Abstract
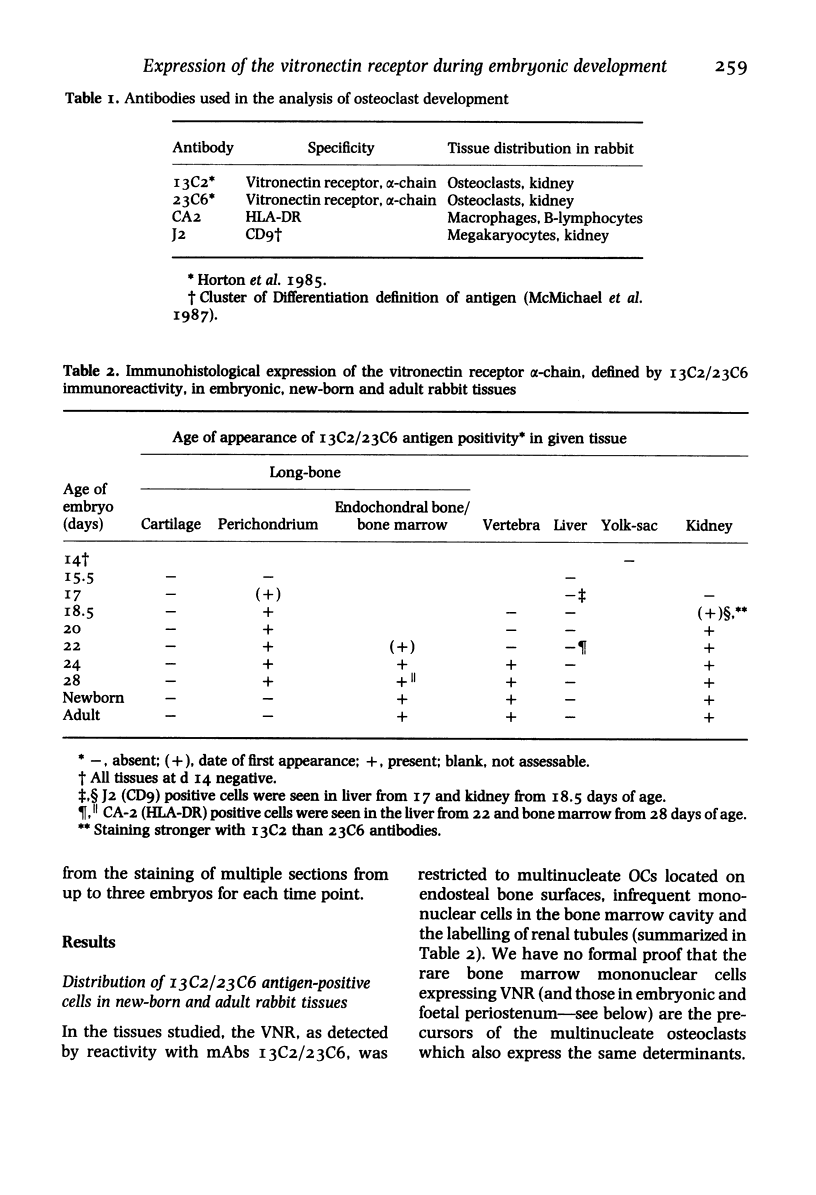
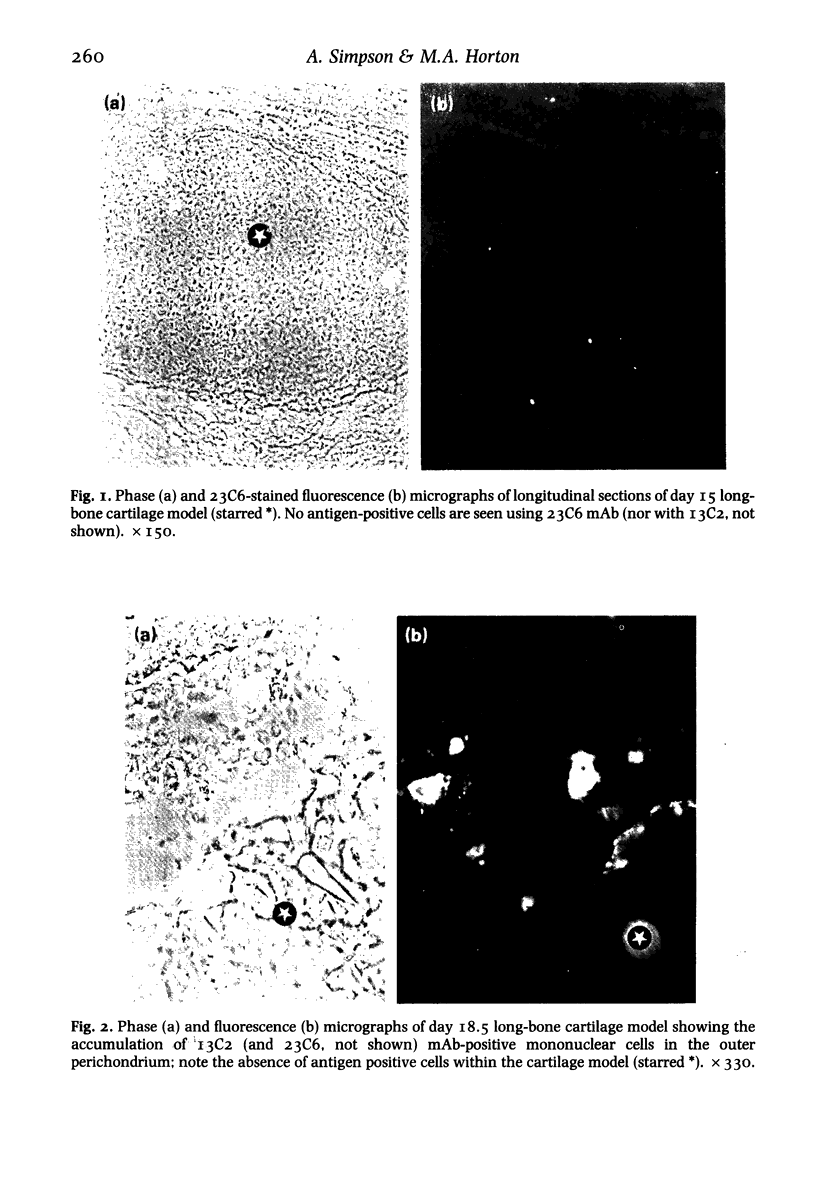
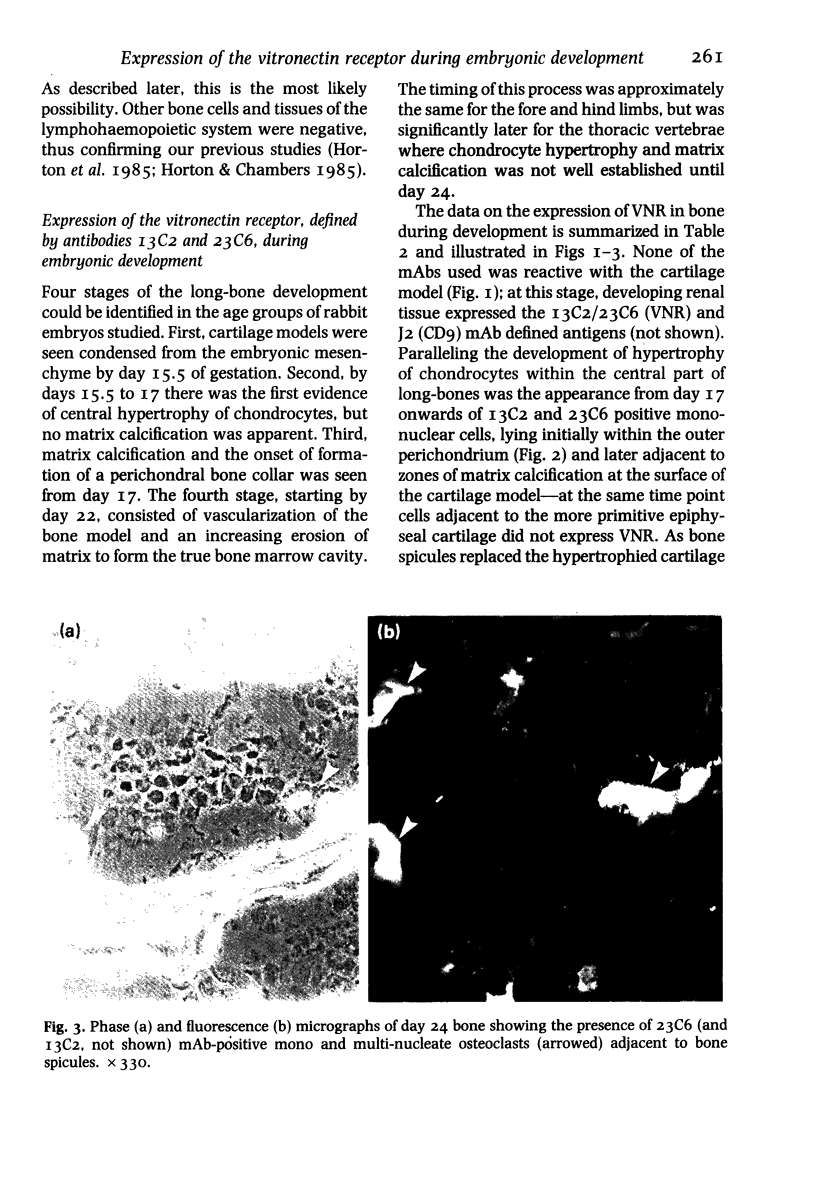
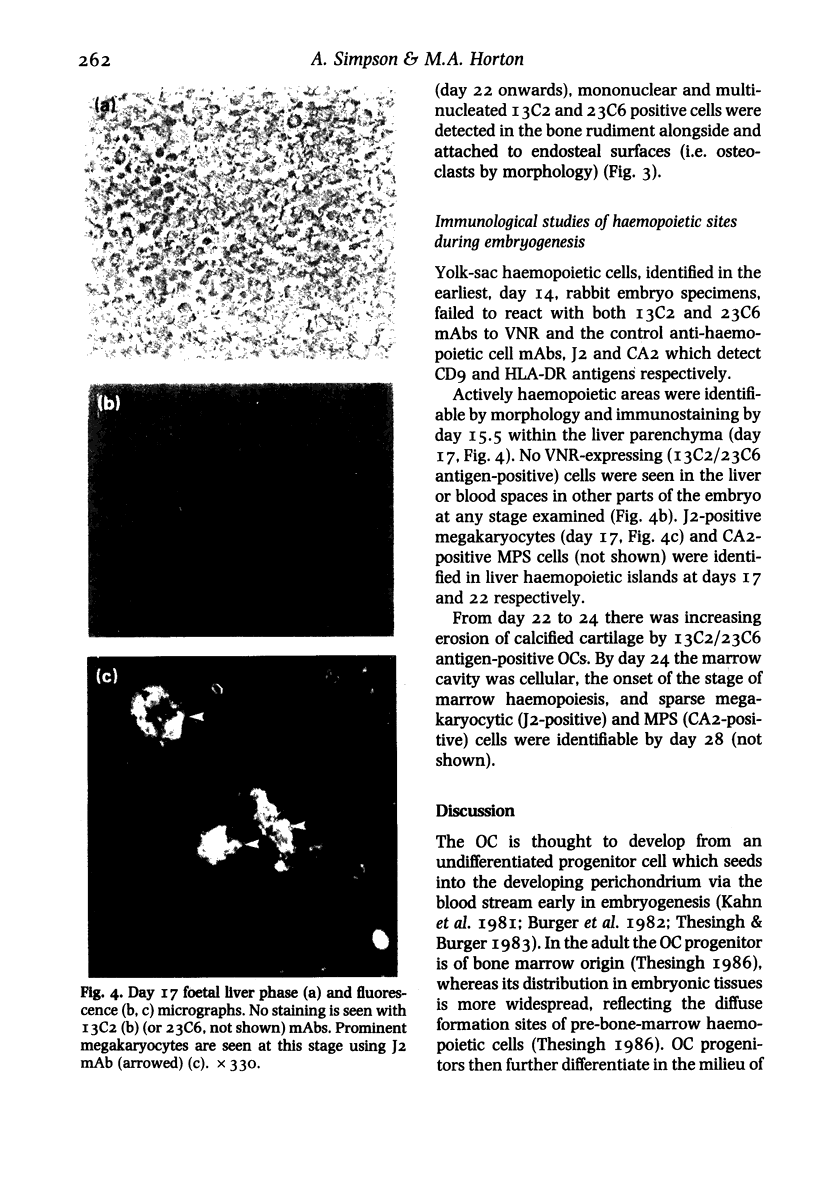
The development of the osteoclast during embryogenesis was studied in the rabbit by immunohistological techniques. Two monoclonal antibodies, 13C2 and 23C6, which react with the alpha-chain of the vitronectin receptor were used to define mono and multi-nucleate osteoclasts; being unreactive with other haemopoietic cells these antibodies could discriminate between osteoclasts and cells of the mononuclear phagocyte system. Staged rabbit embryos, from 14 to 28 days of age, were analysed and compared with findings from newborn and adult rabbits. No 13C2/23C6 immunoreactivity was seen in any of the tissues studied prior to day 17. 13C2/23C6-positive, mononuclear cells--presumptive osteoclast precursors--were first observed in the outer perichondrium of long-bones adjacent to the zone of hypertrophic cartilage in day 17 embryos. From day 17 onwards mono and multi-nucleate cells accumulated progressively in the perichondrium/periosteum, and by day 22 within the developing bone marrow cavity attached to bone spicules. No cells expressing the