [PubMed] [Google Scholar]
- 12.Cintolesi F, Ritz T, Kay CWM, Timmel CR, Hore PJ. Anisotropic recombination of an immobilized photoinduced radical pair in a 50 µT magnetic field: a model avian photomagnetoreceptor. Chem Phys. 2003;294:384–399. [Google Scholar]
- 13.Timmel CR, Cintolesi F, Brocklehurst B, Hore PJ. Model calculations of magnetic field effects on the recombination reactions of radicals with anisotropic hyperfine interactions. Chem Phys Lett. 2001;334:337. [Google Scholar]
- 14.Woodward JR, Timmel CR, McLauchlan KA, Hore PJ. Radio frequency magnetic field effects on electron-hole recombination. Phys Rev Lett. 2001;87:Art. No. 077602. doi: 10.1103/PhysRevLett.87.077602. [DOI] [PubMed] [Google Scholar]
- 15.Henbest KB, Kukura P, Rodgers CT, Hore PJ, Timmel CR. Radio frequency magnetic field effects on a radical recombination reaction: a diagnostic test for the radical pair mechanism. JACS. 2004;126:8102–8103. doi: 10.1021/ja048220q. [DOI] [PubMed] [Google Scholar]
- 16.Rodgers CT, Henbest KB, Kukura P, Timmel CR, Hore PJ. Low-field optically detected EPR spectroscopy of transient photoinduced radical pairs. J Phys Chem A. 2005;109:5035–5041. doi: 10.1021/jp050765z. [DOI] [PubMed] [Google Scholar]
- 17.Ritz T, Thalau P, Phillips JB, Wiltschko R, Wiltschko W. Resonance effects indicate a radical-pair mechanism for avian magnetic compass. Nature. 2004;429:177–180. doi: 10.1038/nature02534. [DOI] [PubMed] [Google Scholar]
- 18.Thalau P, Ritz T, Stapput K, Wiltschko R, Wiltschko W. Magnetic compass orientation of migratory birds in the presence of a 1.315 MHz oscillating field. Naturwiss. 2005;92:6–90. doi: 10.1007/s00114-004-0595-8. [DOI] [PubMed] [Google Scholar]
- 19.Ahmad M, Cashmore AR. HY4 gene of A. thaliana encodes a protein with characteristics of a blue-light photoreceptor. Nature. 1993;366:162–166. doi: 10.1038/366162a0. [DOI] [PubMed] [Google Scholar]
- 20.Gindt YM, Vollenbroek E, Westphal K, Sackett H, Sancar A, et al. Origin of the transient electron paramagnetic resonance signals in DNA photolyase. Biochemistry. 1999;38:3857–3866. doi: 10.1021/bi981191+. [DOI] [PubMed] [Google Scholar]
- 21.Aubert C, Vos MH, Mathis P, Eker AP, Brettel K. Intraprotein radical transfer during photoactivation of DNA photolyase. Nature. 2000;405:586–590. doi: 10.1038/35014644. [DOI] [PubMed] [Google Scholar]
- 22.Weber S, Kay CWM, Mögling H, Möbius K, Hitomi K, et al. Photoactivation of the Flavin Cofactor in Xenopus laevis (6-4) Photolyase: Observation of a Transient Tyrosyl Radical by Time-Resolved Electron Paramagnetic Resonance. Proc Natl Acad Sci USA. 2002;99:1319–1322. doi: 10.1073/pnas.032469399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mouritsen H, Janssen-Bienhold U, Liedvogel M, Feenders G, Stalleicken J, et al. Cryptochrome and activity markers co-localize in bird retina during magnetic orientation. Proc Natl Acad Sci USA. 2004;101:14294–14299. doi: 10.1073/pnas.0405968101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Möller A, Sagasser S, Wiltschko W, Schierwater B. Retinal cryptochrome in a migratory passerine bird: a possible transducer for the avian magnetic compass. Naturwiss. 2004;91:585–588. doi: 10.1007/s00114-004-0578-9. [DOI] [PubMed] [Google Scholar]
