Abstract
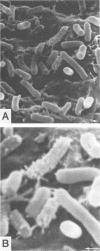
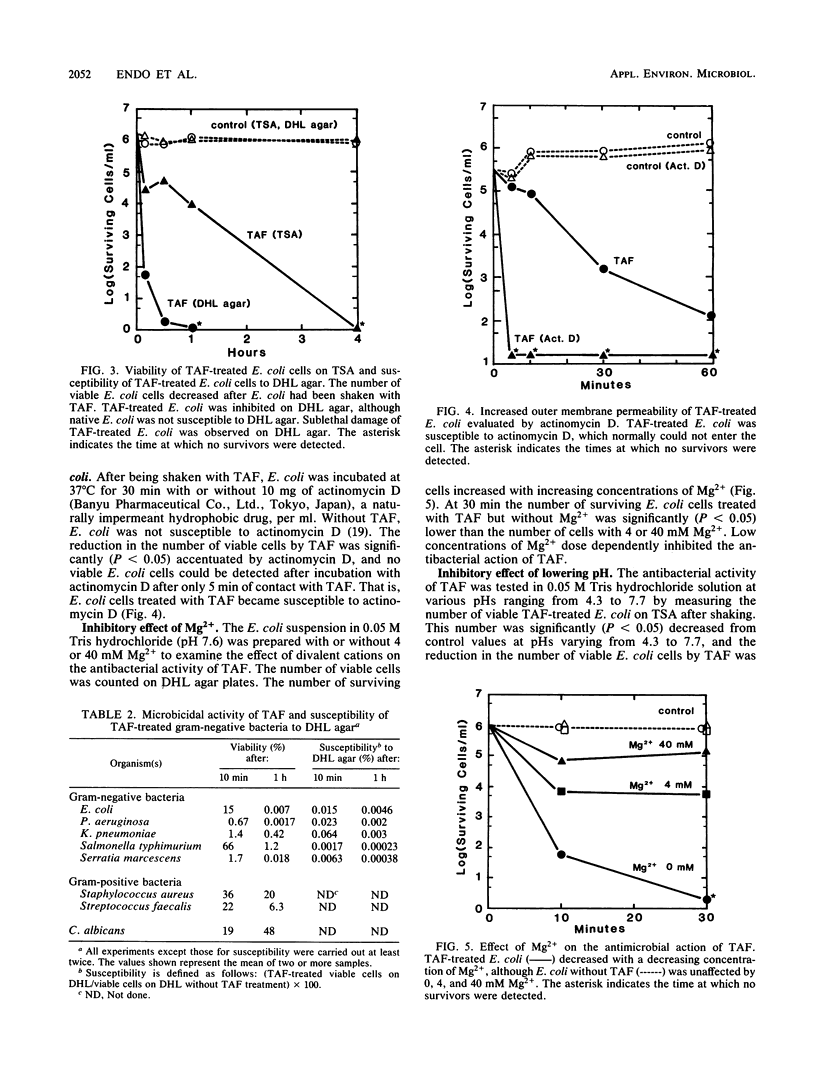
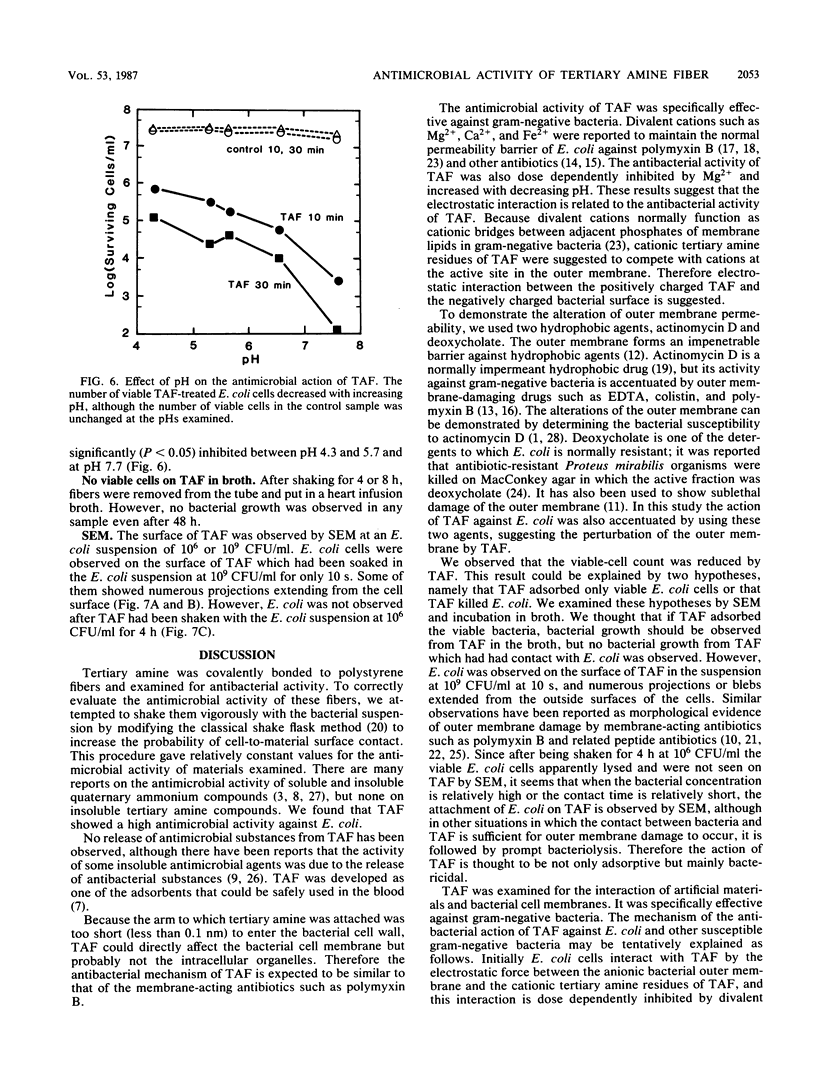
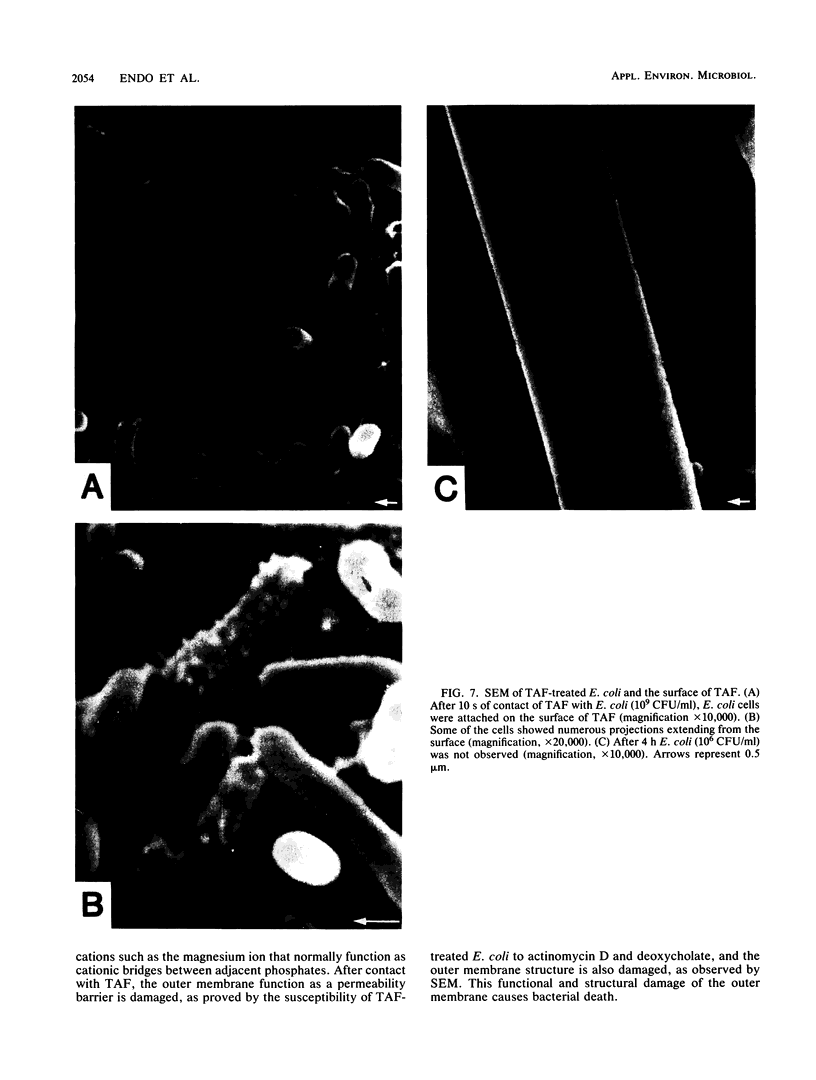
Tertiary amine was covalently bonded to a polystyrene fiber and examined for antibacterial activity. The tertiary amine covalently bonded to a polystyrene fiber (TAF) showed a high antimicrobial activity against Escherichia coli. TAF exhibited a stronger antibacterial activity against gram-negative bacteria (E. coli, Pseudomonas aeruginosa, Klebsiella pneumoniae, Salmonella typhimurium, and Serratia marcescens) than against gram-positive bacteria (Staphylococcus aureus and Streptococcus faecalis) or Candida albicans. This activity against E. coli was accentuated by 0.1% deoxycholate or 10 mg of actinomycin D per ml, to which E. coli is normally not susceptible. This implies that TAF causes an increase of the bacterial outer membrane permeability. On the other hand, the antimicrobial activity was inhibited by adding Mg2+ or by lowering the pH. This suggest an electrostatic interaction between the bacterial cell wall and TAF. Scanning electron microscopy showed that E. coli cells were initially attached to TAF, with many projections on the cell surface, but then were apparently lysed after contact for 4 h. Taken together, these results imply that bacteria initially interact with TAF by an electrostatic force between the anionic bacterial outer membrane and the cationic tertiary amine residues of TAF and that longer contact with TAF damages the bacterial outer membrane structure and increases its permeability.