vitronectin receptor were seen at sites of embryonic or foetal haemopoiesis in yolk sac or foetal liver, that is, prior to the formation of the marrow cavity. Macrophages, defined by cross-reactivity with an antibody to human HLA-DR, first appeared in developing marrow spaces 11 days after the first osteoclast precursor appeared, suggesting that osteoclasts and definitive macrophages might develop from separate cell lineages, or that they diverge at an early stage of differentiation of haemopoietic stem cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burger E. H., Van der Meer J. W., van de Gevel J. S., Gribnau J. C., Thesingh G. W., van Furth R. In vitro formation of osteoclasts from long-term cultures of bone marrow mononuclear phagocytes. J Exp Med. 1982 Dec 1;156(6):1604–1614. doi: 10.1084/jem.156.6.1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers T. J. The pathobiology of the osteoclast. J Clin Pathol. 1985 Mar;38(3):241–252. doi: 10.1136/jcp.38.3.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemler M. E. Adhesive protein receptors on hematopoietic cells. Immunol Today. 1988 Apr;9(4):109–113. doi: 10.1016/0167-5699(88)91280-7. [DOI] [PubMed] [Google Scholar]
- Horton M. A., Chambers T. J. Human osteoclast-specific antigens are expressed by osteoclasts in a wide range of non-human species. Br J Exp Pathol. 1986 Feb;67(1):95–104. [PMC free article] [PubMed] [Google Scholar]
- Horton M. A., Lewis D., McNulty K., Pringle J. A., Chambers T. J. Monoclonal antibodies to osteoclastomas (giant cell bone tumors): definition of osteoclast-specific cellular antigens. Cancer Res. 1985 Nov;45(11 Pt 2):5663–5669. [PubMed] [Google Scholar]
- Horton M. A., Rimmer E. F., Lewis D., Pringle J. A., Fuller K., Chambers T. J. Cell surface characterization of the human osteoclast: phenotypic relationship to other bone marrow-derived cell types. J Pathol. 1984 Dec;144(4):281–294. doi: 10.1002/path.1711440410. [DOI] [PubMed] [Google Scholar]
- Le Douarin N. M. Cell migrations in embryos. Cell. 1984 Sep;38(2):353–360. doi: 10.1016/0092-8674(84)90490-2. [DOI] [PubMed] [Google Scholar]
- Marks S. C., Jr The origin of osteoclasts: evidence, clinical implications and investigative challenges of an extra-skeletal source. J Oral Pathol. 1983 Aug;12(4):226–256. doi: 10.1111/j.1600-0714.1983.tb00337.x. [DOI] [PubMed] [Google Scholar]