- 25.Mouritsen H, Feenders G, Liedvogel M, Wada K, Jarvis ED. A night vision brain area in migratory songbirds. Proc Natl Acad Sci USA. 2005;102:8339–8344. doi: 10.1073/pnas.0409575102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liedvogel M, Feenders G, Wada K, Troje NF, Jarvis ED, et al. Lateralized activation of Cluster N in the brains of migratory songbirds. Euro J Neurosci. 2007;25:1166–1173. doi: 10.1111/j.1460-9568.2007.05350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mouritsen H, Ritz T. Magnetoreception and its use in bird navigation. Curr Opin Neurobiol. 2005;15:406–414. doi: 10.1016/j.conb.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 28.Mouritsen H, Feenders G, Liedvogel M, Kropp W. Migratory birds use head scans to detect the direction of the Earth's magnetic field. Curr Biol. 2004;14:1946–1949. doi: 10.1016/j.cub.2004.10.025. [DOI] [PubMed] [Google Scholar]
- 29.Brautigam CA, Smith BS, Ma Z, Palnitkar M, Tomchick DR, et al. Structure of the photolyase-like domain of cryptochrome 1 from Arabidopsis thaliana. Proc Natl Acad Sci USA. 2004;101:12142–12147. doi: 10.1073/pnas.0404851101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin C, Todo T. The cryptochromes. Gen Biol. 2005;6:220.1–220.9. doi: 10.1186/gb-2005-6-5-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luckow VA, Summers MD. Trends in the development of. baculovirus expression vectors. Bio/Technology. 1988;6:47–55. [Google Scholar]
- 32.Blissard GW, Rohrmann GF. Baculovirus diversity and molecular biology. Annu Rev Entomol. 1990;35:127–155. doi: 10.1146/annurev.en.35.010190.001015. [DOI] [PubMed] [Google Scholar]
- 33.Cashmore AR, Jarillo JA, Wu Y-J, Liu D. Cryptochromes: blue light receptors for plants and animals. Science. 1999;284:760–765. doi: 10.1126/science.284.5415.760. [DOI] [PubMed] [Google Scholar]
- 34.Sancar A. Cryptochrome: the second photoactive pigment in the eye and its role in circadian photoreception. Annu Rev Biochem. 2000;69:31–67. doi: 10.1146/annurev.biochem.69.1.31. [DOI] [PubMed] [Google Scholar]
- 35.Ghisla S, Massey V, Lhoste JM, Mayhew SG. Fluorescence and optical characteris tics of reduced flavines and flavoproteins. Biochemistry. 1974;13:589–597. doi: 10.1021/bi00700a029. [DOI] [PubMed] [Google Scholar]
- 36.Lin C, Robertson DE, Ahmad M, Raibekas AA, Jorns MS, et al. Association of flavin adenine dinocelotide with the Arabidopsis blue light receptor Cry1. Science. 1995;269:968–970. doi: 10.1126/science.7638620. [DOI] [PubMed] [Google Scholar]
- 37.Giovani B, Byrdin M, Ahmad M, Brettel K. Light-induced electron transfer in a cryptochrome blue-light photoreceptor. Nature Struct Biol. 2003;10:489–490. doi: 10.1038/nsb933. [DOI] [PubMed] [Google Scholar]
- 38.Berndt A, Kottke T, Breitkreuz H, Dvorsky R, Hennig S, et al. A novel photoreaction mechanism for the circadian blue light photoreceptor Drosophila cryptochrome. J Biol Chem. 2007;282:13011–13021. doi: 10.1074/jbc.M608872200. [DOI] [PubMed] [Google Scholar]
- 39.Broadhurst RW, Dobson CM, Hore PJ, Radford SE, Rees ML. A photocemically induced dynamic nuclear-polarization study of denatured states of lysozyme. Biochemistry. 1991;30:405–412. doi: 10.1021/bi00216a015. [DOI] [PubMed] [Google Scholar]
