Abstract
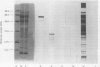
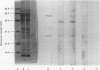
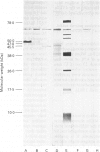
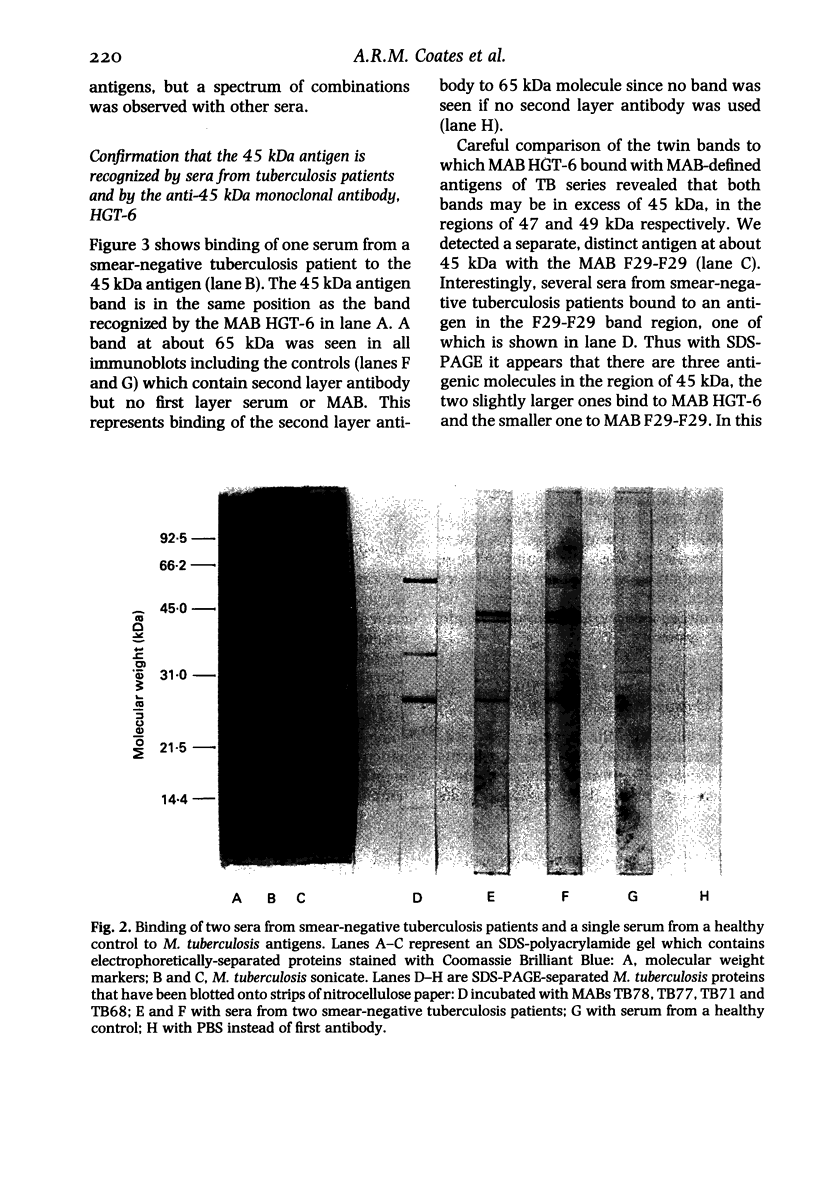
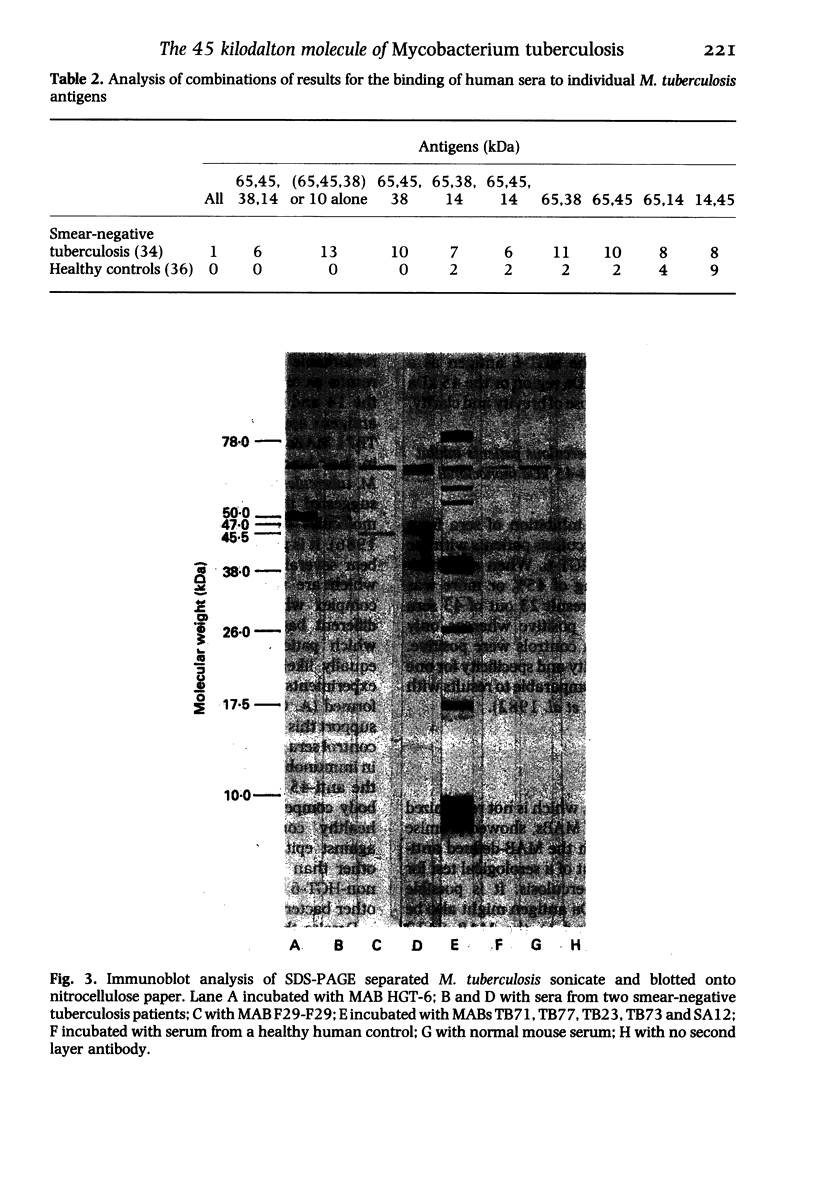
The object of this study was to discover new M. tuberculosis antigens which are recognized by patients with tuberculosis, because effective serodiagnostic tests are likely to require combinations of different antigens. In our early experiments using immunoblotting, the findings suggested that human sera from smear-negative tuberculosis patients bound to an antigen in the 45 kDa region. Subsequently, estimates of molecular weight in the immunoblots confirmed that the murine monoclonal antibody (MAB) HGT-6 and sera from patients both recognized the same 45 kDa molecule. An antibody-antibody competition assay between MAB HGT-6 and sera from smear-positive tuberculosis patients yielded a positive result in 23 out of 43 sera from patients, but in only four out of 23 from controls. This is further evidence that the 45 kDa antigen is recognized by tuberculous patients. We analysed whether a combination of the 45 kDa antigen results and those of known antigens might better discriminate between minimal smear-negative disease and healthy controls than could test with single antigens. There is no clinically useful laboratory test for smear-negative tuberculosis. In immunoblotting, combining the results with the 65, 45, 38 and 10 kDa antigens gave the best discrimination. This suggests that future serodiagnostic tests for minimal disease, such as the antibody-antibody competition assay, should contain a MAB against the 45 kDa antigen and possibly also against the 10 kDa antigen.
