Abstract
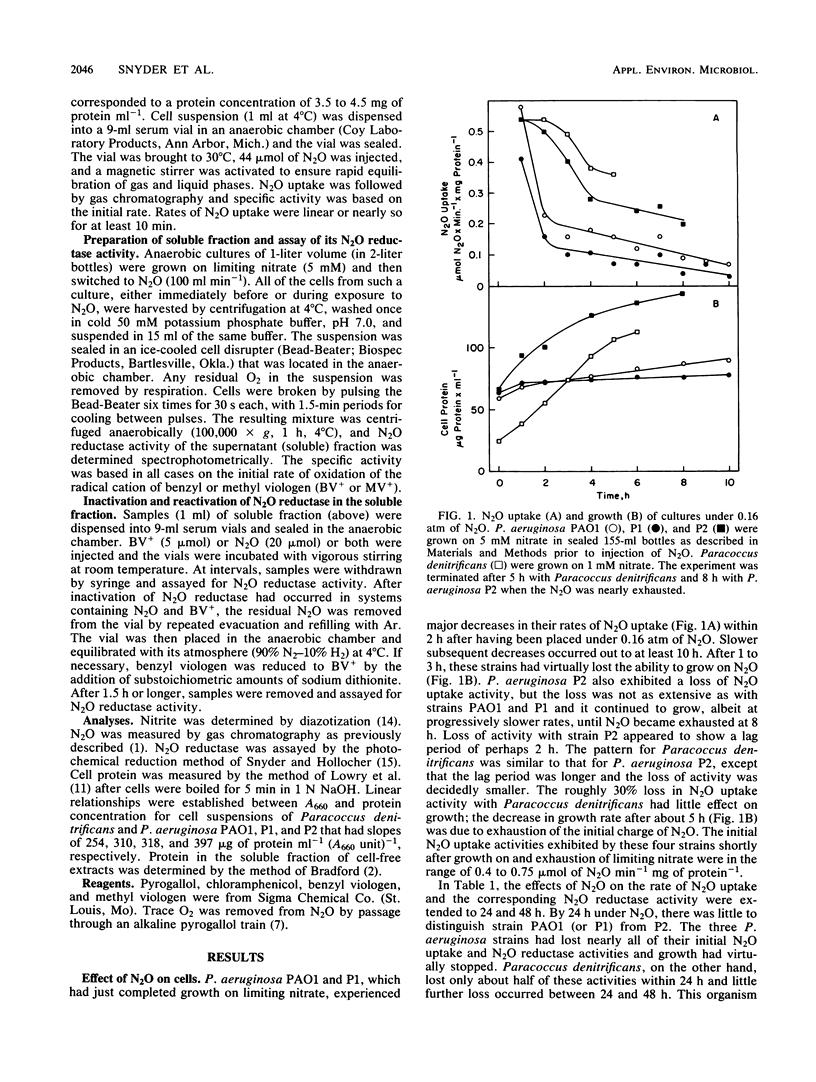
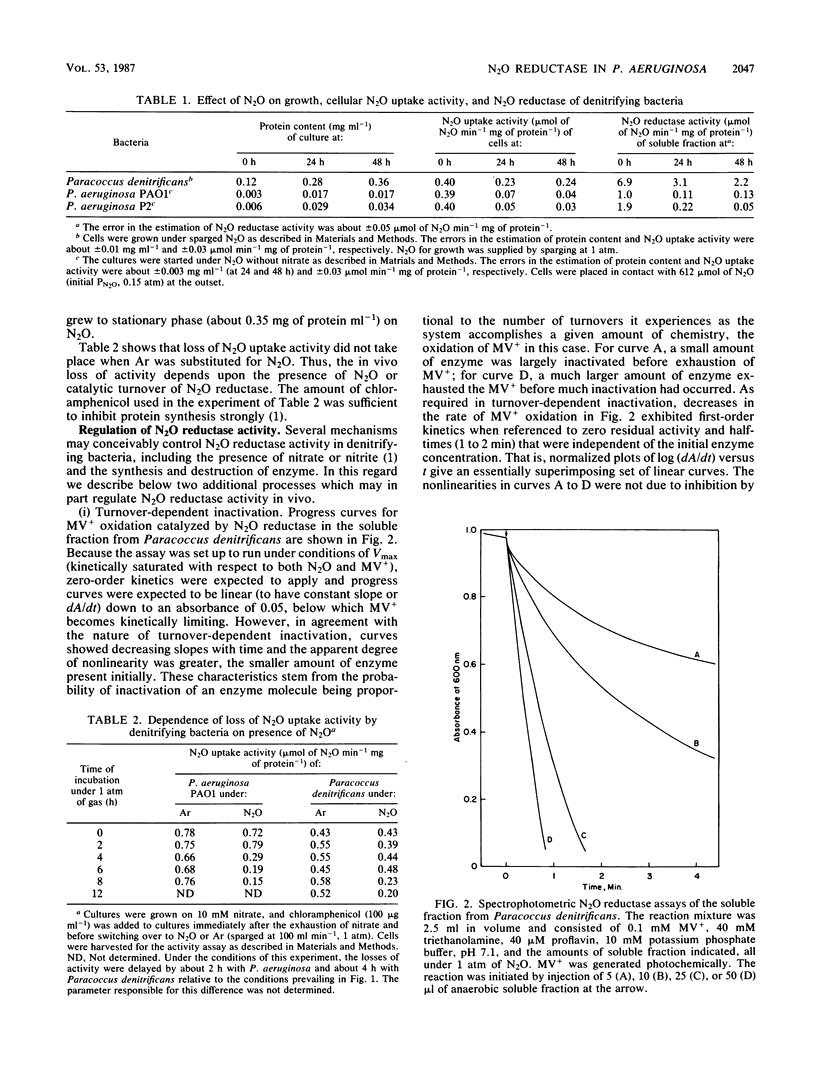
N2O uptake activity of cells and N2O reductase activity of the soluble fraction from denitrifying bacteria were assayed. Pseudomonas aeruginosa strains PAO1 and P1 lost most of their N2O uptake activity and the ability to grow well on N2O within 2 to 5 h after exposure to N2O. Extensive loss of N2O reductase activity accompanied the nearly complete loss of N2O uptake activity under N2O. Paracoccus denitrificans retained much, but not all, of both activities and the ability to grow vigorously on N2O. The pattern with P. aeruginosa strain P2 resembled that for PAO1 and P1 except that loss of the activities proceeded at a slower rate and growth could continue for up to 12 h after exposure to N2O. The inability of a number of P. aeruginosa strains to grow well on N2O is therefore a direct consequence of the nearly complete loss of N2O reductase activity. Turnover-dependent inactivation of N2O reductase and its reactivation under reducing conditions occurred in vitro for the enzyme from P. aeruginosa and Paracoccus denitrificans. These events may be significant in determining the activity level of N2O reductase in denitrifying bacteria during N2O respiration.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bazylinski D. A., Soohoo C. K., Hollocher T. C. Growth of Pseudomonas aeruginosa on nitrous oxide. Appl Environ Microbiol. 1986 Jun;51(6):1239–1246. doi: 10.1128/aem.51.6.1239-1246.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bryan B. A., Jeter R. M., Carlson C. A. Inability of Pseudomonas stutzeri denitrification mutants with the phenotype of Pseudomonas aeruginosa to grow in nitrous oxide. Appl Environ Microbiol. 1985 Nov;50(5):1301–1303. doi: 10.1128/aem.50.5.1301-1303.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson C. A., Ingraham J. L. Comparison of denitrification by Pseudomonas stutzeri, Pseudomonas aeruginosa, and Paracoccus denitrificans. Appl Environ Microbiol. 