- Minkin C. Bone acid phosphatase: tartrate-resistant acid phosphatase as a marker of osteoclast function. Calcif Tissue Int. 1982 May;34(3):285–290. doi: 10.1007/BF02411252. [DOI] [PubMed] [Google Scholar]
- Naidet C., Sémériva M., Yamada K. M., Thiery J. P. Peptides containing the cell-attachment recognition signal Arg-Gly-Asp prevent gastrulation in Drosophila embryos. Nature. 1987 Jan 22;325(6102):348–350. doi: 10.1038/325348a0. [DOI] [PubMed] [Google Scholar]
- Nijweide P. J., Burger E. H., Feyen J. H. Cells of bone: proliferation, differentiation, and hormonal regulation. Physiol Rev. 1986 Oct;66(4):855–886. doi: 10.1152/physrev.1986.66.4.855. [DOI] [PubMed] [Google Scholar]
- Pytela R., Pierschbacher M. D., Ruoslahti E. A 125/115-kDa cell surface receptor specific for vitronectin interacts with the arginine-glycine-aspartic acid adhesion sequence derived from fibronectin. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5766–5770. doi: 10.1073/pnas.82.17.5766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rifkin B. R., Brand J. S., Cushing J. E., Coleman S. J., Sanavi F. Fine structure of fetal rat calvarium; provisional identification of preosteoclasts. Calcif Tissue Int. 1980;31(1):21–28. doi: 10.1007/BF02407163. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Pierschbacher M. D. Arg-Gly-Asp: a versatile cell recognition signal. Cell. 1986 Feb 28;44(4):517–518. doi: 10.1016/0092-8674(86)90259-x. [DOI] [PubMed] [Google Scholar]