Full text
PDF


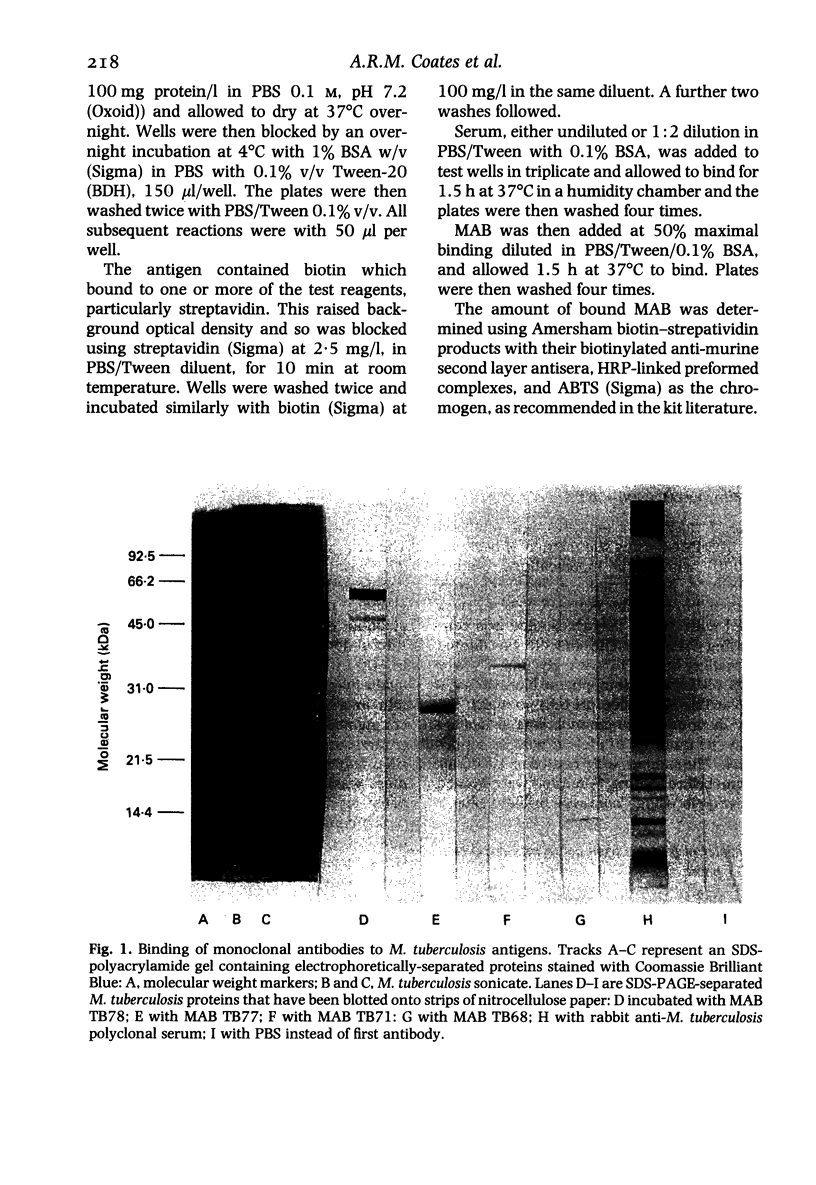





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrew P. W., Rees A. D., Scoging A., Dobson N., Matthews R., Whittall J. T., Coates A. R., Lowrie D. B. Secretion of a macrophage-activating factor distinct from interferon-gamma by human T cell clones. Eur J Immunol. 1984 Oct;14(10):962–964. doi: 10.1002/eji.1830141018. [DOI] [PubMed] [Google Scholar]
- Benjamin R. G., Daniel T. M. Serodiagnosis of tuberculosis using the enzyme-linked immunoabsorbent assay (ELISA) of antibody to Mycobacterium tuberculosis antigen 5. Am Rev Respir Dis. 1982 Dec;126(6):1013–1016. doi: 10.1164/arrd.1982.126.6.1013. [DOI] [PubMed] [Google Scholar]
- Chakrabarty A. K., Maire M. A., Lambert P. H. SDS-PAGE analysis of M. leprae protein antigens reacting with antibodies from sera from lepromatous patients and infected armadillos. Clin Exp Immunol. 1982 Sep;49(3):523–531. [PMC free article] [PubMed] [Google Scholar]
- Closs O., Harboe M., Axelsen N. H., Bunch-Christensen K., Magnusson M. The antigens of Mycobacterium bovis, strain BCG, studied by crossed immunoelectrophoresis: a reference system. Scand J Immunol. 1980;12(3):249–263. doi: 10.1111/j.1365-3083.1980.tb00065.x. [DOI] [PubMed] [Google Scholar]
- Coates A. R., Hewitt J., Allen B. W., Ivanyi J., Mitchison D. A. Antigenic diversity of Mycobacterium tuberculosis and Mycobacterium bovis detected by means of monoclonal antibodies. Lancet. 1981 Jul 25;2(8239):167–169. doi: 10.1016/s0140-6736(81)90355-x. [DOI] [PubMed] [Google Scholar]
- Daniel T. M., Ellner J. J., Todd L. S., McCoy D. W., Payne V. D., Anderson P. A., Bhe F. T. Immunobiology and species distribution of Mycobacterium tuberculosis antigen 5. Infect Immun. 1979 Apr;24(1):77–82. doi: 10.1128/iai.24.1.77-82.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmrich F., Thole J., van Embden J., Kaufmann S. H. A recombinant 64 kilodalton protein of Mycobacterium bovis bacillus Calmette-Guerin specifically stimulates human T4 clones reactive to mycobacterial antigens. J Exp Med. 1986 Apr 1;163(4):1024–1029. doi: 10.1084/jem.163.4.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grange J. M. The humoral immune response in tuberculosis: its nature, biological role and diagnostic usefulness. Adv Tuberc Res. 1984;21:1–78. [PubMed] [Google Scholar]
- Hewitt J., Coates A. R., Mitchison D. A., Ivanyi J. The use of murine monoclonal antibodies without purification of antigen in the serodiagnosis of tuberculosis. J Immunol Methods. 1982 Dec 17;55(2):205–211. doi: 10.1016/0022-1759(82)90032-1. [DOI] [PubMed] [Google Scholar]