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beckerdite S., Mooney C., Weiss J., Franson R., Elsbach P. Early and discrete changes in permeability of Escherichia coli and certain other gram-negative bacteria during killing by granulocytes. J Exp Med. 1974 Aug 1;140(2):396–409. doi: 10.1084/jem.140.2.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey R. A., Greco R. S. The noncovalent bonding of antibiotics to a polytetrafluoroethylene-benzalkonium graft. Ann Surg. 1981 Nov;194(5):642–647. doi: 10.1097/00000658-198111000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idezuki Y., Hamaguchi M., Hamabe S., Moriya H., Nagashima T., Watanabe H., Sonoda T., Teramoto K., Kikuchi T., Tanzawa H. Removal of bilirubin and bile acid with a new anion exchange resin: experimental background and clinical experiences. Trans Am Soc Artif Intern Organs. 1981;27:428–433. [PubMed] [Google Scholar]
- Isquith A. J., Abbott E. A., Walters P. A. Surface-bonded antimicrobial activity of an organosilicon quaternary ammonium chloride. Appl Microbiol. 1972 Dec;24(6):859–863. doi: 10.1128/am.24.6.859-863.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy J. F., Tun H. C. Active insolubilized antibiotics based on cellulose and cellulose carbonate. Antimicrob Agents Chemother. 1973 May;3(5):575–579. doi: 10.1128/aac.3.5.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike M., Iida K., Matsuo T. Electron microscopic studies on mode of action of polymyxin. J Bacteriol. 1969 Jan;97(1):448–452. doi: 10.1128/jb.97.1.448-452.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaPorte D. C., Rosenthal K. S., Storm D. R. Inhibition of Escherichia coli growth and respiration by polymyxin B covalently attached to agarose beads. Biochemistry. 1977 Apr 19;16(8):1642–1648. doi: 10.1021/bi00627a019. [DOI] [PubMed] [Google Scholar]