- 40.Hore PJ, Kaptein R. Proton nuclear magnetic resonance assignments and surface accessibility of tryptophan residues in lysozyme. Biochemistry. 1983;22:1906–1911. doi: 10.1021/bi00277a026. [DOI] [PubMed] [Google Scholar]
- 41.Jorns MS, Sancar GB, Sancar A. Identification of a neutral flavin radical and characterization of a second chromophore in E. coli DNA photolyase. Biochemistry. 1984;23:2673–2679. doi: 10.1021/bi00307a021. [DOI] [PubMed] [Google Scholar]
- 42.Sancar A. Structure and function of DNA photolyase. Biochemistry. 1994;33:2–9. doi: 10.1021/bi00167a001. [DOI] [PubMed] [Google Scholar]
- 43.Yasui A, Eker AP, Yasuhira S, Yajima H, Kobayashi T, et al. A new class of DNA photolyases present in various organisms including aplacental mammals. EMBO J. 1994;13:6143–6151. doi: 10.1002/j.1460-2075.1994.tb06961.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bouly JP, Schleicher E, Dionisio-Sese M, Vandenbussche F, van der Straeten E, et al. Cryptochrome blue light photoreceptors are activated through interconversion of flavin redox states. J Biol Chem. 2007;282:9383–9391. doi: 10.1074/jbc.M609842200. [DOI] [PubMed] [Google Scholar]
- 45.Johnsen S, Mattern E, Ritz T. Light-dependent magnetoreception: quantum catches and opponency mechanisms of possible photosensitive molecules. J Exp Biol. 2007;210:3171–3178. doi: 10.1242/jeb.007567. [DOI] [PubMed] [Google Scholar]
- 46.Solov'yov IA, Chandler DE, Schulten K. Magnetic field effects in Arabidopsis thaliana cryptochrome-1. Biophys J. 2007;92:2711–2726. doi: 10.1529/biophysj.106.097139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Byrdin M, Eker APM, Vos MH, Brettel K. Dissection of the triple tryptophan electron transfer chain in Escherichia coli DNA photolyase: Trp382 is the primary donor in photoactivation. Proc Natl Acad Sci USA. 2003;100:8676–8681. doi: 10.1073/pnas.1531645100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Park HW, Kim ST, Sancar A, Deisenhofer J. Crystal structure of DNA photolyase from Escherichia coli. Science. 1995;268:1866–1872. doi: 10.1126/science.7604260. [DOI] [PubMed] [Google Scholar]
- 49.Weaver J, Vaughan T, Astumian D. Biological sensing of small differences by magnetically sensitive chemical reactions. Nature. 2000;405:707–709. doi: 10.1038/35015128. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
NMR measurements and transient absorption control experiments.
(0.04 MB DOC)
Aromatic region of the 600 MHz 1H NMR spectrum of gwCRY1a protein. Aromatic region of the 600 MHz 1H NMR spectrum of the CRY1a sample used for the transient absorption experiments before (blue) and after (red) addition of 20 µM FAD. Note the additional signals from FAD in the latter spectrum and their absence in the former.
(3.63 MB TIF)
Transient absorption signals at 550 nm for garden warbler cryptochrome and equimolar mixtures of FAD with hen lysozyme. Transient absorption signals at 550 nm recorded for aqueous solutions of 20 µM cryptochrome (purple) and for equimolar mixtures (20, 40 and 80 µM) of FAD with hen lysozyme (blue, orange and red, respectively). The abscissa is time (µs); the ordinate is absorbance. Note the much stronger and much longer lived signal from flavin radicals in the case of the cryptochrome compared to that observed for reactions of free flavin with lysozyme.
(4.19 MB TIF)