- Kadival G. V., Chaparas S. D. Production, characterization, and species specificity of five monoclonal antibodies to Mycobacterium tuberculosis. J Clin Microbiol. 1987 Jan;25(1):76–80. doi: 10.1128/jcm.25.1.76-80.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan M. H., Chase M. W. Antibodies to mycobacteria in human tuberculosis. II. Response to nine defined mycobacterial antigens with evidence for an antibody common to tuberculosis and lepromatous leprosy. J Infect Dis. 1980 Dec;142(6):835–843. doi: 10.1093/infdis/142.6.835. [DOI] [PubMed] [Google Scholar]
- Klatser P. R., van Rens M. M., Eggelte T. A. Immunochemical characterization of Mycobacterium leprae antigens by the SDS-polyacrylamide gel electrophoresis immunoperoxidase technique (SGIP) using patients' sera. Clin Exp Immunol. 1984 Jun;56(3):537–544. [PMC free article] [PubMed] [Google Scholar]
- Lefford M. J., McGregor D. D., Mackaness G. B. Immune response to Mycobacterium tuberculosis in rats. Infect Immun. 1973 Aug;8(2):182–189. doi: 10.1128/iai.8.2.182-189.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews R., Scoging A., Rees A. D. Mycobacterial antigen-specific human T-cell clones secreting macrophage activating factors. Immunology. 1985 Jan;54(1):17–23. [PMC free article] [PubMed] [Google Scholar]
- Minden P., Kelleher P. J., Freed J. H., Nielsen L. D., Brennan P. J., McPheron L., McClatchy J. K. Immunological evaluation of a component isolated from Mycobacterium bovis BCG with a monoclonal antibody to M. bovis BCG. Infect Immun. 1984 Nov;46(2):519–525. doi: 10.1128/iai.46.2.519-525.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison D. A. Examination of sputum by smear and culture in case-finding. Bull Int Union Tuberc. 1968 Dec;41:139–147. [PubMed] [Google Scholar]
- Nassau E., Nelstrop A. E. Sepcific tubercle antigen. Tubercle. 1976 Sep;57(3):197–201. doi: 10.1016/0041-3879(76)90028-3. [DOI] [PubMed] [Google Scholar]
- Reggiardo Z., Aber V. R., Mitchison D. A., Devi S. Hemagglutination tests for tuberculosis with mycobacterial glycolipid antigens. Results in patients with active pulmonary tuberculosis before and during chemotherapy and in healthy tuberculosis contacts. Am Rev Respir Dis. 1981 Jul;124(1):21–25. doi: 10.1164/arrd.1981.124.1.21. [DOI] [PubMed] [Google Scholar]
- Reggiardo Z., Middlebrook G. Serologically active glycolipid families from Mycobacterium bovis BCG. I. Extraction, purification and immunologic studies. Am J Epidemiol. 1974 Dec;100(6):469–476. doi: 10.1093/oxfordjournals.aje.a112059. [DOI] [PubMed] [Google Scholar]
- Reggiardo Z., Middlebrook G. Serologically active glycolipid families from Mycobacterium bovis BCG. II. Serologic studies on human sera. Am J Epidemiol. 1974 Dec;100(6):477–486. doi: 10.1093/oxfordjournals.aje.a112060. [DOI] [PubMed] [Google Scholar]
- Results of a World Health Organization-sponsored workshop to characterize antigens recognized by mycobacterium-specific monoclonal antibodies. Infect Immun. 1986 Feb;51(2):718–720. doi: 10.1128/iai.51.2.718-720.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]