- Sminia T., Dijkstra C. D. The origin of osteoclasts: an immunohistochemical study on macrophages and osteoclasts in embryonic rat bone. Calcif Tissue Int. 1986 Oct;39(4):263–266. doi: 10.1007/BF02555216. [DOI] [PubMed] [Google Scholar]
- Suzuki S., Argraves W. S., Arai H., Languino L. R., Pierschbacher M. D., Ruoslahti E. Amino acid sequence of the vitronectin receptor alpha subunit and comparative expression of adhesion receptor mRNAs. J Biol Chem. 1987 Oct 15;262(29):14080–14085. [PubMed] [Google Scholar]
- Thesingh C. W., Burger E. H. The role of mesenchyme in embryonic long bones as early deposition site for osteoclast progenitor cells. Dev Biol. 1983 Feb;95(2):429–438. doi: 10.1016/0012-1606(83)90044-1. [DOI] [PubMed] [Google Scholar]
- van de Wijngaert F. P., Burger E. H. Demonstration of tartrate-resistant acid phosphatase in un-decalcified, glycolmethacrylate-embedded mouse bone: a possible marker for (pre)osteoclast identification. J Histochem Cytochem. 1986 Oct;34(10):1317–1323. doi: 10.1177/34.10.3745910. [DOI] [PubMed] [Google Scholar]