- Laforce F. M., Boose D. S. Sublethal damage of Escherichia coli by lung lavage. Am Rev Respir Dis. 1981 Dec;124(6):733–737. doi: 10.1164/arrd.1981.124.6.733. [DOI] [PubMed] [Google Scholar]
- Leive L. Studies on the permeability change produced in coliform bacteria by ethylenediaminetetraacetate. J Biol Chem. 1968 May 10;243(9):2373–2380. [PubMed] [Google Scholar]
- Muschel L. H., Jackson J. E. Reversal of the bactericidal reaction of serum by magnesium ion. J Bacteriol. 1966 Apr;91(4):1399–1402. doi: 10.1128/jb.91.4.1399-1402.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEWTON B. A. Reversal of the antibacterial activity of polymyxin by divalent cations. Nature. 1953 Jul 25;172(4369):160–161. [PubMed] [Google Scholar]
- NEWTON B. A. The properties and mode of action of the polymyxins. Bacteriol Rev. 1956 Mar;20(1):14–27. doi: 10.1128/br.20.1.14-27.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima K., Kawamata J. Effect of colistin on actinomycin sensitivity of Escherichia coli. Biken J. 1965 Jun;8(2):115–118. [PubMed] [Google Scholar]
- Nakajima K. Structure-activity relationship of colistins. Chem Pharm Bull (Tokyo) 1967 Aug;15(8):1219–1224. doi: 10.1248/cpb.15.1219. [DOI] [PubMed] [Google Scholar]
- Nikaido H., Nakae T. The outer membrane of Gram-negative bacteria. Adv Microb Physiol. 1979;20:163–250. doi: 10.1016/s0065-2911(08)60208-8. [DOI] [PubMed] [Google Scholar]
- Rosenthal K. S., Swanson P. E., Storm D. R. Disruption of Escherichia coli outer membranes by EM 49. A new membrane active peptide. Biochemistry. 1976 Dec 28;15(26):5783–5792. doi: 10.1021/bi00671a015. [DOI] [PubMed] [Google Scholar]
