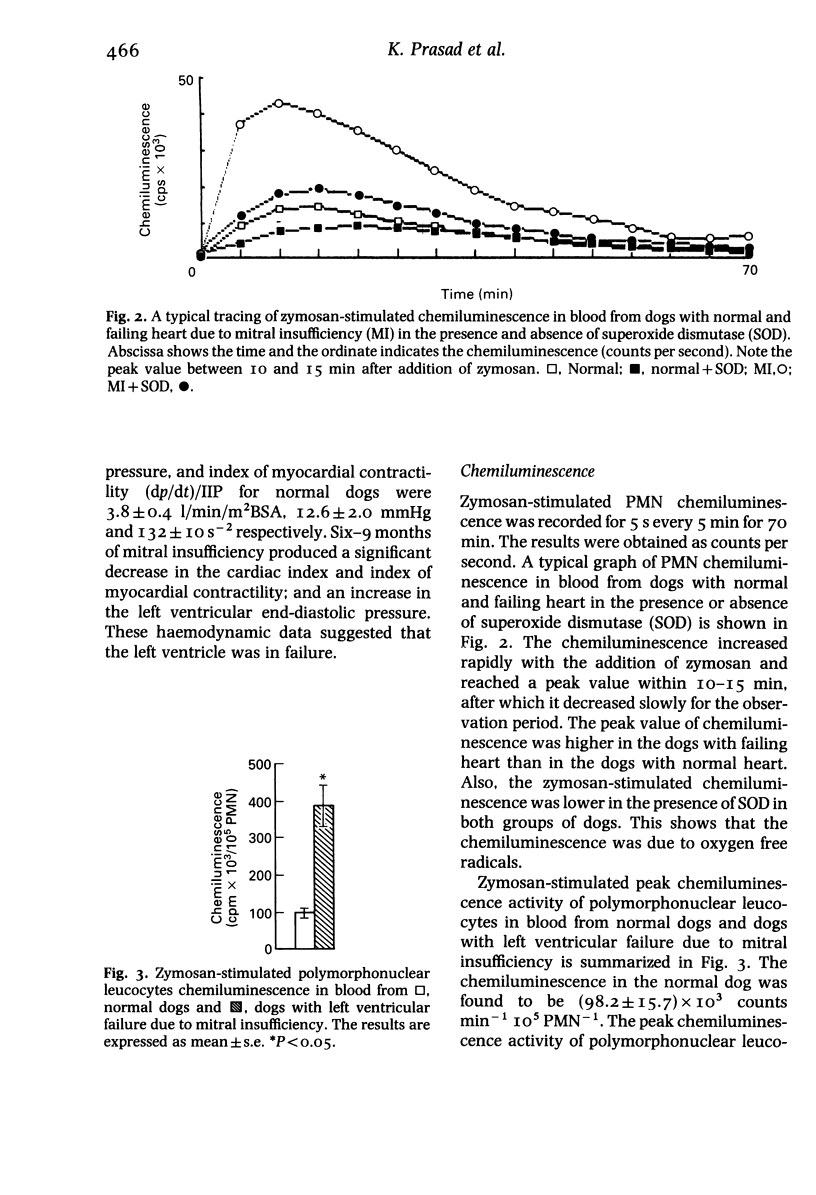
Abstract
Polymorphonuclear leucocyte (PMN) stimulation is known to generate oxygen free radicals. Exogenous oxygen free radicals, generated by xanthine and xanthine oxidase, have been implicated in the decrease of cardiac contractility. It is possible that PMN have increased capacity to release oxygen free radicals in failing heart. It was, therefore, decided to investigate PMN chemiluminescence (oxygen free radicals) from blood in dogs with heart failure due to chronic volume overload. The dogs were divided into two groups: (A) normal, six dogs; (B) dogs with mitral insufficiency (MI) of 6-9 months' duration, six dogs. Haemodynamic parameters were recorded to assess cardiac failure. Mixed venous blood was collected to measure PMN chemiluminescence. Stimulation of PMN was initiated by addition of opsonized zymosan and chemiluminescence was monitored using a luminometer. The haemodynamic parameters in dogs with MI showed that these dogs had left ventricular failure. The peak chemiluminescent activity of PMN in blood of dogs with left ventricular failure was approximately four times that in the blood from normal dogs. This increase in chemiluminescence reflects an increase in the generation of oxygen free radicals from PMN in dogs with chronic heart failure. The decrease in the myocardial contractility in cardiac failure might be due to an increase in the oxygen free radicals produced by the PMN.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DeChatelet L. R., Shirley P. S. Evaluation of chronic granulomatous disease by a chemiluminescence assay of microliter quantities of whole blood. Clin Chem. 1981 Oct;27(10):1739–1741. [PubMed] [Google Scholar]
- Del Maestro R. F. An approach to free radicals in medicine and biology. Acta Physiol Scand Suppl. 1980;492:153–168. [PubMed] [Google Scholar]
- Ford-Hutchinson A. W., Bray M. A., Doig M. V., Shipley M. E., Smith M. J. Leukotriene B, a potent chemokinetic and aggregating substance released from polymorphonuclear leukocytes. Nature. 1980 Jul 17;286(5770):264–265. doi: 10.1038/286264a0. [DOI] [PubMed] [Google Scholar]
- Guarnieri C., Flamigni F., Caldarera C. M. Role of oxygen in the cellular damage induced by re-oxygenation of hypoxic heart. J Mol Cell Cardiol. 1980 Aug;12(8):797–808. doi: 10.1016/0022-2828(80)90081-4. [DOI] [PubMed] [Google Scholar]
- Jennings R. B., Reimer K. A. Lethal myocardial ischemic injury. Am J Pathol. 1981 Feb;102(2):241–255. [PMC free article] [PubMed] [Google Scholar]
- Jolly S. R., Kane W. J., Bailie M. B., Abrams G. D., Lucchesi B. R. Canine myocardial reperfusion injury. Its reduction by the combined administration of superoxide dismutase and catalase. Circ Res. 1984 Mar;54(3):277–285. doi: 10.1161/01.res.54.3.277. [DOI] [PubMed] [Google Scholar]
- Mullane K. M., Read N., Salmon J. A., Moncada S. Role of leukocytes in acute myocardial infarction in anesthetized dogs: relationship to myocardial salvage by anti-inflammatory drugs. J Pharmacol Exp Ther. 1984 Feb;228(2):510–522. [PubMed] [Google Scholar]
- Tono-Oka T., Ueno N., Matsumoto T., Ohkawa M., Matsumoto S. Chemiluminescence of whole blood. 1. A simple and rapid method for the estimation of phagocytic function of granulocytes and opsonic activity in whole blood. Clin Immunol Immunopathol. 1983 Jan;26(1):66–75. doi: 10.1016/0090-1229(83)90174-5. [DOI] [PubMed] [Google Scholar]