1983 Apr;45(4):1247–1253. doi: 10.1128/aem.45.4.1247-1253.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle C. L., Zumft W. G., Kroneck P. M., Körner H., Jakob W. Nitrous oxide reductase from denitrifying Pseudomonas perfectomarina. Purification and properties of a novel multicopper enzyme. Eur J Biochem. 1985 Dec 16;153(3):459–467. doi: 10.1111/j.1432-1033.1985.tb09324.x. [DOI] [PubMed] [Google Scholar]
- Koike I., Hattori A. Energy yield of denitrification: an estimate from growth yield in continuous cultures of Pseudomonas denitrificans under nitrate-, nitrite- and oxide-limited conditions. J Gen Microbiol. 1975 May;88(1):11–19. doi: 10.1099/00221287-88-1-11. [DOI] [PubMed] [Google Scholar]
- Kristjansson J. K., Hollocher T. C. First practical assay for soluble nitrous oxide reductase of denitrifying bacteria and a partial kinetic characterization. J Biol Chem. 1980 Jan 25;255(2):704–707. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Payne W. J., Riley P. S., Cox C. D., Jr Separate nitrite, nitric oxide, and nitrous oxide reducing fractions from Pseudomonas perfectomarinus. J Bacteriol. 1971 May;106(2):356–361. doi: 10.1128/jb.106.2.356-361.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder S. W., Hollocher T. C. Nitrous oxide reductase and the 120,000 MW copper protein of N2-producing denitrifying bacteria are different entities. Biochem Biophys Res Commun. 1984 Mar 15;119(2):588–592. doi: 10.1016/s0006-291x(84)80289-2. [DOI] [PubMed] [Google Scholar]
- St John R. T., Hollocher T. C. Nitrogen 15 tracer studies on the pathway of denitrification in Pseudomonas aeruginosa. J Biol Chem. 1977 Jan 10;252(1):212–218. [PubMed] [Google Scholar]
- Zumft W. G., Döhler K., Körner H. Isolation and characterization of transposon Tn5-induced mutants of Pseudomonas perfectomarina defective in nitrous oxide respiration. J Bacteriol. 1985 Sep;163(3):918–924. doi: 10.1128/jb.163.3.918-924.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]