- Schindler P. R., Teuber M. Action of polymyxin B on bacterial membranes: morphological changes in the cytoplasm and in the outer membrane of Salmonella typhimurium and Escherichia coli B. Antimicrob Agents Chemother. 1975 Jul;8(1):95–104. doi: 10.1128/aac.8.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm D. R., Rosenthal K. S., Swanson P. E. Polymyxin and related peptide antibiotics. Annu Rev Biochem. 1977;46:723–763. doi: 10.1146/annurev.bi.46.070177.003451. [DOI] [PubMed] [Google Scholar]
- Sud I. J., Feingold D. S. Effect of polymyxin B on antibiotic-resistant Proteus mirabilis. Antimicrob Agents Chemother. 1972 May;1(5):417–421. doi: 10.1128/aac.1.5.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suganuma A., Hara K., Kishida T., Nakajima K., Kawamata J. Cytological changes of Escherichia coli caused by polymyxin E. Biken J. 1968 Jun;11(2):149–155. [PubMed] [Google Scholar]
- Taylor S. L., Fina L. R., Lambert J. L. New water disinfectant: an insoluble quaternary ammonium resin-triiodide combination that releases bactericide on demand. Appl Microbiol. 1970 Nov;20(5):720–722. doi: 10.1128/am.20.5.720-722.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters P. A., Abbott E. A., Isquith A. J. Algicidal activity of a surface-bonded organosilicon quaternary ammonium chloride. Appl Microbiol. 1973 Feb;25(2):253–256. doi: 10.1128/am.25.2.253-256.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss J., Victor M., Elsbach P. Role of charge and hydrophobic interactions in the action of the bactericidal/permeability-increasing protein of neutrophils on gram-negative bacteria. J Clin Invest. 1983 Mar;71(3):540–549. doi: 10.1172/JCI110798. [DOI] [PMC free article] [PubMed] [Google Scholar]